Abstract
Avelumab is a human anti-PD-L1 checkpoint inhibitor with clinical activity in multiple solid tumors. Here, we describe the rationale and design for JAVELIN Ovarian 200 (NCT02580058), the first randomized Phase III trial to evaluate the role of checkpoint inhibition in women with ovarian cancer. This three-arm trial is comparing avelumab administered alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in patients with platinum-resistant/refractory recurrent ovarian, fallopian tube or peritoneal cancer. Eligible patients are not preselected based on PD-L1 expression and may have received up to three prior lines of chemotherapy for platinum-sensitive disease, but none for resistant disease. Overall survival and progression-free survival are primary end points, and secondary end points include biomarker evaluations and pharmacokinetics.
Keywords::
Ovarian, fallopian tube, and primary peritoneal cancers, collectively known as epithelial ovarian cancer (EOC), represent the fifth most common cause of cancer mortality in women and are associated with an estimated 239,000 new cases and 152,000 deaths worldwide each year [Citation1]. The incidence of EOC increases with age, and >80% of patients are aged >50 years when diagnosed [Citation2]. A high proportion of patients present with advanced disease, and the 5-year survival of patients with advanced EOC is <30% [Citation3]. Seventy percent of patients relapse within 3 years of first-line therapy [Citation2].
Standard front-line systemic treatment for EOC consists of platinum-based chemotherapy, with the option to add the antiangiogenic agent bevacizumab (anti-VEGF antibody) according to local approval and availability [Citation2]. Although response rates are high, disease recurrence is common and almost all patients eventually become refractory or resistant to platinum-based therapy, defined as lack of response or disease progression during or within 1 month of platinum-based chemotherapy or relapse 1–6 months after response to platinum-based treatment, respectively [Citation4–6]. In either case, the prognosis for patients with platinum-resistant/refractory disease is poor, and the aims of treatment are focused primarily on symptom control and quality of life (QoL) [Citation2,Citation7].
The standard of care for patients with platinum-resistant/refractory disease includes single nonplatinum agents with or without bevacizumab. Pegylated liposomal doxorubicin (PLD) monotherapy is a commonly used treatment option in this setting and has been associated with objective response rates (ORRs) of 10–20%, a median progression-free survival (PFS) of 2.1–3.7 months, and median overall survival (OS) of 8.4–16.8 months in platinum-resistant ovarian cancer [Citation8–12]. Combining single agents such as PLD, paclitaxel, or topotecan with bevacizumab has increased efficacy and QoL compared with chemotherapy alone [Citation13,Citation14], as shown in the AURELIA trial (n = 361; bevacizumab plus chemotherapy vs chemotherapy alone: ORR, 30.9 versus 12.6% [p < 0.001] and median PFS, 6.7 vs 3.4 months [p < 0.001]) [Citation13]. However, patients may receive bevacizumab during first-line treatment or following platinum-sensitive relapse, and the efficacy of bevacizumab retreatment is unknown. In patients with BRCA mutation-positive tumors, poly(ADP-ribose) polymerase inhibitors are an option for some patients [Citation15,Citation16]. The paucity of effective therapies for patients with platinum-resistant/refractory EOC underscores the need for new therapeutic options for this patient population.
Immune checkpoint inhibitors have revolutionized the treatment of cancer. PD-L1 and its receptor, PD-1, are immune checkpoint proteins that have an established role in suppressing T-cell responses [Citation17]. PD-1 expression is upregulated in activated immune cells, and binding of PD-L1 expressed on tumor cells to PD-1 on CD8+ T cells delivers a strong inhibitory signal. The efficacy and tolerability of anti-PD-1 and anti-PD-L1 antibodies seen in several trials has led to regulatory approval in various tumor types [Citation18].
EOC displays immunogenic characteristics [Citation19]. In particular, tumor-infiltrating lymphocytes are present in approximately one-half of cases, and are an established prognostic factor in EOC [Citation20,Citation21]. EOC is also characterized by PD-L1 expression on tumor cells and tumor-infiltrating lymphocytes [Citation22,Citation23], providing a rationale for clinical studies of anti-PD-L1/PD-1 monoclonal antibodies [Citation24]. However, immune infiltrates in EOC contain an increased proportion of regulatory T cells [Citation25], suggesting immunosuppressive activity. EOC cells have a lower frequency of PD-L1 expression (≈40% of tumors are PD-L1+) [Citation26] and lower mutational burden compared with established immunosensitive tumors, such as melanoma, non-small-cell lung cancer, and bladder cancer [Citation27,Citation28]. Because mutational burden is an independent predictor of response to checkpoint inhibitor therapy across different cancers [Citation29], strategies may be needed to augment the activity of checkpoint inhibitors in EOC.
