Abstract
Venetoclax is a highly selective, potent BCL-2 inhibitor that is approved for some patients previously treated for chronic lymphocytic leukemia, and has shown promising activity in adult studies across several hematologic malignancies. Preclinical studies have demonstrated venetoclax activity in pediatric patient-derived xenograft models and cell lines; however, clinical studies in pediatric patients have yet to be conducted. The prognosis is poor for children with most relapsed/refractory malignancies, and limited treatment options result in unmet clinical need. Herein, we describe the rationale and design of the first study of venetoclax in pediatric patients with relapsed/refractory malignancies: a Phase I trial investigating the safety and pharmacokinetics of venetoclax monotherapy followed by the addition of chemotherapy (Trial registration: EudraCT 2017-000439-14; NCT03236857).
Members of the BCL-2 protein family are key regulators of the intrinsic apoptotic pathway [Citation1]. The antiapoptotic proteins of the BCL-2 family prevent activation of proapoptotic proteins that lead to initiation of the mitochondrial apoptosis pathway, thereby promoting cell survival. Overexpression of BCL-2 family members commonly occurs in hematologic malignancies and certain solid tumors, resulting in enhanced cancer cell survival [Citation2]. Therefore, targeting antiapoptotic proteins is a promising therapeutic avenue to explore for pediatric cancer patients.
With new oral targeted therapies becoming more widely available, the landscape of anticancer treatment has changed considerably. However, drug development in early phase studies of pediatric cancers has been met with several challenges. These include low single-site patient enrollment numbers due to the relative rarity of many pediatric cancers, often requiring multicenter and multinational studies to ensure sufficient enrollment, and the occurrence of disease progression during single-agent studies, particularly for acute leukemias [Citation3]. New study designs are required that can balance single-agent toxicity, pediatric pharmacokinetics and efficacy with the mechanism of action, and a strong desire of the pediatric research community to incorporate compelling new agents with a rationally chosen chemotherapy regimen [Citation4,Citation5].
Venetoclax
Venetoclax is a highly selective, potent, orally bioavailable small-molecule inhibitor of the antiapoptotic BCL-2 protein. Venetoclax selectively binds to BCL-2, displacing proapoptotic proteins and leading to mitochondrial outer membrane permeabilization, activation of caspases and subsequent restoration of apoptosis () [Citation1,Citation6]. Venetoclax is a first-in-class novel BCL-2 inhibitor and the first to be approved in the USA, the EU and other countries for selected adults with relapsed/refractory chronic lymphocytic leukemia (CLL) [Citation7,Citation8]. Venetoclax is being evaluated in clinical studies of adult patients with other hematologic malignancies, including multiple myeloma, acute myeloid leukemia (AML) and non-Hodgkin lymphoma (NHL), both alone and in combination regimens on the basis of BCL-2 overexpression data in these malignancies [Citation1,Citation9–11].
MOMP: Mitochondrial outer membrane permeabilization.
Adapted from Cancer Discovery, 2016, 6(10), 1106–1117, Konopleva M, Pollyea DA, Potluri J et al. Efficacy and biological correlates of response in a Phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia, with permission from AACR [Citation6].
![Figure 1. Mechanism of action of venetoclax.MOMP: Mitochondrial outer membrane permeabilization.Adapted from Cancer Discovery, 2016, 6(10), 1106–1117, Konopleva M, Pollyea DA, Potluri J et al. Efficacy and biological correlates of response in a Phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia, with permission from AACR [Citation6].](/cms/asset/c2a1b07b-2943-48e2-8fe3-169448780da6/ifon_a_12331960_f0001.jpg)
Data from ongoing adult studies of hematologic malignancies that also occur in children (AML and NHL) provide a compelling rationale to study venetoclax in children with these malignancies. Promising clinical activity has been reported for venetoclax monotherapy or combination with other therapies in clinical studies of adult patients with AML () [Citation1,Citation6,Citation9,Citation10,Citation12–29]. Single-agent venetoclax (800 mg daily) demonstrated preliminary activity in a Phase II study (NCT01994837) of 32 adult patients with relapsed/refractory AML or newly diagnosed patients unfit for induction chemotherapy. The median age was 71 years (19–84 years) and median time on venetoclax monotherapy was 63.5 days (range: 14–246 days). The objective response rate (ORR) was 19%, with complete response (CR) of 6% and CR with incomplete marrow recovery (CRi) of 13% [Citation6].
Table 1. Mechanism-based selection of pediatric tumor types.
Data from this study prompted two additional studies of venetoclax in combination with chemotherapy (low-dose cytarabine) or a hypomethylating agent (azacitidine or decitabine), therapies commonly used to treat AML, for adult patients with treatment-naive AML. The combination regimens showed preclinical synergistic cytotoxic activity, and can alter the ratio of BCL-2 family members, leading to an increase in leukemic-cell dependence on BCL-2 for survival and sensitivity to venetoclax [Citation30]. A Phase I/II study (NCT02287233) evaluating venetoclax (600 or 800 mg daily) in combination with low-dose cytarabine 20 mg/m2 demonstrated preliminary activity. The maximum tolerated dose (MTD) was not reached, and daily venetoclax at 600 mg was chosen as the recommended Phase II dose (RP2D) in this combination, due to dose-limiting toxicities (DLTs) that occurred in two of ten patients in the 800-mg dose group (grade 4 thrombocytopenia lasting >42 days without evidence of residual leukemia) [Citation31]. Of 61 patients treated at the RP2D of venetoclax, 64% achieved an ORR, with CR + CRi of 62% () [Citation1,Citation6,Citation9,Citation10,Citation12–29]. Another Phase Ib study (NCT02203773) evaluating venetoclax (400, 800 or 1200 mg) in combination with a hypomethylating agent (decitabine or azacitidine) also demonstrated significant activity. Efficacy data from 145 patients aged ≥65 years reported an ORR of 67%, with CR + CRi of 66%. The MTD was not identified, and the RP2D of venetoclax in this combination was identified as 400 mg, as the best benefit:risk profile is observed at this dose () [Citation1,Citation6,Citation9,Citation10,Citation12–29]. Both combinations (venetoclax + low-dose cytarabine and venetoclax + azacitidine) are being evaluated in Phase III studies in patients with treatment-naive AML (NCT03069352, NCT02993523) [Citation32].
