ABSTRACT
Pancreatic cancer is one of our most lethal malignancies. Despite substantial improvements in the survival rates for other major cancer forms, pancreatic cancer survival rates have remained relatively unchanged since the 1960s. Pancreatic cancer is usually detected at an advanced stage and most treatment regimens are ineffective, contributing to the poor overall prognosis. Herein, we review the current understanding of pancreatic cancer, focusing on central aspects of disease management from radiology, surgery and pathology to oncology.
Data taken from NORDCAN [Citation16].
![Figure 1. Time trends in the 5-year age standardized relative survival for pancreatic cancer in the Nordic countries, 1964–2013.Data taken from NORDCAN [Citation16].](/cms/asset/957ddae1-8935-4f8e-812b-02fcca6b106c/ifon_a_12331277_f0001.jpg)
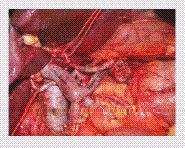
By courtesy of Professor Philippe Bachellier.
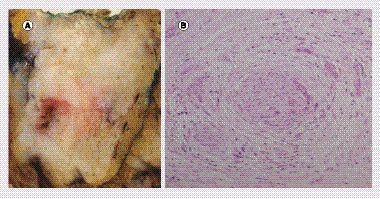
(A) Gross examination shows a 4.1 cm tumor in the head of the pancreas. The consistency is firm and the appearance after cross section is gray–white. (B) Microscopic examination reveals a moderately differentiated ductal adenocarcinoma with a poorly differentiated component. Infiltrating malignant glands are seen here embedded in a dense fibrotic stroma.
Adapted with permission from [Citation135].
![Figure 5. Targetable mechanisms in pancreatic cancer.Adapted with permission from [Citation135].](/cms/asset/0ee15edd-adbb-4add-9fc7-19af7fdffbff/ifon_a_12331277_f0005.jpg)
Historical remarks & present state
The first known description of pancreatic cancer is attributed to Giovanni Battista Morgagni in his 1761 publication ‘de Sedibus Et Causis Morborum Per Anatomen Indagatis Libri Quinque’ [Citation1]. However, the lack of a microscopic evaluation makes the true diagnosis of ductal adenocarcinoma uncertain. The next important advancement in our understanding of pancreatic cancer did not come until 1858, when Jacob Mendez Da Costa revisited Morgagni’s original work and also described the first microscopic diagnosis of adenocarcinoma, manifesting pancreatic cancer as a true disease entity [Citation2].
The history of pancreatic surgery is fairly recent and involves a combination of brave surgical pioneers, the development of surgical anesthesia and modern aseptic techniques. Some landmarks in the history of pancreatic surgery deserve to be mentioned. The first reported attempt at a pancreaticoduodenectomy was performed in 1898 by the Italian surgeon Alessandro Codivilla for a tumor involving the head of the pancreas [Citation3]; however, the patient did not survive the postoperative period. In the same year, William Stewart Halsted from Johns Hopkins Hospital performed the first successful resection for ampullary cancer by excising portions of the duodenum and the pancreas [Citation4]. The first successful pancreaticoduodenectomy is credited to the German surgeon Walther Carl Eduard Kausch, as part of a two-stage procedure [Citation5]. In 1914, Georg Hirschel performed the first successful pancreaticoduodenectomy in one stage [Citation6] and then in 1935, Allen Oldfather Whipple presented the results of a two-stage procedure involving the resection of the head of the pancreas and duodenum for ampullary carcinoma at the annual meeting of the American Surgical Association, which renewed interest in pancreatic surgery [Citation7]. During his career, Whipple performed 37 pancreaticoduodenectomies, with the procedure evolving into a one-stage technique [Citation8,Citation9], and Whipple is generally credited with popularizing the operation that still bears his name. In 1937, Alexander Brunschwig performed the first successful pancreaticoduodenectomy for pancreatic adenocarcinoma [Citation10]. Today, with the concentration of experience in high-volume hospitals, pancreatic surgery has become a safe procedure associated with an operative mortality below 3% [Citation11–13].
While major advances have been made in the surgical management of pancreatic cancer since the era of Whipple, the long-term survival for pancreatic cancer patients is still extremely poor. In the Nordic countries, as in the rest of the World, the 5-year survival rates for pancreatic cancer have remained stagnant since the 1960s (). Pancreatic cancer is currently the fourth leading cause of cancer death in the USA and Europe, but is expected by 2020 to become the second cause of death from cancer after lung cancer [Citation14,Citation15].
Risk groups: how do we find pancreatic cancer earlier?
The lifetime risk of developing pancreatic cancer is 1.49%, which is about one in 67 cases [Citation17]. Pancreatic cancer occurs at all ages but the peak incidence is between 60 and 80 years of age. The symptoms of pancreatic cancer are insidious in onset and gradually progress over time, including midepigastric pain sometimes radiating to the back, weight loss, malaise, nausea and fatigue. Jaundice is a characteristic sign of cancers in the head of the pancreas due to compression of the common bile duct. Sometimes a tumor may extend to the duodenum or stomach, leading to gastric outlet obstruction. The diagnosis of pancreatic cancer usually depends upon the symptoms. Therefore, the diagnosis is often late when there is no chance for cure. However, if the tumor is suspected in individuals with an increased risk of pancreatic cancer or with a family history of pancreatic cancer, earlier diagnosis may be possible.
Approximately 90% of all cases of pancreatic cancer are sporadic, while 10% are hereditary. Smoking is the most important environmental risk factor for pancreatic cancer [Citation18], while obesity has been associated with a higher incidence of pancreatic cancer in epidemiological studies [Citation19]. The etiological role of alcohol in pancreatic cancer has been hard to ascertain because of the close association between alcohol and chronic pancreatitis, a known risk factor for pancreatic cancer [Citation20]. Overall, moderate alcohol consumption is not a risk factor for pancreatic cancer. Recent pooled analyses of case–control and cohort studies suggest that heavy alcohol consumption may increase the risk of pancreatic cancer independent of pancreatitis or tobacco smoking [Citation21,Citation22]. The link between diabetes and pancreatic cancer has been studied for many years. Long-standing diabetes mellitus is a modest risk for pancreatic cancer [Citation23], while new-onset diabetes mellitus may be a manifestation of pancreatic cancer, especially in individuals aged over 50 years [Citation24]. Certain precursor lesions have been associated with the development of pancreatic cancer, including pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) [Citation25]. Of those with hereditary pancreatic cancer, a subgroup of patients may have identified genetic alterations. Known genetic mutations include the STK11 mutation recognized in Peutz-Jeghers syndrome, the cationic trypsinogen gene PRSS1 and SPINK1 mutations associated with hereditary pancreatitis, the p16 mutation linked to familial atypical multiple mole melanoma (FAMMM), the adenomatous polyposis coli (APC) mutation responsible for familial adenomatous polyposis (FAP), BRCA1 and BRCA2 mutations increasing the risk for hereditary breast ovary cancer (HBOC), MLH1 and MSH2 gene mutations associated with hereditary nonpolyposis colon cancer (HNPCC) and the ataxia telangiectasia (ATM) mutation [Citation26].
Pancreatic cancer progresses from noninvasive precursor lesions to invasive cancer over a variable time period. It takes on average 10 years from when a mutation occurs until a primary tumor forms and another decade before the patient dies of the disease [Citation27]. Thus, a tumor usually grows in silence for a long time, which means that there is an opportunity for early diagnosis.
Improving survival will require the accurate diagnosis of early pancreatic cancer. All pancreatic tumors are classified according to the TNM staging system ( & ) [Citation28]. This is based on the evaluation of the primary tumor, lymph node status and the presence of metastatic disease. The R classification is an auxiliary classification and describes the likelihood of any residual tumor after treatment [Citation29]. Currently, most patients with pancreatic cancer are diagnosed with distant metastases and have a poor 5-year survival rate. There is strong evidence that the detection of pancreatic cancer at an earlier, resectable stage has a favorable impact on long-term survival, and even with the potential for micrometastases the survival is better for patients with surgical resection than patients who do not undergo surgery [Citation30]. The 5-year survival rate of localized pancreatic cancer is about 25%, while being 10% for regional disease and 2% for distant disease according to the American Cancer Society [Citation31]. Surgery alone is generally considered curative for patients with PanIN-3, IPMN or MCN with high-grade dysplasia that have not progressed to invasive carcinoma.