Chemotherapy agents, especially anthracyclines, have a range of potentially immunogenic effects against tumor cells, including enhancement of antigen presentation and immunogenicity and increasing susceptibility to immune attack [Citation30], suggesting that combining immunotherapy and chemotherapy has the potential for synergistic activity. Preclinical studies have demonstrated that chemotherapy increases tumor responsiveness to checkpoint inhibitors [Citation31]. In particular, doxorubicin has been identified as an inducer of immunogenic cell death, a form of apoptosis that triggers an adaptive immune response [Citation30]. In mouse models, treatment with liposomal doxorubicin in combination with an anti-PD-L1 antibody decreased the percentage of regulatory T cells and increased the percentage of CD8+ T cells among tumor-infiltrating lymphocytes compared with either agent given as monotherapy; these effects were associated with an increased rate of complete responses (CRs) and survival [Citation32]. Thus, clinical studies combining a checkpoint inhibitor with PLD in EOC are of major interest, and trials have been initiated with different agents (NCT02580058, NCT02839707, and NCT02431559).
Avelumab
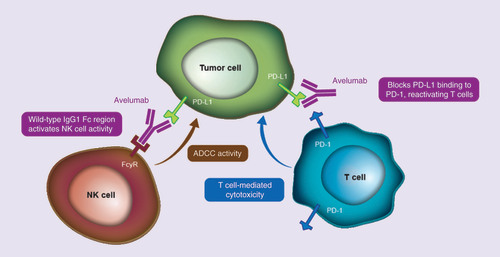
Avelumab (Bavencio®) is a human immunoglobulin G1 anti-PD-L1 monoclonal antibody with a wild-type Fc region that was designed to specifically bind to PD-L1 and inhibit its interaction with PD-1 () [Citation33]. Unlike other anti-PD-L1/PD-1 antibodies that have received regulatory approval or are in advanced clinical development, avelumab has been shown to induce antibody-dependent cell-mediated cytotoxicity of tumor cells in preclinical studies, suggesting it may have an additional mechanism of action compared with similar agents [Citation34]. The safety, pharmacokinetic (PK), and clinical activity of avelumab have been investigated in Phase I studies in patients with various advanced solid tumors (JAVELIN Solid Tumor [NCT01772004] and JAVELIN Solid Tumor JPN [NCT01943461]) [Citation35,Citation36]. Treatment with avelumab 10 mg/kg administered intravenously (iv.) every 2 weeks has a half-life of 3.9–4.1 days and >90% target occupancy for PD-L1 [Citation35]. Doses up to 20 mg/kg every 2 weeks were administered safely during dose-escalation studies.
ADCC: Antibody-dependent cell-mediated cytotoxicity; FcγR: Fc-gamma receptor; IgG1: Immunoglobulin G1; NK: Natural killer.
In Phase Ib dose-expansion cohorts of the JAVELIN Solid Tumor trial, durable responses and disease stabilization were seen with avelumab treatment in several tumor types, including ovarian cancer, urothelial carcinoma, non-small-cell lung cancer, gastric cancer, and breast cancer [Citation37–41]. In addition, avelumab has shown durable clinical activity in a Phase II trial in patients with metastatic Merkel cell carcinoma (MCC), a rare and aggressive skin malignancy [Citation42]. To date, avelumab has been approved in the US and European Union for the treatment of metastatic MCC (since March and September 2017, respectively), in Japan for treatment of curatively unresectable MCC (since September 2017), and in the US for the treatment of advanced urothelial carcinoma after progression during or after platinum-containing chemotherapy (since May 2017) [Citation43–45].