Similarly, venetoclax has shown antitumor activity in adult patients with BCL-2-driven relapsed/refractory NHL in three early phase studies () [Citation1,Citation6,Citation9,Citation10,Citation12–29]. A first-in-human dose-escalation Phase I study (NCT01328626) evaluated venetoclax monotherapy (200–1200 mg daily) in 106 adult patients with relapsed/refractory NHL. Median age for the overall population was 66 years (25–86 years). The MTD was not reached. 34 patients had diffuse large B-cell lymphoma (DLBCL), a subtype that occurs in pediatric patients, and among those with DLBCL, the ORR was 18%, with a CR rate of 12% () [Citation1,Citation6,Citation9,Citation10,Citation12–29]. Venetoclax (50–1200 mg daily) in combination with bendamustine and rituximab (R) was evaluated in an adult Phase I study (NCT01594229). Of 60 patients enrolled, 22 had DLBCL; the ORR among these patients was 41%, with a CR of 14% () [Citation1,Citation6,Citation9,Citation10,Citation12–29]. The MTD was not reached; the RP2D for venetoclax in this combination was 800 mg daily [Citation26], which has an acceptable benefit:risk ratio in other studies of NHL [Citation33]. An ongoing Phase Ib/II study (NCT02055820) is evaluating venetoclax with either R or obinutuzumab (G) combined with standard doses of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) in B-cell NHL patients who received one or more prior therapy. The Phase I portion enrolled a total of 56 patients, 17 with DLBCL. Preliminary data showed 80% of evaluable DLBCL patients achieved CR, and of the eight BCL-2/Myc double-expressing DLBCL patients, six (75%) achieved CR. The 200-mg daily dose of venetoclax in cohort 1 was not tolerated. After three patients experienced DLTs in the first cohort, one patient receiving R-CHOP and two patients receiving G-CHOP (200-mg venetoclax daily dose), venetoclax was reduced from daily dosing to 10 days of the R-CHOP 21-day cycle. A 10-day dosing regimen allowed dose escalation to 800 mg venetoclax. The RP2D for venetoclax in combination with R-CHOP is 800 mg venetoclax 10 days of the 21-day cycle, with acceptable incidence of DLTs [Citation34].
Venetoclax has demonstrated a tolerable safety profile across all studies and indications. The most common any-grade adverse events (AEs) occurring in ≥20% of patients with CLL (n = 240) were neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia and fatigue. The most common grade 3 or 4 AEs occurring in ≥5% of these patients were neutropenia, anemia, thrombocytopenia, febrile neutropenia and pneumonia. Serious AEs were reported in 43.8% of patients [Citation7].
Initial dose-finding studies in patients with relapsed/refractory CLL resulted in some patients experiencing clinical tumor lysis syndrome (TLS) [Citation1,Citation35]. To mitigate the risk of TLS, CLL studies thereafter implemented a dose ramp-up of venetoclax and TLS prophylaxis measures. Data from adult studies suggest that the risk of TLS is low in other disease settings, though TLS prophylaxis and/or a dose ramp-up tailored to these settings is used. In AML studies, no clinical TLS has been observed to date, and one patient had laboratory TLS that was managed [Citation6,Citation12,Citation13]. No clinical TLS was observed in NHL studies; however, six patients had laboratory TLS that was managed [Citation25,Citation34].
Pharmacokinetic data available from adult studies have shown peak venetoclax concentrations at 5–8 h following the first dose. The terminal half-life ranged from 17–41 h following a single dose of venetoclax. In all clinical studies, venetoclax has been administered with food, as the bioavailability of venetoclax is increased by approximately three- to five-fold. Venetoclax is predominantly metabolized by the intestinal and hepatic enzyme cytochrome P450 (CYP)3A4. Concomitant use of venetoclax with strong CYP3A inhibitors at initiation and during dose ramp-up is contraindicated, and use of moderate CYP3A inhibitors, strong or moderate inducers, P-gp inhibitors or narrow therapeutic index P-gp substrates should be avoided with venetoclax on the basis of clinical drug–drug interaction studies [Citation7,Citation36–38]. However, if a patient requires use of CYP3A and/or P-gp strong or moderate inhibitors, or in cases where a concomitant chemotherapeutic is a CYP3A/P-gp inhibitor and will be used after the ramp-up period, venetoclax dose reduction can be followed according to the prescribing information [Citation7,Citation39].