To be successful a screening program for pancreatic cancer needs to find and treat patients with T1N0M0 cancer with negative resection margins or highly dysplastic precursor lesions. According to the International Cancer of Pancreas Screening (CAPS) Consortium [Citation32], the screening should not be done on a population basis, but in groups according to defined criteria, such as those with two first-degree relatives with pancreatic cancer or known genetic syndromes associated with an increased risk of pancreatic cancer. Initial screening should include endoscopic ultrasound (EUS) or MRI/magnetic resonance cholangiopancreatography. Screening typically begins at age 40 years in PRSS1 mutation carriers with hereditary pancreatitis due earlier onset of pancreatic cancer. In other high-risk populations, there is no consensus at what age to begin and end screening. Newly diagnosed diabetes mellitus in patients over 50 years is a very interesting group that has so far not been subjected to screening [Citation33]. However, the role of screening EUS/MRI even in high-risk individuals is still unclear. Abnormal findings are frequent and the management of these detected lesions is still unclear. To be cost effective and practical, easier methods for screening are required, for example, biomarkers that can be measured in blood samples. CA 19-9, a Sialyl Lewis carbohydrate antigen, is the only serum marker for pancreatic cancer that has been used in clinics and has a sensitivity and specificity of around 80% [Citation34]. Its sensitivity for early-stage pancreatic cancer is even lower, which means that it cannot be used for screening purposes. CA 19-9 can be falsely elevated by cholestasis and some patients lack the Lewis antigen, and therefore cannot express CA 19-9. The clinical utility of CA 19-9 is currently only for therapeutic monitoring to follow the dynamics of an individual patient’s disease [Citation35]. In summary, there are currently no methods of screening for sporadic pancreatic cancer. Novel biomarkers for pancreatic cancer are urgently needed as most pancreatic cancer cases occur in patients without evidence of a hereditary origin. The search for pancreatic cancer biomarkers is an intense research field.
Radiological examination
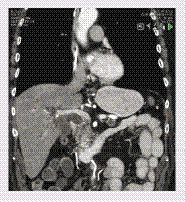
Computed tomography (CT) remains the standard examination for pancreatic cancer (). The direct sign of pancreatic cancer on a CT is a low attenuation, hypovascular lesion, which may be iso-dense in about 11% of cases. Dual-energy CT-perfusion is a promising new technique and possibly a tool for small iso-attenuating tumors [Citation36]. Indirect signs of pancreatic cancer are the dilation of the biliary tree and the main pancreatic duct, as well as atrophy of the distal pancreas. MRI and EUS may be of value in uncertain cases. MRI has poorer spatial resolution and tissue diagnosis than CT, but is good for the imaging of ductal structures. Stenosis of the main pancreatic duct is suggestive of underlying malignancy and should always be followed up [Citation37]. MRI is also useful for the detection of cystic tumors of the pancreas. Advances in MRI instrumentation over the past years include the improvement of magnet and gradient coil technology, radiofrequency electronics and computer systems, leading to improved image quality, faster exams and higher throughput. Diffusion-weighted (DW) MRI is a relatively new MRI technique that reflects the random Brownian motion of water molecules in different tissues. The measurement of the movement of water molecules in DW MRI provides an additional feature for lesion characterization that may further increase the specificity of MRI for classifying pancreatic lesions [Citation38]. The high resolution of EUS enables the visualization of lesions as small as 1 mm, which is useful for the detection of early pancreatic cancer [Citation39]; however, the poor specificity of EUS imaging remains a challenge. EUS-guided fine needle aspiration (FNA) may aid in the differentiation of benign from malignant lesions, but false-negative and false-positive diagnoses are common, and may be attributed to sampling error and cytological misinterpretation [Citation30]. The added value of PET for staging and diagnosis of pancreatic cancer is controversial [Citation40]. In addition to conventional imaging techniques, biomarker-based imaging using molecular probes, nanoparticles or antibodies, are being evaluated in experimental settings and may ultimately aid in early cancer detection and development of novel therapeutic strategies [Citation41,Citation42].
Operability assessment
The preoperative evaluation of pancreatic cancer includes the assessment of the relationship between the tumor and the adjacent major vessels, portal vein (PV), superior mesenteric vein (SMV), superior mesenteric artery (SMA), celiac axis (CA), hepatic artery (HA), together with the presence of metastases (most commonly liver, peritoneum, lungs). With current imaging methods it is challenging to distinguish between true tumor infiltration of the vessel wall and inflammatory changes caused by a tumor. The value of diagnostic imaging can be improved by using structured protocols, such as the one developed by radiologists in the USA [Citation43].
Operability assessment also involves a comprehensive consideration of the risk–benefit of pancreatic resection. This involves weighing the patient’s physical and mental resources in relation to the stress caused by surgery. Age in itself is not a factor to exclude patients from potentially curative surgery, but with increasing age comes increasing co-morbidity [Citation44].
Currently, we have no specific methods to assess operative risk. Cardiopulmonary exercise testing that measures oxygen consumption and carbon dioxide elimination may identify a subgroup of patients with increased operative risk but mortality is low, so the use of the test as a discriminatory factor is not fully adequate [Citation45].
Many patients with pancreatic cancer are malnourished at the time of diagnosis. In severe cases, malnutrition may progress to cancer cachexia, which is characterized by the loss of lean body mass, muscular atrophy and impaired function of the immune system [Citation46]. Low preoperative albumin is a strong risk factor for complications after pancreatic resection [Citation47]. Approximately 25% of all patients with resectable pancreatic cancer suffer from sarcopenia, a relatively new concept reflecting the degenerative loss of skeletal muscle mass. It has been shown that sarcopenia is a significant predictor of major complications (Clavien grade ≥III), hospitalization, intensive care unit stay, as well as infectious and cardiopulmonary complications after pancreatic cancer surgery. Furthermore, patients with sarcopenia have impaired long-term survival [Citation48].
Jaundice is a common symptom of pancreatic cancer. It has become clear over time that obstructive jaundice impairs the outcome of pancreatic resection. However, a randomized study showed that if surgical resection is performed within 1 week after the diagnosis of jaundice, the overall morbidity rate following surgery is decreased compared with preoperative biliary drainage with endoscopic retrograde cholangiopancreatography (ERCP) [Citation49]. Stent placement has been found to be associated with complications such as cholangitis and pancreatitis. Although early surgery without preoperative biliary drainage remains the treatment of choice, fully covered self-expandable metal stents may be preferred over plastic stents whenever preoperative biliary drainage is indicated [Citation50]. In severely jaundiced patients with serum bilirubin levels ≥300 µmol/l, biliary stenting is appropriately recommended [Citation51].
Perioperative mortality after pancreatic resection is governed by the complications, and nearly half of all patients who undergo Whipple’s operation develop one or more complications. Mortality is driven by serious morbidity, which largely depends on pancreatic fistula formation, representing a failure in healing of the pancreatic anastomosis or a parenchymal leak not directly related to an anastomosis, and this leakage can result in sepsis and bleeding. Numerous attempts have been made to reduce the frequency of pancreatic fistula using different surgical techniques, but none have proved superior. A number of meta-analyses indicate that a pancreaticogastrostomy has a slightly lower rate of leakage compared with a pancreaticojejunostomy [Citation52,Citation53]. Other risk factors for pancreatic fistulas include a high BMI, a small pancreatic duct diameter and a soft pancreatic texture [Citation54,Citation55].
It is important that the patient does not only survive the operation, but also leaves hospital with an acceptable quality of life. It has been shown that operated patients have a higher quality of life 6 months postoperatively than before surgery [Citation56].