As mentioned earlier, the clinical activity of avelumab in EOC was investigated initially in a Phase Ib cohort of the JAVELIN Solid Tumor trial, and early data (after a median follow-up of 12.4 months) have been reported, most recently at the 2016 annual meeting of the American Society of Clinical Oncology. All 124 patients enrolled in this cohort had recurrent or refractory EOC, were heavily pretreated (65.3% had received ≥3 prior therapies in the advanced/metastatic setting), and were unselected for PD-L1 expression [Citation41]. Avelumab showed antitumor activity, including an ORR of 9.7% (all partial responses [PRs]), disease control rate (i.e., patients with response or stable disease) of 54.0%, and median OS of 10.8 months. Responses occurred irrespective of tumor PD-L1 expression. Avelumab was associated with an acceptable safety profile, including a low rate of grade 3/4 treatment-related toxicities (6.5%) and no deaths related to avelumab [Citation41]. To date, this is the largest reported data set of patients with EOC treated with an anti-PD-L1/PD-1 antibody. These data indicate that avelumab has promising activity and tolerability in recurrent/refractory EOC and provide a rationale for further assessment of avelumab, either alone or in combination with immunogenic chemotherapy, such as PLD, in a randomized controlled trial in this setting.
JAVELIN Ovarian 200 trial
We describe the design of the randomized, open-label, Phase III trial JAVELIN Ovarian 200 (NCT02580058) comparing avelumab administered alone or in combination with PLD versus PLD alone in patients with platinum-resistant/refractory EOC.
Objectives
The primary objective of this trial is to demonstrate that avelumab, given alone or in combination with PLD, is superior to PLD alone in prolonging OS or PFS in patients with platinum-resistant/refractory EOC. Secondary objectives include the evaluation of antitumor activity, candidate biomarkers of sensitivity or resistance to avelumab with or without PLD, and safety profiles of avelumab with or without PLD versus PLD alone. This trial will also characterize the PK of avelumab and PLD and assess the immunogenicity of avelumab and patient-reported outcomes (PROs).
Key eligibility criteria
Eligible patients have histologically confirmed EOC (epithelial ovarian, fallopian tube or peritoneal cancer), including malignant mixed Müllerian tumors with a high-grade serous component, and measurable disease by Response Evaluation Criteria In Solid Tumors (RECIST) v1.1. Patients must have platinum-resistant or platinum-refractory disease, defined as disease progression within 180 days following the last administered dose of platinum therapy (resistant) or lack of response or disease progression while receiving the most recent platinum-based therapy (refractory). Patients may have received up to three prior lines of therapy for platinum-sensitive disease but must not have received prior treatment for platinum-resistant disease. Other eligibility criteria are listed in Box 1. Patients are not preselected based on PD-L1 tumor expression status because no difference in ORR between patients with PD-L1+ or PD-L1− tumors based on archival tissue was seen in the Phase Ib EOC cohort [Citation41].
Study design
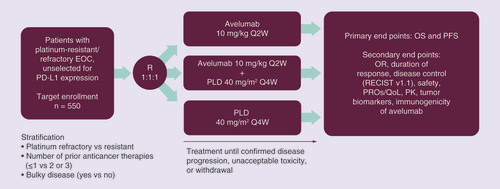
JAVELIN Ovarian 200 is a global, open-label, multicenter, parallel three-arm, Phase III trial (). Approximately 550 patients (target enrollment) will be randomized 1:1:1 to receive avelumab 10 mg/kg monotherapy as a 1-h iv. infusion once every 2 weeks; avelumab 10 mg/kg every 2 weeks plus PLD 40 mg/m2 every 4 weeks, each as 1-h iv. infusions; or PLD alone as a 1-h iv. infusion every 4 weeks. The patients are stratified by platinum-refractory versus platinum-resistant disease, number of previous anticancer therapies, and the presence or absence of bulky disease (defined as a tumor ≥5 cm). Treatment is given until confirmed progression, unacceptable toxicity, or any other protocol-specified criterion for withdrawal occurs. However, because of the potential for pseudoprogression during checkpoint inhibitor therapy, avelumab (but not PLD) may be continued beyond disease progression at the investigator’s discretion while awaiting radiological confirmation of disease progression. Avelumab monotherapy may also be continued if a patient with confirmed disease progression is still experiencing clinical benefit, based on the judgment of the investigator. Crossover between study arms is not permitted. The primary end points of the trial are OS and PFS (Box 2). Secondary end points include objective response, duration of response, disease control, safety and tolerability, PK parameters, candidate biomarker assessments, and PROs. Immune-related efficacy assessments are assessed as an exploratory end point.
EOC: Epithelial ovarian cancer; OR: Objective response; OS: Overall survival; PFS: Progression-free survival; PK: Pharmacokinetics; PLD: Pegylated liposomal doxorubicin; PRO: Patient-reported outcome; Q2W: Once every 2 weeks; Q4W: Once every 4 weeks; QoL: Quality of life; R: Randomization; RECIST: Response Evaluation Criteria In Solid Tumors.