In addition to clinical data from relevant adult studies, preclinical data were analyzed to identify additional pediatric tumors with greatest potential to respond to venetoclax. To date, venetoclax has not been evaluated in patients with acute lymphoblastic leukemia (ALL). Preclinical studies have demonstrated BCL-2 overexpression and activity in human-derived pediatric cancer cell lines and several murine tumor models () [Citation1,Citation6,Citation9,Citation10,Citation12–29]. In particular, tumor models harboring the t(4;11) translocation are especially sensitive to venetoclax, most likely due to the direct transcriptional upregulation of BCL-2 expression by the MLL–AF4 fusion protein [Citation14]. The Pediatric Preclinical Testing Program assessed venetoclax in 21 pediatric patient-derived xenograft (PDX) models of ALL. Three of nine B-cell precursor ALL PDX models showed objective responses (two CR and one partial response [PR]), and three of six mixed lineage leukemia (MLL) ALL PDX models showed strong responses to venetoclax (two CR and one PR) [Citation20]. In another study, venetoclax demonstrated potent in vitro and in vivo activity in MLL-rearranged ALL xenografts, with significantly delayed progression in 11 of 19 (58%) xenografts. MLL-rearranged and B-cell precursor subtypes had the longest median growth delay [Citation21]. BCL-2 expression is upregulated by TCF3–HLF, the product of the t(17;19)(q22;p13) translocation and hypodiploid ALL, both of which are associated with extremely poor outcomes [Citation16]. Specific sensitivity to venetoclax was achieved in both ex vivo and in vivo TCF3–HLF-positive ALL samples [Citation20,Citation40,Citation41] as well as hypodiploid ALL cell lines and PDXs [Citation17].
Most patient-derived neuroblastoma cell lines are particularly sensitive to venetoclax treatment in vitro, most likely due to BCL-2 expression and its sequestration of the prodeath protein BIM [Citation27]. Studies in neuroblastoma murine xenografts showed that single-agent venetoclax treatment led to long-term survival in 20% of mice. When combined with cyclophosphamide, survival rates were substantially increased to 60% () [Citation1,Citation6,Citation9,Citation10,Citation12–29].
Phase I trial
Herein we describe the design of the venetoclax pediatric trial (M13–833), an open-label, global Phase I study (EudraCT 2017–000439–14; NCT03236857) evaluating the safety, pharmacokinetics and preliminary efficacy of venetoclax in pediatric and young adult patients with relapsed/refractory malignancies. This trial is the first study of venetoclax in pediatric patients, and incorporates a novel study design. The study will be conducted in accordance with the protocol, International Conference on Harmonization Good Clinical Practice guidelines and applicable regulations and guidelines governing clinical study conduct and ethical principles that have their origin in the Declaration of Helsinki. All patients must provide written informed consent (in addition to pediatric assent, if required) approved by an independent ethics committee/institutional review board, prior to initiation of any screening or study-specific procedures.
Objectives
The primary study objectives are to evaluate the safety, determine the DLT and RP2D and assess the pharmacokinetics of venetoclax monotherapy in pediatric patients. The secondary objectives are to determine the preliminary efficacy of venetoclax monotherapy, and to evaluate the safety and preliminary efficacy of venetoclax in combination with chemotherapy appropriate to the diseases of patients enrolled. The exploratory objectives are to evaluate pharmacodynamic and predictive biomarkers (including BCL-2 family expression), and to assess minimal residual disease (MRD) in peripheral blood and bone marrow (particularly for patients with acute leukemias).
Study design
This trial will follow a two-part study design () consisting of dose determination (Part 1) and cohort expansion (Part 2). Part 1 will enroll pediatric and young adult patients with any relapsed/refractory tumor type who have no available curative options. During Part 2, four tumor cohorts will enroll patients with relapsed/refractory ALL, AML, NHL or neuroblastoma. An exploratory fifth cohort will enroll patients with any tumor that has evidence of BCL-2 expression and no available treatment options, or patients with TCF3–HLF ALL confirmed during frontline induction.
†The first ten patients will be pediatric patients <18 years of age; a maximum of 20 patients may enroll.
‡Excluding brain tumors.
§Potential to de-escalate or escalate.
¶Must have evidence of BCL-2 expression and includes patients with TCF3-HLF ALL.
ALL: Acute lymphoblastic leukemia; AML: Acute myeloid leukemia; NHL: Non-Hodgkin lymphoma; PK: Pharmacokinetic.
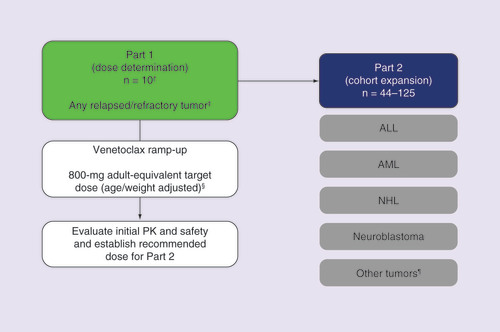
Treatment
Two formulations of venetoclax will be available. Tablets for oral suspension, an age-appropriate formulation, will be used in Part 1. In a relative bioavailability study in healthy participants, the bioavailability of the tablets for oral suspension was found to be similar to that of the oral adult tablet when taken with a high-fat meal. Since the pharmacokinetics of the two formulations are similar, in Part 2, patients will choose between the approved oral tablet formulation, or tablets for oral suspension if they are unable to swallow tablets [Citation7,Citation8].