Surgical treatment: recent trends & advancements
Resectable pancreatic cancer is defined by the absence of distant metastasis, the lack of SMV or PV distortion and clear fat planes around the CA, HA and SMA [Citation57]. A relatively new concept in pancreatic surgery is borderline resectable pancreatic cancer. This concept includes tumors that involve the mesentericoportal or arterial axis, constituting an intermediate stage between upfront resectable and technically unresectable disease. At diagnosis, approximately 20% of the patients are found to have resectable disease, 20–25% have locally advanced disease and 50–55% have distant metastases. The group of borderline resectable patients are found in the group with locally advanced disease, where the patients with vessel involvement now are considered for resection [Citation58]. The International Study Group of Pancreatic Surgery (ISGPS) has presented a consensus report to avoid misclassification of borderline resectable tumors as unresectable [Citation59]. Borderline resectable tumors are characterized by the lack of distant metastasis and may show venous involvement with narrowing or occlusion of the PV/SMV, but with suitable vessels proximally and distally allowing for safe vein resection and reconstruction. On the arterial side, borderline tumors in the pancreatic head may have gastroduodenal artery encasement and short encasement of the HA, but less than 50% abutment of the SMA around the vessel wall and no involvement of the CA [Citation57,Citation59]. For borderline tumors in the body and tail, contact with the CA is allowed but only if the encasement is less than 50%.
Is there any benefit to performing vein resection? The first case of pancreaticoduodenectomy with vein resection was reported in 1951 by Moore et al. [Citation60]. Vein resections were performed in the 1960s and 1970s in order to increase the resectability rates [Citation61,Citation62], but enthusiasm waned because of the high morbidity and mortality associated with the procedure. Today, vein resections can be performed safely. Isolated vein involvement is not a contraindication to resection, with comparable short and long-term outcomes comparable to those of a standard resection [Citation63]. Techniques that can be used for reconstruction of the PV or SMV are wedge resection with venoplasty, end-to-end anastomosis (), patches or vein interposition grafts [Citation64,Citation65]. Direct end-to-end anastomosis of the PV system is safe, but when tension-free anastomosis cannot be guaranteed; generally, in cases requiring ≥3 cm of SMV/PV resection, venous autografting may decrease the likelihood of anastomotic stenosis [Citation66].
How should the splenic vein (SV) be dealt with? The SV can be safely ligated if there is preserved collateral blood flow in the SV-inferior mesenteric vein (IMV), left gastric vein, splenocolic collaterals (Barkow) or middle colic vein. Potential risks of ligating the splenic vein include left-sided portal hypertension, splenomegaly, esophageal varices and hypertensive gastropathy. If the SV-IMV confluence is absent or impossible to preserve, drainage of the SV may be re-established by construction of an anastomosis between the SV and IMV to avoid left-sided portal hypertension [Citation67].
The ISGPS does not recommend arterial resection on a routine basis [Citation59], as pancreatic resection with simultaneous arterial resection is associated with a greatly increased perioperative mortality (>5-times) and halved 1-year survival rate compared with similar patients without arterial resection [Citation68]. However, arterial resection is associated with more favorable survival rates compared with patients who did not undergo resection for locally advanced disease. Patients categorized as borderline resectable on the basis of the features of arterial involvement seen by imaging, should undergo surgical exploration in order to obtain further verification of any arterial infiltration. In cases where arterial involvement is verified, palliative treatment is the standard care.
Extended lymphadenectomy has been studied in an effort to improve survival rates in pancreatic cancer. Extended lymphadenectomy involves the resection of the lymph nodes along the left side of the SMA and around the celiac trunk, splenic artery or left gastric artery [Citation69]. The reason why ISGPS does not recommend an extended lymphadenectomy is that there is no evidence of survival benefit for the patient [Citation69,Citation70]. Positive lymph nodes outside the standard lymphadenectomy are considered to be metastatic disease. Selective removal of suspected lymph nodes during surgery has not been reported to influence survival. The standard lymphadenectomy should always be performed together with the primary pancreatic resection.
In recent years fast-track or enhanced recovery after surgery (ERAS) programs have emerged with the aim to accelerate postoperative recovery by decreasing the body’s stress reaction to surgery. Key constituents of these fast-track programs include preoperative education, avoidance of fluid overload, multimodal analgesia, early oral intake and enforced mobilization. Fast-track programs for pancreaticoduodenectomy are safe and feasible, and reduce complications such as delayed gastric emptying, as well as length of hospital stay and costs [Citation71,Citation72].
Pathological evaluation: how do we improve prognostic accuracy?
It is rarely necessary to provide a preoperative histopathological diagnosis for a patient with a resectable pancreatic tumor. A negative FNA biopsy does not exclude an adenocarcinoma because of the possibility of sampling error. FNA is generally restricted to patients with borderline or unresectable disease to provide a histological diagnosis before initiating chemotherapy [Citation73]. To avoid the risk of tumor seeding in the needle track, biopsies should be taken with EUS.
Approximately 70% of all pancreatic cancers occur in the head of the pancreas, 20% in the body of the pancreas and 10% appear in the tail. Macroscopically, pancreatic cancers are associated with a prominent desmoplastic reaction and appear as gray-white, firm masses (). At the microscopic level, ductal adenocarcinomas form more or less well defined duct structures, and are embedded in an extensive, fibrotic stroma (). Immunohistochemically, pancreatic cancers may express CEA, CA 19-9, CA125, SMAD4, as well as cytokeratins (CK 5/6, 7, 8, 18, and 19) and mucins (MUC1, MUC4, MUC5AC, and MUC6). Increasing tumor size and invasion correlate with poor patient survival [Citation74]. Lymph node involvement is a major prognostic factor in pancreatic cancer, and prognosis has been proposed to be affected by the number of positive lymph nodes [Citation75,Citation76]. The histological grade is based on glandular differentiation, mucin production intensity, number of mitoses per high power field (HPF) and nuclear pleomorphism grade. Grade 1 tumors form well-differentiated duct-like glands, have intensive mucin production and show few mitoses and little pleomorphism. Grade 2 tumors are composed of moderately differentiated duct-like structures and have irregular mucin production, moderate mitoses and moderate pleomorphism. Grade 3 tumors form poorly differentiated glands, with little mucin production, high mitotic rate (>10 per HPF) and distinct pleomorphism. Variations in the rate of differentiation within the same tumor are common. Poorly differentiated tumors exhibit a very aggressive behavior and carry a poor prognosis [Citation77,Citation78]. Vascular, lymphatic and perineural invasion have also been shown to worsen the prognosis of pancreatic cancer [Citation79].
The R classification of pancreatic cancer involves the evaluation of the surgical specimen to exclude any tumor cells at the resection margins. R0 resection indicates that there are no tumor cells at any of the margins, while microscopic residual tumor is defined as R1. Given the complex nature of pancreatic surgery, sometimes not all macroscopic tumor tissue can be removed at the time of surgical exploration. This is known as an incomplete resection or R2 resection. The vast majority of patients with pancreatic cancer who undergo surgical resection eventually develop local or systemic recurrence. Historically, R0 frequency has been reported at 70–80%, which is significantly higher than the 5-year survival rate after curatively aimed surgery. This observation is partly explained by the lack of a standardized assessment of tumor specimens. In recent years standardized protocols have been developed for the pathological examination of Whipple specimens, which have increased the rate of R1 resections [Citation80,Citation81]; yet both 0 and 1 mm are still used to define R0 resections. The R0 resection rate is 70–74% when using a 0 mm margin and decreases to 29–49% when using a 1 mm margin [Citation82]. The most commonly involved margins are the SMV/PV, medial/SMA and posterior margins.