Evaluations
Response and PFS are evaluated per RECIST v1.1 based on blinded independent central review. Response assessments include MRI or computed tomography scans every 8 weeks and mandatory imaging of chest, abdomen, and pelvis. Safety is assessed throughout and is graded based on National Cancer Institute Common Terminology Criteria for Adverse Events v4.03. For avelumab, any adverse event that is suspected to be potentially immune related is considered an adverse event of special interest. Blood samples are collected at various time points to analyze PK profiles and immunogenicity of avelumab (antidrug antibodies). PK parameters being investigated include trough concentration for avelumab and maximum concentration, volume of distribution, clearance, and area under the concentration-time curve for doxorubicin (PLD). PROs and QoL are assessed using European Organization for Research and Treatment of Cancer QLQ-C30 [Citation46] and QLQ-OV28 [Citation47] and EuroQol5 Dimension 5L [Citation48] questionnaires.
Candidate predictive biomarkers being investigated in tumor tissue (archived tumor tissue and de novo biopsies taken at enrollment and at end of treatment) include, but may not necessarily be limited to, PD-L1 expression and the presence or absence of tumor-infiltrating CD8+ T-lymphocytes based on immunohistochemistry. BRCA1/2 germline mutational status is also collected when available at baseline or during study participation, but testing is not mandated.
Statistical analysis methods
OS is defined as the time from randomization to the date of death due to any cause. PFS is defined as the time from randomization to the date of the first documented progression of disease or death due to any cause and is based on blinded independent central review assessment of the primary end point. OS and PFS will be summarized using the Kaplan–Meier method, and the Cox proportional hazards model will be fitted to compute hazard ratios and corresponding 95% CIs. Objective response is defined as a CR or PR, as determined by RECIST v1.1. Duration of response is defined for patients with an objective response as the time from first documentation of a PR or CR to the first documentation of disease progression or death due to any cause. Disease control is defined as a CR, PR, or stable disease, as determined by RECIST v1.1.
Discussion & future perspective
The JAVELIN Ovarian 200 trial is the first Phase III trial of an immune checkpoint inhibitor in a population of patients with EOC. Enrollment began in December 2015, and the estimated date of primary end point data availability is March 2018. Results from this trial will determine whether avelumab, given as monotherapy or in combination with PLD, has the potential to improve PFS or OS compared with PLD alone in patients with platinum-refractory/resistant disease. PFS was added as a primary end point via a protocol amendment because an improvement in OS may be difficult to observe in study populations with a long duration of survival post progression (e.g., >12 months) due to effects of post-study treatments. As a result, PFS may be considered a clinically meaningful measure of efficacy in this disease setting [Citation5,Citation49–51]. In addition, because of the importance of evaluating the effects of treatment from the patient perspective in terms of symptom palliation and side effects, which are particularly relevant in a setting of incurable disease [Citation49], the trial is also assessing PROs as a secondary end point using validated questionnaires.
Checkpoint inhibitors may also have the potential to improve outcomes of patients with EOC in the first-line setting. To test this hypothesis, a second Phase III trial of avelumab in EOC has been initiated. JAVELIN Ovarian 100 (NCT02718417) is a global Phase III trial of avelumab in combination with and/or following carboplatin and paclitaxel chemotherapy in previously untreated patients with EOC. This is a three-arm trial that will compare avelumab given in combination with platinum-based chemotherapy followed by avelumab maintenance, avelumab given only as maintenance after platinum-based chemotherapy, or platinum-based chemotherapy alone. These two Phase III trials of avelumab will therefore provide important information on treatment sequencing with an anti-PD-L1 antibody in EOC, including benefits of treatment in the first-line and platinum-resistant/refractory settings, and evaluation of monotherapy, combination therapy, and maintenance therapy strategies.
Phase III studies of anti-PD-1/PD-L1 antibodies in combination with chemotherapy or as maintenance therapy are ongoing in various other tumor types and have shown promising activity to date [Citation24]. In addition, data for combinations of anti-PD-1/PD-L1 antibodies with other agents (e.g., poly(ADP-ribose)) polymerase inhibitors or bevacizumab) would be of interest in EOC, and Phase III trials have been initiated.