Oral venetoclax monotherapy will be administered once daily, continuously. The initial target dose in Part 1 will be an exposure-equivalent dose to the 800-mg venetoclax dose used in adults, that will be weight or age adjusted [Citation4]. Venetoclax is metabolized by CYP3A, which is not fully mature in humans until they reach the age of 2 years. Therefore, this may affect hepatic elimination of venetoclax. In this trial, patients <2 years old will be dosed primarily according to their age ( & ) [Citation42]. Patients who are 2 years of age and older will be dosed according to their weight ( & ). Ultimately, pharmacokinetic data from this study will be used to assess the impact of age and weight on venetoclax pharmacokinetic parameters (i.e., apparent clearance and volume of distribution) to determine dosing instructions for pediatric patients. The pediatric target doses are currently projected on the basis of matching safe and efficacious exposures achieved in adults at the 800-mg dose, and using population pharmacokinetic modeling techniques to select doses; at least ten pediatric patients are required.
Table 2. Venetoclax dose ramp-up in patients with hematologic malignancies or lymphomas.
Table 3. Venetoclax dose ramp-up in patients with solid tumors.
To mitigate TLS risk, venetoclax doses will ramp up to the target dose. Due to the increased risk of TLS in patients with leukemia or lymphoma, these patients will ramp up over 3 days (adult exposure-equivalent doses of 200 mg day 1, 400 mg day 2 and 800 mg day 3), detailed in & . Patients with solid tumors will ramp up over 2 days (adult exposure-equivalent doses of 400 mg day 1 and 800 mg day 2), detailed in & . TLS has rarely been observed in pediatric patients with solid tumors [Citation43]. All patients will receive TLS prophylaxis measures, including hydration, a uric acid-reducing agent (allopurinol or rasburicase) and laboratory monitoring as clinically indicated, but at a minimum of predose, 4, 8 and 24 h postdose until venetoclax target dose is achieved ( & B).
(A) Patients with ALL, AML and NHL. (B) Patients with neuroblastoma and other solid tumors.
†Longer durations of therapy may be allowed.
ALL: Acute lymphoblastic leukemia; AML: Acute myeloid leukemia; IV: Intravenous; NHL: Non-Hodgkin lymphoma; TLS: Tumor lysis syndrome.
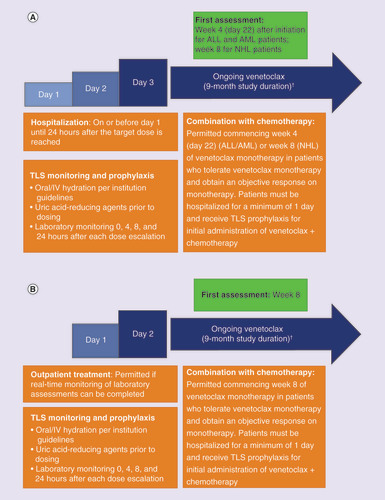
Evidence from preclinical and adult studies suggests venetoclax is most likely to be effective in combination with chemotherapy; therefore, alongside establishing the safety of the single agent, patients will be permitted to receive combination of venetoclax with chemotherapy after a venetoclax monotherapy window, as determined by their diagnosis. During Part 1 and 2, patients may receive venetoclax in combination with chemotherapy following documentation of response to monotherapy at the first disease assessment (this may include disease progression at the first assessment or earlier if suspected and confirmed). For patients with leukemia, combination with chemotherapy will be allowed commencing week 4 (day 22), and for patients with lymphoma and solid tumors, commencing week 8. Allowed chemotherapies were selected on the basis of available venetoclax + chemotherapy data from clinical trials in adults (for AML and NHL patients), salvage regimens commonly used for relapsed pediatric tumors or preclinical data, as summarized in . Patients who progress on venetoclax + chemotherapy will come off the study.
Table 4. Allowed chemotherapy regimens in combination with venetoclax for each tumor type.
Part 1 (dose determination)
In Part 1, a minimum of ten pediatric patients (<18 years of age) will be enrolled. On the basis of pharmacokinetic parameter variability observed with the tablets for oral suspension, pharmacokinetic data from ten patients will yield a two-sided 95% CI within 60 and 140% of the geometric mean parameter estimate with approximately 95% probability. Additional patients (up to 20) may be enrolled to ensure sufficient enrollment across age, weight ranges and tumor types, if the population of enrolled patients is not balanced and additional pharmacokinetic data are required to evaluate other ages and weights. Of the population enrolled in Part 1, at least three patients must have bone marrow involvement, and at least three patients must have disease without bone marrow involvement. The collected pharmacokinetics data from ten pediatric patients will be evaluated using population pharmacokinetics approaches, to enable understanding of age and weight effects on venetoclax pharmacokinetics. The initial target dose will be an adult exposure-equivalent dose of 800 mg daily. Safe and efficacious exposures achieved in adults at the 800-mg dose will be matched in pediatrics at the projected doses, as shown in & . Sufficient exposure in children is identified as within twofold of the target exposure, to account for uncertainty in these projections and observed pharmacokinetic variability in adults. The pediatric doses will be adjusted if needed, based on collected pharmacokinetics data from Part 1 of the study, with the objective of achieving the mean and within twofold of venetoclax-targeted efficacious area under the concentration–time curve steady state values.
For the purposes of dose-escalation decisions, DLTs will be assessed during the first 21 days of venetoclax monotherapy. Separate DLT criteria will be utilized depending on tumor type (leukemias vs solid tumors), to account for the prevalence of bone marrow involvement in patients with leukemia. As a result, hematologic toxicities will not be used to define DLT in patients with ALL/AML. Safety data from the first three patients enrolled in Part 1 will be evaluated after completion of the DLT assessment period and prior to enrollment of additional patients.