Oncological treatment
What is the evidence for adjuvant treatment of resectable pancreatic cancer? Several randomized trials have been published on the adjuvant setting for resectable pancreatic cancer (). In the 1980s, the small GITSG trial indicated that combined radiation and fluorouracil (5-FU) was superior to observation alone [Citation83]. The treatment group received 2 years of 5-FU following chemoradiation and it might be speculated that it was the long adjuvant treatment with 5-FU that benefited the patients. The ESPAC 1 study [Citation84] compared 5-FU, chemoradiotherapy, chemoradiotherapy followed by 5-FU and observation, and the results showed that 5-FU prolongs survival while chemoradiotherapy shortens survival. The CONKO 001 trial [Citation85,Citation86] randomized patients to either gemcitabine or observation. The 5-year survival rate in the gemcitabine group was 21% and the median survival was 23 months, while patients that received no adjuvant treatment had a 5-year survival rate of 10% and a median survival of 20 months. The RTOG-9704 trial [Citation87,Citation88] compared gemcitabine with 5-FU before and after 5-FU-based chemoradiation and found no significant difference in survival rates between the treatment arms. In the ESPAC 3 study [Citation89], patients were randomized to gemcitabine or 5-FU. There was no significant difference in median survival time between the gemcitabine and 5-FU groups (23.6 vs 23.0 months), but more toxicity was reported with 5-FU. The results of the JASPAC 01 study [Citation90] from Japan was presented at the ASCO meeting in 2013, and in this study gemcitabine was compared with S-1. The results are very interesting, with a median survival time for gemcitabine of 26 months compared with 46 months for S-1. However, it should be mentioned that there may be a difference in the S-1 metabolism between Asians and Europeans. Thus, the current standard for adjuvant treatment is still gemcitabine, but for Asian patients S-1 could be a superior alternative but long-term follow-up has yet to be reported.
What studies are in the pipeline for adjuvant therapy? ESPAC 4 (ISRCTN96397434) is a randomized study designed to compare gemcitabine with gemcitabine-capecitabine; this study has been completed, but the data have not yet been published. APACT (NCT01964430) is an ongoing study that aims to compare gemcitabine × 6 with gemcitabine/nab-paclitaxel (Abraxane®) × 6. PRODIGE24/ACCORD24 (NCT01526135) is another ongoing study designed to compare gemcitabine × 6 with mFOLFIRINOX × 12. The upcoming ESPAC 6 study draws inspiration from JASPAC 01 and is scheduled to include S-1, but is awaiting data from ESPAC 4 before the study design is determined.
What is the evidence for neoadjuvant therapy in resectable or borderline pancreatic cancer? There are no Phase III studies in this area. Currently, neoadjuvant therapy is not standard in resectable cancer. In borderline resectable cancer, common praxis is neoadjuvant treatment with chemotherapy, chemoradiotherapy or chemotherapy followed by chemoradiotherapy. ESPAC 5F (ISRCTN89500674) is a four-arm Phase II study on borderline resectable pancreatic cancer. The purpose of the study is to compare neoadjuvant gemcitabine–capecitabine × 2, neoadjuvant FOLFIRINOX × 4, neoadjuvant chemoradiotherapy and immediate surgery. All patients undergoing surgical resection will receive adjuvant gemcitabine or 5-FU. NEPAFOX is a Phase II/III trial (NCT02172976), where the aim is to compare surgery and adjuvant gemcitabine × 6 with perioperative FOLFIRINOX given for six cycles preoperatively and six cycles postoperatively.
What is the evidence for palliative chemotherapy in locally advanced pancreatic cancer? In clinical practice, the same regimens are administered as in metastatic disease. For palliative chemoradiotherapy there are promising data from small retrospective studies [Citation91,Citation92]. However, in the Phase III study LAP-07 [Citation93], administering chemoradiotherapy to patients with stable disease showed no improvement compared with continuing chemotherapy in patients with locally advanced disease.
What is the evidence for chemotherapy in metastatic pancreatic cancer? summarizes the outcomes of randomized trials in this area. 5-FU has been well studied and has shown beneficial effects compared with best supportive care, although in 1997 Burris et al. [Citation94] reported that gemcitabine was superior to 5-FU. Gemcitabine was associated with a median survival time of 5.7 months compared with 4.4 months for 5-FU. The 1-year survival rate for gemcitabine reached 18% with a clinical benefit of 24% compared with 2 and 5% for 5-FU, respectively. The NCIC CTG PA.3 trial [Citation95] compared single-agent gemcitabine with combination therapy using the EGFR inhibitor erlotinib. The addition of erlotinib was associated with a statistically significant improvement of survival time by 2 weeks, from 5.9 to 6.2 months, but due to this limited survival benefit and the added toxicity, this regimen has not become widespread. The study PRODIGE4/ACCORD 11 [Citation96] randomized patients to gemcitabine or FOLFIRINOX. Median survival time increased from 6.8 months to, in this context, an impressive 11.1 months in the FOLFIRINOX group. The proportion who responded to treatment was 9% in the gemcitabine group and 32% in the FOLFIRINOX group. The study included only patients with an ECOG performance status of 0–1 and the toxicity associated with FOLFIRINOX must be taken into account. In the MPACT study [Citation97] gemcitabine was compared with gemcitabine/nab-paclitaxel (Abraxane). Survival times increased from 6.7 to 8.5 months, and the percentage who responded to treatment increased from 7 to 23% with the addition of nab-paclitaxel. Regarding second-line treatment after gemcitabine failure, the CONKO-003 part 1 trial [Citation98] indicated that OFF (oxaliplatin, folinic acid/leucovorin and 5-FU) significantly increased survival times compared with best supportive care. This study was followed by the CONKO-003 part 2 trial [Citation99], which found that OFF significantly prolonged survival time compared with 5-FU and leucovorin alone. However, the PANCREOX study [Citation100] failed to confirm the findings of the CONKO-003 studies and reported that the addition of oxaliplatin to 5-FU and leucovorin in the second-line treatment may be detrimental. In the PANCREOX study, there were some imbalances in the treatment groups with regard to performance status and subsequent treatments. More patients withdrew in the mFOLFOX6 group than in the 5-FU/LV group due to adverse events (20 vs 2%). The addition of oxaliplatin should be carefully considered in frail patients due to concerns regarding toxicity and efficacy in the second-line setting. On 22 October 2015, the US FDA approved nanoliposomal irinotecan (MM-398/Onivyde®) for use in combination with 5-FU and leucovorin to treat patients with metastatic pancreatic cancer whose disease has progressed after gemcitabine-based chemotherapy. The NAPOLI-1 trial [Citation101] randomly assigned patients to either nanoliposomal irinotecan plus 5-FU and leucovorin, nanoliposomal irinotecan monotherapy or 5-FU and leucovorin. The results showed that nanoliposomal irinotecan plus 5-FU and leucovorin significantly increased median overall survival time from 4.2 months with 5-FU and leucovorin to 6.1 months with acceptable toxicity.
Palliation: pain relief, nutrition & jaundice
Palliative care is a cornerstone of comprehensive pancreatic cancer management. Palliative care aims to improve the quality of life for patients and their families by preventing and relieving physical, mental, social and spiritual problems. A named contact nurse should be recommended to all patients.
Pain treatment for pancreatic cancer requires a multimodal approach. The WHO analgesic ladder proposes that the treatment of pain should begin with nonopioid medication, such as paracetamol or a NSAID. If the pain is not sufficiently controlled a weak opioid can then be introduced, and subsequently a strong opioid. Steroids have been shown to reduce cancer-related pain [Citation103]. For neuropathic pain, amitriptyline, sodium valproate or gabapentin may be administered [Citation104]. A celiac plexus block may be used for severe, intractable pain [Citation105], and the procedure can be performed intraoperatively or guided via CT or EUS. For all pain management, follow-up and evaluation are important.
Malnutrition affects most patients with pancreatic cancer. Reasons for this include gastric outlet obstruction, pancreatic exocrine and endocrine insufficiency, pain, nausea and cachexia. Pancreatic enzymes and nutritional drinks lead to significantly better weight stability [Citation106]. This is important because weight stability has been linked to prolonged survival and improved quality of life in patients with unresectable pancreatic cancer [Citation107]. Steroids have also been associated with improved quality of life and a reduction in cancer-related fatigue [Citation108].
Endoscopic metal stents are preferred over plastic stents in patients with unresectable pancreatic cancer and obstructive jaundice [Citation109]. The use of metallic biliary stents is also preferred over surgical biliary bypass. Furthermore, duodenal self-expanding metal stents appear to be superior to surgical bypass for symptomatic gastric outlet obstruction [Citation110].
Translational research: what’s beyond the horizon?