Conclusion
The JAVELIN Ovarian 200 trial will demonstrate whether avelumab as monotherapy or in combination with chemotherapy can improve PFS or OS in patients with platinum-refractory/resistant disease compared with standard chemotherapy. It is hoped that this Phase III trial, along with others in progress, will lead to the availability of new treatment options that can improve outcomes for patients with EOC.
Inclusion criteria
Age ≥18 years (≥20 years in Japan)
Histologically confirmed epithelial ovarian, fallopian tube or peritoneal cancer, including malignant mixed Müllerian tumors with high-grade serous component
Platinum-resistant/refractory disease, defined as disease progression within 180 days following the last administered dose of platinum therapy (resistant) or lack of response or disease progression while receiving the most recent platinum-based therapy (refractory), respectively
Up to three prior lines of chemotherapy for platinum-sensitive disease, most recently platinum containing, and no prior therapy for platinum-resistant disease
Measurable disease by RECIST v1.1 with ≥1 unidimensional measurable lesion not previously irradiated
ECOG performance status score of 0–1
Estimated life expectancy of ≥3 months
Tumor sample collected before enrollment (mandatory unless there is a documented clinical contradiction), with availability of archived tumor tissue; a new tumor sample is not required if tumor tissue was collected within 3 months of enrollment and no intervening treatment was received
Adequate bone marrow function, including absolute neutrophil count of ≥1.5 × 109/L, platelet count of ≥100 × 109/L, and hemoglobin of ≥9 g/dL
Adequate liver function, including total bilirubin level ≤1.5 × ULN and aspartate aminotransferase and alanine aminotransferase levels ≤2.5 × ULN
Adequate renal function (creatinine clearance of ≥50 mL/min, as calculated using the Cockcroft–Gault equation)
Exclusion criteria
Nonepithelial tumor or ovarian tumor with low malignant potential (i.e., borderline tumor)
Prior therapy with any drug targeting a T-cell co-regulatory protein
PLD-resistant disease, defined as lack of response or progression within 6 months of the last dose of PLD
Active brain metastases requiring steroids
Concurrent anticancer treatment within 28 days before study entry (except for palliative radiotherapy), major surgery within 28 days before study entry (excluding diagnostic biopsy), use of hormonal agents within 7 days before study entry, or use of any investigational drug in the 28 days before study entry; patients receiving bisphosphonate or denosumab are eligible if treatment started ≥14 days before study entry
Diagnosis of any other malignancy within 5 years before registration, except for adequately treated basal cell or squamous cell skin cancer or carcinoma in situ of the breast or cervix
Any one of the following currently or in the previous 6 months: myocardial infarction, congenital long QT syndrome, Torsades de Pointes, arrhythmias (including sustained ventricular tachyarrhythmia and ventricular fibrillation, bradycardia defined as <50 bpm), right bundle branch block and left anterior hemiblock (bifascicular block), unstable angina, coronary/peripheral artery bypass graft, symptomatic congestive heart failure (New York Heart Association class III or IV), cerebrovascular accident, transient ischemic attack or symptomatic pulmonary embolism
Ongoing cardiac dysrhythmias of NCI CTCAE grade ≥3, atrial fibrillation of any grade, or QTcF interval >470 ms at screening (average of triplicate electrocardiography)
Left ventricular ejection fraction <50% by multiple-gated acquisition or echocardiography
Prior anthracycline-related cardiotoxicity or prior anthracycline exposure approaching the lifetime limit
Prior organ transplantation including allogeneic stem-cell transplantation
Known history of a positive test for HIV or AIDS-related illness
Active infection requiring systemic therapy
Hepatitis B virus or hepatitis C virus (HCV) infection at screening (positive hepatitis B virus surface antigen or HCV RNA if anti-HCV antibody screening test positive)
Administration of a live vaccine within 30 days prior to study entry
Current or prior use of an immunosuppressive medication within 7 days before randomization, except intranasal, inhaled, topical steroids or local steroid injections, systemic corticosteroids ≤10 mg/day of prednisone or equivalent, or steroids as premedication for hypersensitivity reactions
Active autoimmune disease that could deteriorate; patients with type 1 diabetes, vitiligo, psoriasis; patients with hypo/hyperthyroid disease not requiring immunosuppressive treatments are eligible
Known hypersensitivity to monoclonal antibodies or liposomal preparations
Persisting grade ≥2 toxicity related to prior therapy (grade 2 sensory neuropathy is acceptable)
Severe gastrointestinal conditions in the 4 weeks before enrollment or a history of inflammatory bowel disease
Other severe acute or chronic medical conditions, including pneumonitis or psychiatric conditions, that could increase the risk of participation or interfere with the interpretation of the study results