To be evaluable for DLTs, patients must complete the first 21 days of venetoclax monotherapy with 80% drug compliance, and be assessable for toxicities or experience a DLT within the 21-day DLT period. Patients who do not complete the 21-day venetoclax monotherapy DLT period without a DLT will be considered unevaluable and will be replaced, to obtain sufficient pharmacokinetic and safety data for analysis.
Dose de-escalation and escalation decisions will be made utilizing a Bayesian optimal interval (BOIN) design that considers the cumulative number of patients who experience a DLT at the current venetoclax dose [Citation44]. The target toxicity rate utilized for the BOIN design is 33% and is applied to all tumor types. An initial cohort of three patients will be enrolled and a safety evaluation performed prior to enrollment of additional patients. The cohort size for subsequent patients is not similarly restricted. If 800-mg adult-equivalent venetoclax is not tolerated, a lower dose of 400-mg adult-equivalent venetoclax will be evaluated as dose level-1. The MTD for venetoclax monotherapy has not been reached in adult studies evaluating daily venetoclax monotherapy at doses up to 1200 mg [Citation25,Citation35].
The dose and schedule identified in Part 1 will be utilized in Part 2 of the study. The toxicity of venetoclax combined with chemotherapy will be assessed separately. The DLT evaluation period is based on monotherapy alone.
Part 2 (cohort expansion)
For Part 2, between 44 and 125 patients will be enrolled into four tumor cohorts: relapsed/refractory ALL, AML, NHL and neuroblastoma. A fifth cohort that is exploratory in nature will enroll patients who have any tumor that expresses BCL-2 and no available curative treatment options, or patients with TCF3–HLF ALL diagnosed during frontline induction. As in Part 1, patients will initially be treated with venetoclax monotherapy until first protocol-defined assessment, to fully evaluate the secondary objective of defining preliminary efficacy of venetoclax monotherapy. After the first assessment, patients may receive venetoclax in combination with chemotherapy, beginning week 4 (day 22) for patients with hematologic malignancies, and week 8 for patients with solid tumors, unless a patient progresses before the first assessment at these times.
The four main tumor cohorts will utilize a Gehan two-stage design [Citation45] to minimize unnecessary venetoclax exposure to pediatric patients if there is no sign of activity. In the first stage, 11 patients will be enrolled in each of the four tumor cohorts, and preliminary antitumor activity will be evaluated based on response criteria established for each tumor type. The second stage will enroll 14 additional patients in an individual tumor cohort, provided there is evidence of antitumor activity observed in any of the first 11 patients. The target response rate is 20%; this is appropriate for each tumor type, as these patients would all be considered incurable. In addition, this target response rate corresponds to the adult venetoclax monotherapy response rate observed in AML and NHL. The fifth cohort will be exploratory. Statistical analyses will not be performed for the fifth cohort.
Key eligibility criteria
Eligible patients must be <25 years of age at enrollment; the inclusion of young adults in this trial is based on the continued high unmet clinical need in this patient population that is generally underrepresented in clinical trials [Citation46]. Key eligibility criteria are summarized in .
Table 5. Key inclusion and exclusion criteria.
Safety evaluations
Safety parameters will be evaluated throughout the study. Safety will be assessed by evaluating study drug exposure, AEs according to the National Cancer Institute Common Terminology Criteria for AEs version 4.03 [Citation47], serious AEs, deaths and changes from baseline in laboratory tests. DLTs will be evaluated during the first 21 days of monotherapy during Part 1.
Pharmacokinetic evaluations
Limited pharmacokinetic samples from peripheral blood will be collected during ramp up on days 1–3 of week 1 and on day 8 of week 2, when the target dose exposure is expected to reach steady state. Sparse samples will also be collected during clinical visits on weeks 3, 4, 8, 12, 24 and every 12 weeks thereafter, and at final visit. The sampling time points were selected based on the established pharmacokinetic profile of venetoclax in adults [Citation25,Citation35,Citation48,Citation49], and to accommodate limitations in the number of blood samples that can be drawn from pediatric patients (three-to-five samples/day on two occasions in Part 1, and one sample/visit on specified occasions). Plasma concentrations of venetoclax (and if needed possible metabolite[s]), and pharmacokinetic parameter values will be evaluated. Venetoclax doses will be adjusted if needed, based on pharmacokinetic data from Part 1. Overall analysis of pharmacokinetic data along with safety assessments will inform dosing of venetoclax in Part 2.
Efficacy evaluations
Response will be assessed by the investigator based on standard established response criteria for each tumor type, as summarized in [Citation50–52]. MRD in peripheral blood and/or bone marrow will be assessed by flow cytometry or quantitative PCR as an exploratory objective when relevant. An objective response will be recorded for each patient on venetoclax monotherapy, and for each patient on venetoclax combined with chemotherapy. Best response to both monotherapy (including progressive disease) and combination therapy will be assessed. Patient survival data will be collected until the end of the study.
Table 6. Efficacy assessment criteria for each tumor type.