Biobanking and biobank repositories have become an integral part of pancreatic cancer research. Molecular profiling of biobank specimens, such as tissue and blood, holds significant potential to improve basic and translational research, especially when linked to clinical outcome data. OMICS technology can accelerate our understanding of pancreatic cancer on a molecular level by identifying key drivers of disease progression and biomarkers for diagnosis and targeted intervention [Citation111–116]. In the future, the increased molecular understanding of pancreatic cancer will inevitably lead to more tailored treatment options. Some promising examples include targeting specific signaling pathways, modulating the tumor stroma, immunotherapy, stem cell therapy and inhibiting cancer metabolism. Nanotechnology is an emerging treatment approach that can enhance therapeutic drug delivery.
Targeting key signaling pathways
The use of molecular targeted therapies is based on the notion that blocking particular molecules or predominant signaling pathways regulating tumor growth, progression, invasion, metastasis and chemoresistance can induce tumor regression. Pancreatic cancer is characterized by frequently observed mutations in KRAS and misregulation of diverse signaling pathways, including TGF-β, Wnt/Notch and hedgehog signaling [Citation112]. Several key signaling pathways, covering an average of 63 genetic alterations per tumor, have been identified in pancreatic cancer, involving cellular processes such as apoptosis, DNA damage control, G1/S cell cycle transition and the regulation of invasion. Although molecularly targeted treatments have not yet proven to significantly prolong patient survival [Citation117], they may prove useful within precision or personalized medicine when targeted therapies based on an individual patient’s tumor profile are used. Mutationally activated KRAS is an attractive therapeutic target given its expression in more than 90% of pancreatic cancers and its role in the initiation and progression of the disease. Mutant KRAS was previously believed to be ‘undruggable’ due to the lack of well-defined surface pockets for drug binding. However, this limitation may have been overcome by new innovative approaches, such as siRNA delivery technology [Citation118] (Phase II trial NCT01676259) and small molecule KRAS inhibitors [Citation118].
Stroma targeting
Pancreatic cancer is characterized by a dense desmoplastic reaction with the stroma, comprising more than 80% of the entire tumor volume. The stroma consists of pancreatic stellate cells, infiltrating immune cells, blood vessels and an extracellular matrix. Interactions between pancreatic cancer cells and stromal cells promote tumor growth, invasion, metastasis and chemoresistance. Moreover, the pancreatic tumor stroma creates an immunosuppressive microenvironment, which is conducive to tumor development and progression. A number of approaches are underway in order to disrupt the stroma to improve drug penetrance to the tumor [Citation119]. Potential strategies include PEGylated hyaluronidase (PEGPH20), an enzyme which can deplete hyaluronic acid in the extracellular matrix. Preliminary data from Phase Ib/II studies demonstrate the clinical activity of PEGPH20 and the potential use of hyaluronidase expression as a biomarker for treatment [Citation120,Citation121]. Other strategies directed against the stroma include FG-3019, a monoclonal antibody (mAb) against connective tissue growth factor (Phase II trial NCT02210559)
Immunotherapy
Immunotherapy can be based on either active or passive therapeutical approaches. Active immunotherapy aims to stimulate the pre-existing immune response to recognize tumor-associated antigens and eliminate tumor cells by means of vaccination. Several types of cancer vaccines have been tested in pancreatic cancer, including whole cell vaccines, peptide vaccines, DNA vaccines and antigen pulsed dendritic cells, with promising results in small trials [Citation122]. Whole cell vaccines, such as Algenpentucel-L (NCT01072981; NCT01836432) and GVAX (NCT01896869), are currently being investigated in larger Phase II/III trials. Passive immunotherapy requires the exogenous administration of immunomodulators, including mAbs, checkpoint inhibitors or cytokines. Various mAbs targeting specific antigens expressed on tumor cells are being tested in Phase I/II clinical trials (NCT01040000; NCT01647828). Adoptive T-cell transfer (ACT) is another growing field of immunotherapy for pancreatic cancer. In this strategy, T cells are isolated from resected tumors, modified to enhance their activity, and reintroduced into a patient with the aim of improving the immune system’s anticancer response. Several trials of ACT techniques are currently under way [Citation123].
The combination of different immunotherapies, such as cancer vaccines and checkpoint-inhibitors, or the combination of immunotherapy and chemotherapy, have been shown to work synergistically and may ultimately prove successful [Citation124]. The checkpoint inhibitor ipilimumab (Yervoy®) combined with gemcitabine (Phase I trial NCT01473940) aimed for the treatment of patients diagnosed with inoperable stage III–IV or recurrent pancreatic cancer is currently under investigation.
Stem cell therapy
Pancreatic cancer stem cells constitute a small subpopulation of cells capable of tumor initiation, propagation and metastatic spread. These cells have the ability to self-renew and are highly resistant to conventional chemoradiotherapy, which may account for the rapid and almost universally occurring disease relapse seen in pancreatic cancer. Markers, such as CD44, CD24, ESA, CD133 and ALDH1, are commonly used to isolate and characterize the cancer stem cell population. However, many pancreatic cancer stem cells may express only one or two known surface markers, which may complicate the development of therapeutic strategies [Citation125,Citation126]. Some possibilities for targeting pancreatic cancer stem cells include disrupting signaling pathways that are active in cancer stem cells, such as Sonic hedgehog, Notch and mTOR, or using immunotherapy [Citation127]. Eliminating pancreatic cancer stem cells may ultimately improve the poor prognosis of pancreatic cancer.
Targeting cancer metabolism
One of the first studies on cancer metabolism was performed by Warburg in the 1920s [Citation128,Citation129]. Warburg found that cancer cells utilize cytosolic aerobic glycolysis and lactate fermentation, rather than mitochondrial oxidative phosphorylation of pyruvate, for energy production as in most normal cells, which was later named the Warburg effect. In recent years, the importance of cancer metabolism has received increased attention. Pancreatic cancer is, however, very heterogeneous in its metabolic phenotypes, including the main metabolic pathways, such as KRAS signaling, glutamine-dependent metabolism, aberrant fatty acid and lipid metabolism and autophagy. Understanding aberrant signaling pathways and the complex metabolic networks of pancreatic cancer may open new possibilities for treating pancreatic cancer by selectively starving cancer cells or for the development of other types of therapies targeting tumor metabolism [Citation130–133].
Nanotechnology
Nanoparticles can enter cells more freely than most other molecules due to their small size and can be conjugated with therapeutic drugs in order to increase drug delivery. Nanoparticles show promise in treating pancreatic cancer, and nanoalbumin bound paclitaxel (Abraxane) has been approved for the first-line treatment of metastatic pancreatic cancer in combination with gemcitabine [Citation97]. Nanoparticles in the form of liposomes have also shown an effect in pancreatic cancer, such as the liposomal irinotecan (Onivyde), which has been approved for the second-line treatment of metastatic pancreatic cancer in combination with 5-FU and leucovorin [Citation101]. A possible interesting direction for future research is the idea that nanoparticles can be actively targeted to cancer cells and the stromal compartment through the attachment of epitope-specific targeting molecules [Citation134]. The use of nanoparticles in medicine is certain to expand in the future, potentially enabling simultaneous imaging with therapeutic delivery.
Conclusion
Pancreatic cancer remains one of our most recalcitrant and dismal diseases, with very few improvements in outcomes over recent decades. Despite advancements in surgery and oncological treatment for pancreatic cancer, very few patients achieve a cure. The degrading clinical trends for this disease is likely due to the fact that pancreatic cancer is a systemic disease already at the time of clinical detection.
Future perspective
Focused efforts will hopefully lead to improved prognosis by detecting tumors at an earlier stage, preferably high-grade dysplasia or carcinoma in situ, for example in high-risk patients through novel biomarkers and screening tools, and the development of more effective treatment strategies. Surgery will still have a role in the future but will most likely be used as a debulking procedure, complemented by individualized oncological pharmacotherapy, where all patients receive neoadjuvant treatment. In recent years much effort has been expended in defining the genetic landscape of pancreatic cancer. Building on the genome map of pancreatic cancer, one of the primary objectives of pancreatic cancer research in the coming years will be to link genotype with phenotype characteristics by integrating large-scale genomic, transcriptomic, proteomic and metabolomic data. It is envisioned that in the future, drugs will be administered based on individual patients’ cancer phenotype by targeting one or several capabilities of pancreatic cancer cells (), transforming pancreatic cancer from a uniformly fatal disease to a disease that can be treated.
Table 1. TNM classification for pancreatic cancer.