Current use or anticipated need for treatment with drugs or foods that are known strong CYP3A4 inhibitors or inducers, including their administration within 10 days prior to patient registration; the topical use of these medications (if applicable) is allowed
Current use or anticipated need for drugs that are known strong CYP2D6 inducers or inhibitors, and PgP inducers and inhibitors, including their administration with 10 days prior to patient registration
Primary end points
Overall survival
PFS by blinded ICR according to RECIST v1.1
Secondary end points
Efficacy: objective response, duration of response, and disease control, as determined by blinded ICR and investigators according to RECIST v1.1
PFS as determined by investigators according to RECIST v1.1
Safety and tolerability
PK parameters for avelumab and doxorubicin
Immunogenicity of avelumab
Candidate predictive biomarkers in tumor tissue
Patient-reported outcomes
Exploratory end points
Molecular, cellular, and soluble markers in peripheral blood and/or tumor tissue that may be relevant to immune response or disease progression on any treatment arm
CA125 levels
ir-PFS, ir-objective response, ir-duration of response, and ir-disease control, per irRECIST
Background
Patients with platinum-resistant/refractory epithelial ovarian cancer (EOC) have a poor prognosis, and there is currently no standard-of-care treatment.
It is well established that EOC displays immunologic features, including tumor infiltration by lymphocytes and PD-L1 expression on tumor cells, providing a rationale for immunotherapy.
In preclinical models, treatment with checkpoint inhibitors in combination with liposomal doxorubicin has been shown to increase efficacy.
Avelumab
Avelumab is a human immunoglobulin G1 anti-PD-L1 monoclonal antibody that has shown durable clinical activity in various tumor types and is approved in several countries for the treatment of metastatic Merkel cell carcinoma, and in the US for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-based chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
In a Phase Ib study, in patients with heavily pretreated recurrent or refractory EOC (n = 124), avelumab showed antitumor activity, including an objective response rate of 10%, disease control rate of 54%, and median overall survival of 10.8 months; responses occurred in patients with PD-L1+ and PD-L1- tumors.
JAVELIN Ovarian 200 trial
JAVELIN Ovarian 200 is the first Phase III trial to investigate an immune checkpoint inhibitor in patients with EOC.
Eligible patients have platinum-resistant/refractory epithelial ovarian, fallopian tube or peritoneal cancer and may have received up to three prior lines of therapy for platinum-sensitive disease but must not have received prior treatment for platinum-resistant disease.
Patients are randomized 1:1:1 to receive avelumab monotherapy, avelumab plus pegylated liposomal doxorubicin, or pegylated liposomal doxorubicin alone.
Primary end points are overall survival and progression-free survival.
It is hoped that the results of this trial will lead to the availability of a new treatment option for patients with platinum-resistant/refractory EOC.
Author’s contributions
All authors met the criteria for authorship set forth by the International Committee of Medical Journal Editors, and were involved in conception, preparation, and approval of the manuscript.
Infographic
Download PDF (609.9 KB)Acknowledgements
The authors would like to thank the patients and their families, investigators, co-investigators, and the study teams at each of the participating centers.
Financial & competing interests disclosure
The JAVELIN Ovarian 200 trial discussed in this manuscript is sponsored by Pfizer Inc., New York, NY, USA, and is part of an alliance between Pfizer Inc. and Merck KGaA, Darmstadt, Germany. E Pujade-Lauraine has acted in a consultant/advisory role for AstraZeneca, Pfizer, and Roche and has received travel, accommodation, or expenses from AstraZeneca, Pfizer, and Roche. BJ Monk has acted in a consultant/advisory role for Amgen, AstraZeneca, Clovis, Gradalis, Immunogen, Insys, Mateon, Merck KGaA, Pfizer, Precision Oncology, and Tesaro, and has provided speaker services for AstraZeneca, Clovis, and Tesaro. K Fujiwara has acted in a consultant/advisory role for AstraZeneca, Chugai-Roche, Eisai, GSK, Lilly, Merck & Co, and Pfizer and has provided speaker services for Chugai-Roche, Daiichi-Sankyo, Jannsen, Kyowahakko-Kirin, Lilly, Nippon Kayaku, Novartis, Ono Pharmaceutical, Taiho, Yakult, and Zeria Pharma. K Fujiwara has received research grants from Advanced Oncotherapy, AstraZeneca, Chungai-Roche, Daiichi-Sankyo, Eisai, GSK, ImmunoGen, Kaken-Seiyaku, Lilly, Merck & Co, Ono Pharmaceutical, Pfizer, Shionogi, Taiho, and Zeria Pharma. SS Dychter and G Devgan are employees of Pfizer, Inc. SS Dychter holds stock in Fate Therapeutics and Halozyme Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by Amy Davidson at ClinicalThinking, Inc, Hamilton, NJ, USA, and was funded by Merck KGaA, Darmstadt, Germany and Pfizer Inc, New York, NY, USA.