Biomarker evaluations
Preclinical studies have contributed significantly to our understanding of BCL-2 biology, and have guided the translational biomarker strategy for venetoclax [Citation53]. These studies have shown that high BCL-2 expression is commonly associated with greater in vitro sensitivity to single-agent venetoclax [Citation1]. However, due to the dynamic nature of other antiapoptotic proteins such as BCL-XL and MCL-1, which can sequester proapoptotic proteins liberated from BCL-2, a broader expression profile may provide additional value for predicting responses to venetoclax. Alternatively, high BCL-2 expression has been associated with known genetic lesions [Citation1,Citation11,Citation14,Citation20,Citation40,Citation54] that offer potential surrogate markers of BCL-2 dependency; however, other mechanisms that contribute to venetoclax sensitivity may not be captured, such as aberrantly active cell-signaling pathways or defects in mRNA-mediated regulation. This dynamic interplay between multiple cellular systems may limit the utility of expression-based markers as predictors of venetoclax sensitivity. One potential solution is to determine on which BCL-2 family member a given cell population depends for survival, a method termed ‘BH3 profiling’ [Citation55]. Thus, BH3 profiling may provide an alternative, functional means of assessing tumor sensitivity to venetoclax. Similarly, proximity ligation assays may be employed to extend the capabilities of traditional immunoassays to include detection of direct protein–protein interactions, particularly BCL-2–BIM.
The overall objectives of the exploratory biomarker research in this study are to evaluate potential predictive biomarkers of venetoclax preliminary efficacy, and to gain a better understanding of potential resistance mechanisms to venetoclax. To enable these studies, peripheral blood, bone marrow and tumor tissue samples will be collected according to tumor type at designated time points throughout the study to conduct exploratory biomarker analyses. Biomarkers related to the pathway(s) targeted by the study drug (i.e., BCL-2 family expression levels) or biomarkers related to the disease or to drug response/resistance may be evaluated for association with pharmacokinetics, safety or efficacy. Samples may also be collected for functional and/or phenotypic analyses by BH3 profiling, for hematologic malignancies, or proximity ligation assay for solid tumors. Results will be correlated with response where applicable.
Statistical methods
The BOIN design [Citation44] will be used to guide dose de-escalation/escalation decisions during Part 1; a Gehan two-stage design [Citation45] will be used to minimize unnecessary venetoclax exposure to pediatric patients during Part 2. Plasma concentrations of venetoclax and metabolite(s) and pharmacokinetic parameters will be tabulated for each patient and each dose level. Summary statistics will be provided for each dose level and each parameter. The following efficacy analyses will be assessed: ORR (CR + CRi + CR without platelet recovery [CRp] + PR for hematologic malignancies, CR + PR for NHL, neuroblastoma and solid tumors), CR rate, PR rate, MRD rate, overall survival and progression-free survival. Statistical analysis will not be performed for the exploratory fifth cohort in Part 2 due to the many different tumor types expected to be enrolled. All efficacy analyses will be performed using SAS® version 9.4 (SAS Institute Inc., NC, USA).
Conclusion
Pediatric patients with certain relapsed/refractory malignancies represent a population with extremely poor prognosis. Advances in drug development in this high-risk population are hindered by low numbers of patients and difficulties in enrolling patients onto traditional early phase studies that evaluate single-agent activity in these highly refractory cancers. The venetoclax pediatric trial (M13–833) is a global study and the first to evaluate the safety, pharmacokinetics and preliminary activity of venetoclax, a potent and highly specific BCL-2 inhibitor, in pediatric patients. The current approach was driven by data (monotherapy and combination therapy) in adult patients with malignancies that also occur in children (AML and NHL), and importantly, also by preclinical data across a number of pediatric malignancies. Utilization of preclinical data ensures a mechanism-based approach for tumor type selection and thus increased access to venetoclax for patients who may benefit. For example, an exploratory cohort is included in Part 2 to allow enrollment of patients with tumor types that have insufficient data to warrant a dedicated cohort.
International collaboration among academic clinicians and regulatory agencies (European Medicines Agency/US FDA Oncologic Drugs Advisory Committee, Pediatric Subcommittee) is crucial, and this unique global study incorporates input from the USA, EU, Australia and Canada on how best to design and conduct this clinical trial for the greatest benefit to pediatric patients. This trial represents an example of national and international academic clinicians and industry working in collaboration to streamline novel agents for therapy in pediatric patients [Citation56].
To accelerate drug development efforts in children and young adults with cancer, this trial aims to overcome issues in current pediatric clinical trials that potentially delay progress, in line with new guidelines developed by the Innovative Therapies for Children with Cancer (ITCC) Consortium. For example, this study utilizes a starting target dose equivalent to that examined in adults, without performing dose escalation [Citation4]. Venetoclax has been extensively studied in adult patients with hematologic malignancies, thus providing a data set to inform development in the pediatric setting. The MTD of venetoclax monotherapy has not been reached in adult studies [Citation25,Citation35]. On the basis of these data and pharmacokinetic modeling, a dose-determination design is used in Part 1 to validate that the 800-mg adult-equivalent dose is appropriate, rather than a more traditional dose-escalation design that could require larger numbers of patients, expose patients to subtherapeutic doses, and require longer time to execute. This also aligns with the ITCC Consortium recommendation of a pediatric starting dose of 100% of the RP2D used for adults to validate and/or confirm findings from adults [Citation4]. This approach enables enrollment of a smaller number of patients, with only ten pediatric patients initially required for Part 1 safety pharmacokinetics analysis, and facilitates advancing to Part 2 of the study, which will focus on antitumor activity in specific tumor cohorts. Utilization of expansion cohorts in Part 2 enables evaluation in specific tumor types selected on the basis of preclinical data and data from clinical trials in adults, and will provide information about venetoclax in combination therapy more rapidly than if done in separate, larger subsequent studies of each individual tumor type.