Table 2. Stage grouping.
Table 3. Adjuvant therapy for resectable pancreatic cancer.
Table 4. Chemotherapy for metastatic pancreatic cancer.
Executive Summary
Pancreatic cancer is expected to become the second leading cause of cancer-related death by the year 2020.
Reasons for this include the lack of early detection and effective treatments.
Screening is indicated in individuals with two first-degree relatives with pancreatic cancer or known genetic syndromes associated with an increased risk of pancreatic cancer.
New-onset diabetes mellitus after the age of 50 years is an attractive target group for screening for sporadic pancreatic cancer.
Endoscopic ultrasound and MRI/magnetic resonance cholangiopancreatography may be used for screening for early pancreatic cancer, but these methods are unable to fully distinguish benign from malignant lesions and are not cost effective; therefore, there is an unmet need for biomarkers that can detect early pancreatic cancer with high sensitivity and specificity.
Computed tomography remains the standard examination for staging of pancreatic cancer and determination of resectability.
Pancreatic surgery should be performed in high-volume institutions.
Operability assessment should be individualized; age should not preclude potentially curative surgery.
Patients with borderline resectable pancreatic cancer with invasion into the portomesenteric veins can safely undergo vein resection with comparable outcomes to a standard resection.
Arterial resection is not recommended outside of clinical trials.
The introduction of standardized protocols has improved the pathological assessment of Whipple specimens.
Recent advances in systemic treatments for pancreatic cancer include FOLFIRINOX, nab-paclitaxel (Abraxane®) and liposomal irinotecan (Onivyde®).
Multimodal pain management is central to the palliative care of pancreatic cancer patients.
OMICS technology applied to biobank specimens may help advance our understanding of pancreatic cancer by identifying new biomarkers for diagnosis and treatment, especially when linked to clinical outcome data.
Future treatment options for pancreatic cancer include molecular targeted therapies, stromal modulation, immunotherapy, stem cell therapy, compounds that intervene with metabolic pathways and nanotechnology.
Acknowledgements
The authors wish to thank Philippe Bachellier, MD, Professor of Surgery and Head Department of HPB Surgery and Liver Transplant Hôpital de Hautepierre, Hôpitaux Universitaires de Strasbourg, Strasbourg, France, for providing the intraoperative photograph of venous resection during pancreatectomy, which has been presented in this manuscript.
Financial & competing interests disclosure
Financial support has been provided by SWElife/Vinnova, the Royal Physiographic Society of Lund, the Lars Hierta Research Foundation, the Erik and Angelica Sparre Research Foundation and the Anna-Lisa and Sven-Erik Lundgren Research Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Morgagni G . De Sedibus, et Causis Morborum per Anatomen Indagatis Libri Quinque. Venetiis, Typog Remondiniana (1761).
- Da Costa J . On the Morbid Anatomy and Symptoms of Cancer of the Pancreas. JB Lippincott & Company, Philadelphia, PA, USA (1858).
- Codivilla A . Rendiconto statistico della sezione chirurgica dell’ospedale di Imola. Imola, Italy (1898).
- Halsted W . Contributions to the surgery of the bile passages, especially of the common bile-duct. Boston Med. Surg. J.141, 645–654 (1899).
- Kausch W . Das carcinom der papilla duodeni und seine radikale entfernung. Beitr. Klin. Chir.78, 439–486 (1912).
- Hirschel G . Die resektion des duodenums mit der papille wegen karzinoms. Munch. Med. Wochenschr.61, 1728–1729 (1914).
- Whipple AO , ParsonsWB, MullinsCR. Treatment of Carcinoma of the ampulla of vater. Ann. Surg.102(4), 763–779 (1935).
- Whipple AO . Observations on radical surgery for lesions of the pancreas. Surg. Gynecol. Obstet.82, 623–631 (1946).
- Whipple AO . A reminiscence: pancreaticduodenectomy. Rev. Surg.20, 221–225 (1963).
- Brunschwig A . Resection of the head of pancreas and duodenum for carcinoma – pancreatoduodenectomy. Surg. Gynecol. Obstet.65, 681–684 (1937).
- Ansari D , WilliamssonC, TingstedtB, AnderssonB, LindellG, AnderssonR. Pancreaticoduodenectomy – the transition from a low- to a high-volume center. Scand. J. Gastroenterol.49(4), 481–484 (2014).
- Balzano G , ZerbiA, CaprettiG, RocchettiS, CapitanioV, Di CarloV. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br. J. Surg.95(3), 357–362 (2008).
- Yoshioka R , YasunagaH, HasegawaKet al. Impact of hospital volume on hospital mortality, length of stay and total costs after pancreaticoduodenectomy. Br. J. Surg.101(5), 523–529 (2014).
- Rahib L , SmithBD, AizenbergR, RosenzweigAB, FleshmanJM, MatrisianLM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res.74(11), 2913–2921 (2014).
- Malvezzi M , BertuccioP, RossoTet al. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann. Oncol. 26(4), 779–786 (2015).
- Engholm G , FerlayJ, ChristensenNet al. NORDCAN – a Nordic tool for cancer information, planning, quality control and research. Acta Oncol.49(5), 725–736 (2010).
- Becker AE , HernandezYG, FruchtH, LucasAL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J. Gastroenterol.20(32), 11182–11198 (2014).
- Nitsche C , SimonP, WeissFUet al. Environmental risk factors for chronic pancreatitis and pancreatic cancer. Dig. Dis.29(2), 235–242 (2011).
- Bracci PM . Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol. Carcinog.51(1), 53–63 (2012).
- Raimondi S , LowenfelsAB, Morselli-LabateAM, MaisonneuveP, PezzilliR. Pancreatic cancer in chronic pancreatitis: aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol.24(3), 349–358 (2010).
- Lucenteforte E , La VecchiaC, SilvermanDet al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case–Control Consortium (PanC4). Ann. Oncol.23(2), 374–382 (2012).
- Tramacere I , ScottiL, JenabMet al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int. J. Cancer126(6), 1474–1486 (2010).
- Everhart J , WrightD. Diabetes mellitus as a risk factor for pancreatic cancer: a meta-analysis. JAMA273(20), 1605–1609 (1995).
- Bartosch-Harlid A , AnderssonR. Diabetes mellitus in pancreatic cancer and the need for diagnosis of asymptomatic disease. Pancreatology10(4), 423–428 (2010).
- Hruban RH , MaitraA, KernSE, GogginsM. Precursors to pancreatic cancer. Gastroenterol. Clin. North Am.36(4), 831–849, vi (2007).
- Klein AP . Identifying people at a high risk of developing pancreatic cancer. Nat. Rev. Cancer13(1), 66–74 (2013).
- Yachida S , JonesS, BozicIet al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature467(7319), 1114–1117 (2010).
- AJCC Cancer Staging Manual (7th Edition) . Springer, NY, USA (2010).
- Wittekind C , ComptonCC, GreeneFL, SobinLH. TNM residual tumor classification revisited. Cancer94(9), 2511–2516 (2002).
- Chari ST , KellyK, HollingsworthMAet al. Early detection of sporadic pancreatic cancer: summative review. Pancreas44(5), 693–712 (2015).
- Siegel RL , MillerKD, JemalA. Cancer statistics, 2015. CA Cancer J. Clin.65(1), 5–29 (2015).
- Canto MI , HarinckF, HrubanRHet al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut62(3), 339–347 (2013).
- Bruenderman EH , MartinRC2nd. High-risk population in sporadic pancreatic adenocarcinoma: guidelines for screening. J. Surg. Res.194(1), 212–219 (2015).
- Goonetilleke KS , SiriwardenaAK. Systematic review of carbohydrate antigen (CA 19-19) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol.33(3), 266–270 (2007).
- Duffy MJ , SturgeonC, LamerzRet al. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann. Oncol.21(3), 441–447 (2010).
- Klauss M , StillerW, PahnGet al. Dual-energy perfusion-CT of pancreatic adenocarcinoma. Eur. J. Radiol.82(2), 208–214 (2013).
- Ichikawa T , SouH, ArakiTet al. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology221(1), 107–116 (2001).
- Barral M , TaouliB, GuiuBet al. Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology274(1), 45–63 (2015).