Additional information
Funding
Notes
ECOG: Eastern Cooperative Oncology Group; NCI CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; PLD: Pegylated liposomal doxorubicin; RECIST: Response Evaluation Criteria In Solid Tumors; ULN: Upper limit of normal.
ICR: Independent central review; ir: Immune related; PFS: Progression-free survival; PK: Pharmacokinetic; RECIST: Response Evaluation Criteria In Solid Tumors.
References
- Ferlay J , SoerjomataramI, DikshitRet al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN Int. J. Cancer 136(5), e359–e386 (2015).
- Ledermann JA , RajaFA, FotopoulouCet al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol.24(Suppl. 6), vi24–vi32 (2013).
- Siegel RL , MillerKD, JemalA. Cancer statistics. CA Cancer J. Clin.67(1), 7–30 (2017).
- Griffiths RW , ZeeYK, EvansSet al. Outcomes after multiple lines of chemotherapy for platinum-resistant epithelial cancers of the ovary, peritoneum, and fallopian tube. Int. J. Gynecol. Cancer21(1), 58–65 (2011).
- Wilson MK , Pujade-LauraineE, AokiDet al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recurrent disease. Ann. Oncol.28(4), 727–732 (2017).
- Herzog TJ , MonkBJ. Bringing new medicines to women with epithelial ovarian cancer: what is the unmet medical need?Gynecol. Oncol. Res. Pract.4, 13 (2017).
- Roncolato FT , GibbsE, LeeCKet al. Quality of life predicts overall survival in women with platinum-resistant ovarian cancer: an AURELIA substudy. Ann. Oncol.28(8), 1849–1855 (2017).
- Gordon AN , FleagleJT, GuthrieD, ParkinDE, GoreME, LacaveAJ. Recurrent epithelial ovarian carcinoma: a randomized Phase III study of pegylated liposomal doxorubicin versus topotecan. J. Clin. Oncol.19(14), 3312–3322 (2001).
- Monk BJ , HerzogTJ, KayeSBet al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J. Clin. Oncol.28(19), 3107–3114 (2010).
- Monk BJ , HerzogTJ, KayeSBet al. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: overall survival analysis. Eur. J. Cancer48(15), 2361–2368 (2012).
- Colombo N , KutarskaE, DimopoulosMet al. Randomized, open-label, Phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. J. Clin. Oncol.30(31), 3841–3847 (2012).
- Lawrie TA , BryantA, CameronA, GrayE, MorrisonJ. Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database Syst. Rev. (7), CD006910 (2013).
- Pujade-Lauraine E , HilpertF, WeberBet al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized Phase III trial. J. Clin. Oncol.32(13), 1302–1308 (2014).
- Stockler MR , HilpertF, FriedlanderMet al. Patient-reported outcome results from the open-label Phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J. Clin. Oncol.32(13), 1309–1316 (2014).
- Oza AM , TinkerAV, OakninAet al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from Study 10 and ARIEL. Gynecol. Oncol.147(2), 267–275 (2017).
- Kaufman B , Shapira-FrommerR, SchmutzlerRKet al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol.33(3), 244–250 (2015).
- Pardoll DM . The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer12(4), 252–264 (2012).
- Balar AV , WeberJS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol. Immunother.66(5), 551–564 (2017).
- Kandalaft LE , PowellDJJr, SinghN, CoukosG. Immunotherapy for ovarian cancer: what’s next?J. Clin. Oncol.29(7), 925–933 (2011).
- Zhang L , Conejo-GarciaJR, KatsarosDet al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med.348(3), 203–213 (2003).