The current study does not have a lower age limit, due to the availability of a formulation suitable for infants. Therefore, pharmacokinetic data could be available across a broader age range, including in infants and children under 2 years of age, a group commonly underrepresented in many studies. Though the population for the current study is limited to relapsed/refractory patients, there are certain subtypes of leukemias that occur and can relapse in infancy. Additionally, the inclusion of young adults in this study is an approach to accelerate drug development in this specific patient population that is underrepresented in clinical trials [Citation4,Citation46,Citation56].
This study includes a monotherapy window to assess single-agent activity, and importantly, incorporates a combination therapy portion to efficiently evaluate venetoclax in combination with chemotherapy, and facilitate rapid transition to a larger-scale confirmatory study. The option to combine venetoclax with chemotherapy after a defined period of venetoclax monotherapy balances the need to understand pediatric-specific pharmacology and toxicology of a single agent while optimizing an individual patient’s chance of deriving clinical benefit, and should help optimize accrual of patients whose disease may benefit from targeted inhibition of BCL-2 alone and/or in combination therapy. Preliminary data from ongoing adult studies have shown that a combination approach can lead to higher ORR and CR rates compared with venetoclax monotherapy [Citation12,Citation13,Citation26,Citation57]. It should be noted that pretreatment with venetoclax alone may alter the tumor biologically to affect the efficacy of the venetoclax and chemotherapy combination. However, defining the toxicity of the combination therapy within this trial facilitates starting future combination studies.
In addition, correlative analysis performed in this study will seek to identify whether genetic, immunohistochemistry expression or protein–protein interactions provide better insight into critical biomarkers for venetoclax in pediatric patients, and distinguish specific patient populations that will clinically benefit most from venetoclax therapy.
This study maximizes efficiency in pediatric early phase trial design, by combining multiple elements in a single study with the goal of efficiently advancing to subsequent larger-scale studies in a timely manner. The novelty of the design, correlative analysis and the unique international and industry collaborations that are working to streamline novel therapeutics in pediatric cancer aim to improve the overall risk:benefit ratio in children.
The results of the described clinical trial may lead to a novel therapeutic avenue for pediatric patients who have limited treatment options. Recruitment of this trial began in October 2017.
Novel study designs are required for pediatric patients with certain relapsed/refractory malignancies who have poor prognosis and limited treatment options.
The antiapoptotic BCL-2 protein is overexpressed in many hematologic malignancies and certain solid tumors, and represents a promising therapeutic target for pediatric cancer patients.
Venetoclax
Venetoclax is a highly selective, potent and orally bioavailable first-in-class BCL-2 inhibitor.
Venetoclax has shown promising activity in adult studies across several hematologic malignancies, and studies in pediatric patient-derived xenograft models and cell lines have demonstrated venetoclax activity in acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL) and neuroblastoma.
Phase I trial
The venetoclax pediatric trial (M13–833) is an open-label, global Phase I trial (EudraCT 2017–000439–14; NCT03236857) and the first study of venetoclax in pediatric patients.
The primary objectives of the study are to evaluate the safety, determine the dose-limiting toxicities and the recommended Phase II dose and assess the pharmacokinetics of venetoclax monotherapy.
The secondary objectives are to determine the preliminary efficacy of venetoclax monotherapy, and to evaluate the safety and preliminary efficacy of venetoclax in combination with chemotherapy.
A biomarker strategy is a key exploratory objective of this trial.
Study design & treatment
In Part 1 (dose determination), patients with any relapsed/refractory tumor will receive oral venetoclax once daily and ramp up to the adult venetoclax exposure-equivalent dose of 800 mg that is weight or age adjusted.
Dose-limiting toxicities will be evaluated during the first 21 days of monotherapy.
Part 2 (cohort expansion) will enroll with histologically confirmed relapsed/refractory ALL, AML, NHL or neuroblastoma, and use the venetoclax dose and schedule identified in Part 1. A fifth exploratory cohort will enroll patients with any tumor that expresses BCL-2, or patients with TCF3-HLF ALL diagnosed during frontline induction.
Patients may receive venetoclax in combination with chemotherapy following documentation of response to monotherapy at the first disease assessment (or earlier if progressive disease).
Conclusion
The venetoclax pediatric trial (M13–833) will combine multiple elements of study designs into one trial in an international collaborative academic and industry effort to accelerate drug development in pediatric cancer patients.
Acknowledgements
AbbVie, Genentech, and the authors thank the patients participating in this clinical trial as well as all study investigators and support staff for their contributions.
Financial & competing interests disclosure
AE Place, AbbVie consultant. ML Loh, institutional research funding from AbbVie. L Gore, employment: ARIAD (immediate family member); consulting or advisory role: Celgene, MedImmune, Novartis, ProEd Communications, Genentech/Roche, Amgen, Medscape; leadership: ARIAD (immediate family member); travel, accommodations, expenses: Amgen, Roche/Genentech; patents, royalties, other intellectual property: Patent held for diagnostic discovery and treatment response methodology tools in the use of MR spectroscopy for leukemia; stock and other ownership interests: ARIAD (immediate family member), Amgen, Sanofi, Celgene, ARIAD, Clovis Oncology; Honoraria: Amgen. Y Sanzgiri, D Hoffman, Y Zhou, JA Ross, B Prine, M Shebley, M McNamee, SY Kim, M Verdugo, L Lash-Fleming are AbbVie employees and may own stock. T Farazi, full-time employee of Genentech. CM Zwaan, consulting/advisory role: Pfizer, Roche, Bristol-Myers Squibb, Novartis, Celgene (all institution); travel, accommodations, expenses: Jazz Pharmaceuticals. Josef Vormoor, Roche/Genentech consultant. Venetoclax (ABT-199/GDC-0199) is being developed in a collaboration between AbbVie and Genentech. AbbVie and Genentech provided financial support for the study and participated in the design and study conduct, as well as the writing, review, and approval of the manuscript. This study was supported by research funding from AbbVie and Genentech. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance was utilized in the production of this manuscript. Medical writing support was provided by S Amin, PhD, of TRM Oncology, The Hague, The Netherlands, funded by AbbVie.