- Yasuda I , IwashitaT, DoiS, NakashimaM, MoriwakiH. Role of EUS in the early detection of small pancreatic cancer. Dig. Endosc.23(Suppl. 1), 22–25 (2011).
- Pappas SG , ChristiansKK, TolatPPet al. Staging chest computed tomography and positron emission tomography in patients with pancreatic adenocarcinoma: utility or futility? HPB 16(1), 70–74 (2014).
- Kramer-Marek G , GoreJ, KorcM. Molecular imaging in pancreatic cancer: a roadmap for therapeutic decisions. Cancer Lett.341(2), 132–138 (2013).
- England CG , HernandezR, EddineSB, CaiW. Molecular imaging of pancreatic cancer with antibodies. Mol. Pharm.13(1), 8–24 (2016).
- Al-Hawary MM , FrancisIR, ChariSTet al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology270(1), 248–260 (2014).
- Ansari D , AronssonL, FredrikssonJ, AnderssonB, AnderssonR. Safety of pancreatic resection in the elderly: a retrospective analysis of 556 patients. Ann. Gastroenterol.29(2), 221–225 (2016).
- Junejo MA , MasonJM, SheenAJet al. Cardiopulmonary exercise testing for preoperative risk assessment before pancreaticoduodenectomy for cancer. Ann. Surg. Oncol.21(6), 1929–1936 (2014).
- Argiles JM . Cancer-associated malnutrition. Eur. J. Oncol. Nurs.9(Suppl. 2), S39–S50 (2005).
- La Torre M , ZiparoV, NigriG, CavalliniM, BalducciG, RamacciatoG. Malnutrition and pancreatic surgery: prevalence and outcomes. J. Surg. Oncol.107(7), 702–708 (2013).
- Joglekar S , AsgharA, MottSLet al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J. Surg. Oncol.111(6), 771–775 (2015).
- Van Der Gaag NA , RauwsEA, Van EijckCHet al. Preoperative biliary drainage for cancer of the head of the pancreas. N. Engl. J. Med.362(2), 129–137 (2010).
- Tol JA , Van HooftJE, TimmerRet al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut doi: 10.1136/gutjnl-2014-308762 (2015) ( Epub ahead of print).
- Sauvanet A , BoherJM, PayeFet al. Severe jaundice increases early severe morbidity and decreases long-term survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. J. Am. Coll. Surg.221(2), 380–389 (2015).
- Xiong JJ , TanCL, SzatmaryPet al. Meta-analysis of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Br. J. Surg.101(10), 1196–1208 (2014).
- Menahem B , GuittetL, MulliriA, AlvesA, LubranoJ. Pancreaticogastrostomy is superior to pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Ann. Surg.261(5), 882–887 (2015).
- Roberts KJ , HodsonJ, MehrzadHet al. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB16(7), 620–628 (2014).
- Ansorge C , StrommerL, Andren-SandbergA, LundellL, HerringtonMK, SegersvardR. Structured intraoperative assessment of pancreatic gland characteristics in predicting complications after pancreaticoduodenectomy. Br. J. Surg.99(8), 1076–1082 (2012).
- Chan C , FranssenB, DominguezI, Ramirez-Del ValA, UscangaLF, CampuzanoM. Impact on quality of life after pancreatoduodenectomy: a prospective study comparing preoperative and postoperative scores. J. Gastrointest. Surg.16(7), 1341–1346 (2012).
- National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Version 2 (2015). www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- Mahipal A , FrakesJ, HoffeS, KimR. Management of borderline resectable pancreatic cancer. World. J. Gastrointest. Oncol.7(10), 241–249 (2015).
- Bockhorn M , UzunogluFG, AdhamMet al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery155(6), 977–988 (2014).
- Moore GE , SakoY, ThomasLB. Radical pancreatoduodenectomy with resection and reanastomosis of the superior mesenteric vein. Surgery30(3), 550–553 (1951).
- Asada S , ItayaH, NakamuraK, IsohashiT, MasuokaS. Radical pancreatoduodenectomy and portal vein resection. report of two successful cases with transplantation of portal vein. Arch. Surg.87, 609–613 (1963).
- Fortner JG . Regional resection of cancer of the pancreas: a new surgical approach. Surgery73(2), 307–320 (1973).
- Zhou Y , ZhangZ, LiuY, LiB, XuD. Pancreatectomy combined with superior mesenteric vein-portal vein resection for pancreatic cancer: a meta-analysis. World J. Surg.36(4), 884–891 (2012).
- Kim SM , MinSK, ParkDet al. Reconstruction of portal vein and superior mesenteric vein after extensive resection for pancreatic cancer. J. Korean Surg. Soc.84(6), 346–352 (2013).
- Glebova NO , HicksCW, PiazzaKMet al. Technical risk factors for portal vein reconstruction thrombosis in pancreatic resection. J. Vasc. Surg.62(2), 424–433 (2015).
- Fujii T , NakaoA, YamadaSet al. Vein resections >3 cm during pancreatectomy are associated with poor 1-year patency rates. Surgery157(4), 708–715 (2015).
- Ferreira N , OussoultzoglouE, FuchshuberPet al. Splenic vein-inferior mesenteric vein anastomosis to lessen left-sided portal hypertension after pancreaticoduodenectomy with concomitant vascular resection. Arch. Surg.146(12), 1375–1381 (2011).
- Mollberg N , RahbariNN, KochMet al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann. Surg.254(6), 882–893 (2011).
- Tol JA , GoumaDJ, BassiCet al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery156(3), 591–600 (2014).
- Dasari BV , PasqualiS, VohraRSet al. Extended versus standard lymphadenectomy for pancreatic head cancer: meta-analysis of randomized controlled trials. J. Gastrointest. Surg.19(9), 1725–1732 (2015).
- Williamsson C , KarlssonN, SturessonC, LindellG, AnderssonR, TingstedtB. Impact of a fast-track surgery programme for pancreaticoduodenectomy. Br. J. Surg.102(9), 1133–1141 (2015).
- Ansari D , GianottiL, SchroderJ, AnderssonR. Fast-track surgery: procedure-specific aspects and future direction. Langenbecks Arch. Surg.398(1), 29–37 (2013).
- Andersson R , VagianosCE, WilliamsonRC. Preoperative staging and evaluation of resectability in pancreatic ductal adenocarcinoma. HPB6(1), 5–12 (2004).
- Poruk KE , FirpoMA, AdlerDG, MulvihillSJ. Screening for pancreatic cancer: why, how, and who?Ann. Surg.257(1), 17–26 (2013).
- Strobel O , HinzU, GluthAet al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann. Surg.261(5), 961–969 (2015).
- Basturk O , SakaB, BalciSet al. Substaging of lymph node status in resected pancreatic ductal adenocarcinoma has strong prognostic correlations: proposal for a revised N classification for TNM staging. Ann. Surg. Oncol.22(Suppl. 3), S1187–S1195 (2015).
- Crippa S , PartelliS, ZamboniGet al. Poorly differentiated resectable pancreatic cancer: is upfront resection worthwhile? Surgery 152(3 Suppl. 1), S112–S119 (2012).
- Hruban RH , FukushimaN. Pancreatic adenocarcinoma: update on the surgical pathology of carcinomas of ductal origin and PanINs. Mod. Pathol.20(Suppl. 1), S61–S70 (2007).
- Chen JW , BhandariM, AstillDSet al. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB12(2), 101–108 (2010).
- Esposito I , KleeffJ, BergmannFet al. Most pancreatic cancer resections are R1 resections. Ann. Surg. Oncol.15(6), 1651–1660 (2008).
- Verbeke CS , LeitchD, MenonKV, McmahonMJ, GuillouPJ, AnthoneyA. Redefining the R1 resection in pancreatic cancer. Br. J. Surg.93(10), 1232–1237 (2006).
- Chandrasegaram MD , GoldsteinD, SimesJet al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br. J. Surg.102(12), 1459–1472 (2015).
- Kalser MH , EllenbergSS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch. Surg.120(8), 899–903 (1985).
- Neoptolemos JP , StockenDD, FriessHet al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med.350(12), 1200–1210 (2004).