- Hwang WT , AdamsSF, TahirovicE, HagemannIS, CoukosG. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol. Oncol.124(2), 192–198 (2012).
- Webb JR , MilneK, KroegerDR, NelsonBH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol.141(2), 293–302 (2016).
- Darb-Esfahani S , KunzeCA, KulbeHet al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget7(2), 1486–1499 (2016).
- Gaillard SL , SecordAA, MonkB. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol. Oncol. Res. Pract.3, 11 (2016).
- Woo EY , ChuCS, GoletzTJet al. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res.61(12), 4766–4772 (2001).
- Gatalica Z , SnyderC, ManeyTet al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomarkers Prev.23(12), 2965–2970 (2014).
- Alexandrov LB , Nik-ZainalS, WedgeDCet al. Signatures of mutational processes in human cancer. Nature500(7463), 415–421 (2013).
- Chalmers ZR , ConnellyCF, FabrizioDet al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med.9(1), 34 (2017).
- Goodman AM , KatoS, BazhenovaLet al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther.16(11), 2598–2608 (2017).
- Zitvogel L , GalluzziL, SmythMJ, KroemerG. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity39(1), 74–88 (2013).
- Pfirschke C , EngblomC, RickeltSet al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity44(2), 343–354 (2016).
- Rios-Doria J , DurhamN, WetzelLet al. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia17(8), 661–670 (2015).
- Heery CR , CoyneGHO, MadanRAet al. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies. J. Clin. Oncol.32(Suppl.), Abstract 3064 (2014).
- Boyerinas B , JochemsC, FantiniMet al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol. Res.3(10), 1148–1157 (2015).
- Heery CR , O’Sullivan-CoyneG, MadanRAet al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a Phase 1a, multicohort, dose-escalation trial. Lancet Oncol.18(5), 587–597 (2017).
- Shitara K , YamadaY, YohKet al. Phase I, open-label, multi-ascending dose trial of avelumab (MSB0010718C), an anti-PD-L1 monoclonal antibody, in Japanese patients with advanced solid tumors. J. Clin. Oncol.33(Suppl.), Abstract 3023 (2015).
- Gulley JL , RajanA, SpigelDRet al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, Phase Ib trial. Lancet Oncol.18, 599–610 (2017).
- Apolo AB , InfanteJR, BalmanoukianAet al. Avelumab, an anti-PD-L1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, Phase Ib study. J. Clin. Oncol.35(19), 2117–2124 (2017).
- Chung HC , ArkenauH, WyrwiczLet al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN Solid Tumor Phase Ib trial: analysis of safety and clinical activity. J. Clin. Oncol.34(Suppl.), Abstract 4009 (2016).
- Dirix LY , TakacsI, NikolinakosPet al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a Phase Ib JAVELIN solid tumor trial. Cancer Res.76(Suppl. 4), Abstract S1–04 (2016).
- Disis ML , PatelM, PantSet al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor Phase Ib trial: safety and clinical activity. J. Clin. Oncol.34(Suppl. 15), Abstract 5533 (2016).
- Kaufman HL , RussellJ, HamidOet al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol.17(10), 1374–1385 (2016).
- Bavencio (avelumab) injection [package insert]. Darmstadt, Germany: Merck KGaA; 2017.
- Bavencio (avelumab) injection [prescribing information (Japan)]. Darmstadt, Germany: Merck KGaA; 2017.
- Bavencio (avelumab) injection [summary of product characteristics]. Darmstadt, Germany: Merck KGaA; 2017.
- Aaronson NK , AhmedzaiS, BergmanBet al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst.85(5), 365–376 (1993).
- Greimel E , BottomleyA, CullAet al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. Eur. J. Cancer39(10), 1402–1408 (2003).
- Herdman M , GudexC, LloydAet al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res.20(10), 1727–1736 (2011).
- Herzog TJ , AlvarezRD, SecordAet al. SGO guidance document for clinical trial designs in ovarian cancer: a changing paradigm. Gynecol. Oncol.135(1), 3–7 (2014).
- Broglio KR , BerryDA. Detecting an overall survival benefit that is derived from progression-free survival. J. Natl Cancer Inst.101(23), 1642–1649 (2009).
- Herzog TJ , IsonG, AlvarezRDet al. FDA ovarian cancer clinical trial end points workshop: a Society of Gynecologic Oncology white paper. Gynecol. Oncol.147(1), 3–10 (2017).