- Oettle H , NeuhausP, HochhausAet al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA310(14), 1473–1481 (2013).
- Oettle H , PostS, NeuhausPet al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA297(3), 267–277 (2007).
- Regine WF , WinterKA, AbramsRAet al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA299(9), 1019–1026 (2008).
- Regine WF , WinterKA, AbramsRet al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 Phase III trial. Ann. Surg. Oncol.18(5), 1319–1326 (2011).
- Neoptolemos JP , StockenDD, BassiCet al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA304(10), 1073–1081 (2010).
- Fukutomi A , UesakaK, BokuNet al. JASPAC 01: randomized Phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 for patients with resected pancreatic cancer. J. Clin. Oncol.31(Suppl. 15), 4008 (2013).
- Huguet F , AndreT, HammelPet al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR Phase II and III studies. J. Clin. Oncol.25(3), 326–331 (2007).
- Krishnan S , RanaV, JanjanNAet al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer110(1), 47–55 (2007).
- Hammel P , HuguetF, Van LaethemJet al. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: final results of the international Phase III LAP 07 study. J. Clin. Oncol.31(Suppl. 18), Abstract LBA4003 (2013).
- Burris HA 3rd , MooreMJ, AndersenJet al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol.15(6), 2403–2413 (1997).
- Moore MJ , GoldsteinD, HammJet al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a Phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol.25(15), 1960–1966 (2007).
- Conroy T , DesseigneF, YchouMet al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med.364(19), 1817–1825 (2011).
- Von Hoff DD , ErvinT, ArenaPFet al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med.369(18), 1691–1703 (2013).
- Pelzer U , SchwanerI, StielerJet al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a Phase III-study from the German CONKO-study group. Eur. J. Cancer47(11), 1676–1681 (2011).
- Oettle H , RiessH, StielerJMet al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J. Clin. Oncol.32(23), 2423–2429 (2014).
- Gill S , KoY, CrippsMet al. PANCREOX: a randomized Phase 3 study of 5FU/LV with or without oxaliplatin for second-line advanced pancreatic cancer (APC) in patients (pts) who have received gemcitabine (GEM)-based chemotherapy (CT). J. Clin. Oncol.32(15), 4022 (2014).
- Wang-Gillam A , LiCP, BodokyGet al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, Phase 3 trial. Lancet doi: 10.1016/S0140-6736(15)00986-00981 (2015) ( Epub ahead of print).
- Ueno H , IokaT, IkedaMet al. Randomized Phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J. Clin. Oncol.31(13), 1640–1648 (2013).
- Haywood A , GoodP, KhanSet al. Corticosteroids for the management of cancer-related pain in adults. Cochrane Database Syst. Rev.4, CD010756 (2015).
- Johnson CD , BerryDP, HarrisSet al. An open randomized comparison of clinical effectiveness of protocol-driven opioid analgesia, celiac plexus block or thoracoscopic splanchnicectomy for pain management in patients with pancreatic and other abdominal malignancies. Pancreatology9(6), 755–763 (2009).
- Mercadante S , KlepstadP, KuritaGP, SjogrenP, GiarratanoA. European Palliative Care Research C. Sympathetic blocks for visceral cancer pain management: a systematic review and EAPC recommendations. Crit. Rev. Oncol. Hematol. doi: 10.1016/j.critrevonc.07.014 (2015) ( Epub ahead of print).
- Bruno MJ , HaverkortEB, TijssenGP, TytgatGN, Van LeeuwenDJ. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut42(1), 92–96 (1998).
- Davidson W , AshS, CapraS, BauerJ, Cancer Cachexia StudyG. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin. Nutr.23(2), 239–247 (2004).
- Yennurajalingam S , Frisbee-HumeS, PalmerJLet al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J. Clin. Oncol.31(25), 3076–3082 (2013).
- Moss AC , MorrisE, Mac MathunaP. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst. Rev.2, CD004200 (2006).
- Williamsson C , WennerblomJ, TingstedtB, JonssonC. A wait-and-see strategy with subsequent self-expanding metal stent on demand is superior to prophylactic bypass surgery for unresectable periampullary cancer. HPB doi: 10.1111/hpb.12513 (2015) ( Epub ahead of print).
- Ansari D , AnderssonR, BaudenMPet al. Protein deep sequencing applied to biobank samples from patients with pancreatic cancer. J. Cancer Res. Clin. Oncol.141(2), 369–380 (2015).
- Jones S , ZhangX, ParsonsDWet al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science321(5897), 1801–1806 (2008).
- Waddell N , PajicM, PatchAMet al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature518(7540), 495–501 (2015).
- Biankin AV , WaddellN, KassahnKSet al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature491(7424), 399–405 (2012).
- Hoskins JW , JiaJ, FlandezMet al. Transcriptome analysis of pancreatic cancer reveals a tumor suppressor function for HNF1A. Carcinogenesis35(12), 2670–2678 (2014).
- Daemen A , PetersonD, SahuNet al. Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc. Natl Acad. Sci. USA112(32), E4410–4417 (2015).
- Eltawil KM , RenfrewPD, MolinariM. Meta-analysis of Phase III randomized trials of molecular targeted therapies for advanced pancreatic cancer. HPB14(4), 260–268 (2012).
- Zorde Khvalevsky E , GabaiR, RachmutIHet al. Mutant KRAS is a druggable target for pancreatic cancer. Proc. Natl Acad. Sci. USA110(51), 20723–20728 (2013).
- Xie D , XieK. Pancreatic cancer stromal biology and therapy. Genes Dis.2(2), 133–143 (2015).
- Hingorani S , HarrisW, HendifarAet al. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: Interim results of a randomized Phase II study. J. Clin. Oncol.33(Suppl.), Abstract 4006 (2015).
- Hingorani S , HarrisW, BeckJet al. Final results of a phase Ib study of gemcitabine plus PEGPH20 in patients with stage IV previously untreated pancreatic cancer. J. Clin. Oncol.33(Suppl. 3), Abstract 359 (2015).
- Amedei A , NiccolaiE, PriscoD. Pancreatic cancer: role of the immune system in cancer progression and vaccine-based immunotherapy. Hum. Vaccin. Immunother.10(11), 3354–3368 (2014).
- Houot R , SchultzLM, MarabelleA, KohrtH. T-cell-based immunotherapy: adoptive cell transfer and checkpoint inhibition. Cancer Immunol. Res.3(10), 1115–1122 (2015).
- Lutz ER , KinkeadH, JaffeeEM, ZhengL. Priming the pancreatic cancer tumor microenvironment for checkpoint-inhibitor immunotherapy. Oncoimmunology3(11), e962401 (2014).
- Kure S , MatsudaY, HagioM, UedaJ, NaitoZ, IshiwataT. Expression of cancer stem cell markers in pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinomas. Int. J. Oncol.41(4), 1314–1324 (2012).
- Mohammed A , JanakiramNB, PantS, RaoCV. Molecular targeted intervention for pancreatic cancer. Cancers (Basel)7(3), 1499–1542 (2015).
- Raj D , AicherA, HeeschenC. Concise review: stem cells in pancreatic cancer: from concept to translation. Stem Cells33(10), 2893–2902 (2015).
- Warburg O , WindF, NegeleinE. The metabolism of tumors in the body. J. Gen. Physiol.8(6), 519–530 (1927).
- Warburg O . On the metabolism of carcinoma cells. Biochem. Z.152, 309–344 (1924).
- Blum R , KloogY. Metabolism addiction in pancreatic cancer. Cell Death Dis.5, e1065 (2014).
- Son J , LyssiotisCA, YingHet al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature496(7443), 101–105 (2013).
- Vander Heiden MG . Exploiting tumor metabolism: challenges for clinical translation. J. Clin. Invest.123(9), 3648–3651 (2013).
- Yu M , ZhouQ, ZhouYet al. Metabolic phenotypes in pancreatic cancer. PLoS ONE10(2), e0115153 (2015).
- Lee GY , QianWP, WangLet al. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano7(3), 2078–2089 (2013).
- Hanahan D , WeinbergRA. Hallmarks of cancer: the next generation. Cell144(5), 646–674 (2011).