Additional information
Funding
References
- Souers AJ , LeversonJD, BoghaertERet al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med.19(2), 202–208 (2013).
- Davids MS , LetaiA. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J. Clin. Oncol.30(25), 3127–3135 (2012).
- Mussai FJ , YapC, MitchellC, KearnsP. Challenges of clinical trial design for targeted agents against pediatric leukemias. Front. Oncol.4, 374 (2015).
- Moreno L , PearsonADJ, PaolettiXet al. Early phase clinical trials of anticancer agents in children and adolescents – an ITCC perspective. Nat. Rev. Clin. Oncol.14(8), 497–507 (2017).
- Pearson ADJ , HeroldR, RousseauRet al. Implementation of mechanism of action biology-driven early drug development for children with cancer. Eur. J. Cancer62, 124–131 (2016).
- Konopleva M , PollyeaDA, PotluriJet al. Efficacy and biological correlates of response in a Phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov.6(10), 1106–1117 (2016).
- Venclexta (venetoclax) [prescribing information]. AbbVie Inc., IL, USA (2016). www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf.
- Venclyxto (venetoclax) [summary of product characteristics]. AbbVie Ltd, Maidenhead, UK (2016). www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004106/WC500218800.pdf.
- Bogenberger JM , KornblauSM, PierceallWEet al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia28(8), 1657–1665 (2014).
- Pan R , HogdalLJ, BenitoJMet al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov.4(3), 362–375 (2014).
- Touzeau C , DoussetC, Le GouillSet al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia28(1), 210–212 (2014).
- Wei A , StricklandSA, RobozGJet al. Phase I/II study of venetoclax with low-dose cytarabine in treatment-naive, elderly patients with acute myeloid leukemia unfit for intensive chemotherapy: 1-year outcomes. Blood130(Suppl. 1), Abstract 890 (2017).
- DiNardo CD , PollyeaDA, JonasBAet al. Updated safety and efficacy of venetoclax with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood130(Suppl. 1), Abstract 2628 (2017).
- Benito JM , GodfreyL, KojimaKet al. MLL-rearranged acute lymphoblastic leukemias activate BCL-2 through H3K79 methylation and are sensitive to the BCL-2-specific antagonist ABT-199. Cell Rep.13(12), 2715–2727 (2015).
- Ackler S , OleksijewA, ChenJet al. Clearance of systemic hematologic tumors by venetoclax (Abt-199) and navitoclax. Pharmacol. Res. Perspect.3(5), e00178 (2015).
- de Boer J , YeungJ, ElluJet al. The E2A–HLF oncogenic fusion protein acts through Lmo2 and Bcl-2 to immortalize hematopoietic progenitors. Leukemia25(2), 321–330 (2011).
- Diaz-Flores E , ComeauxEQ, KimKet al. BCL-2, a therapeutic target for high risk hypodiploid B-cell acute lymphoblastic leukemia. Blood128(22), 280 (2016).
- Peirs S , MatthijssensF, GoossensSet al. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood124(25), 3738–3747 (2014).
- Anderson NM , HarroldI, MansourMRet al. BCL2-specific inhibitor ABT-199 synergizes strongly with cytarabine against the early immature LOUCY cell line but not more differentiated T-ALL cell lines. Leukemia28(5), 1145–1148 (2014).
- Jones L , CarolH, EvansKet al. A review of new agents evaluated against pediatric acute lymphoblastic leukemia by the Pediatric Preclinical Testing Program. Leukemia30(11), 2133–2141 (2016).
- Khaw SL , SuryaniS, EvansKet al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood128(10), 1382–1395 (2016).
- Leverson JD , PhillipsDC, MittenMJet al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med.7(279), 279ra40 (2015).
- Bogenberger JM , DelmanD, HansenNet al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk. Lymphoma56(1), 226–229 (2015).
- Li L , PongtornpipatP, TiutanTet al. Synergistic induction of apoptosis in high-risk DLBCL by BCL2 inhibition with ABT-199 combined with pharmacologic loss of MCL1. Leukemia29(8), 1702–1712 (2015).
- Davids MS , RobertsAW, SeymourJFet al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J. Clin. Oncol.35(8), 826–833 (2017).
- Swinnen LJ , FlowersCR, WangDet al. Venetoclax (VEN), bendamustine (B) and rituximab (R) in patients (pts) with relapsed or refractory (R/R) non-Hodgkin lymphoma (NHL): final results of a Phase I study. Hematol. Oncol.35(Suppl. S2), Abstract 79 (2017).
- Bate-Eya LT , den HartogIJ, van der PloegIet al. High efficacy of the BCL-2 inhibitor ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell lines and xenografts and rational for combination with MCL-1 inhibition. Oncotarget7(19), 27946–27958 (2016).
- Tanos R , KarmaliD, NalluriS, GoldsmithKC. Select Bcl-2 antagonism restores chemotherapy sensitivity in high-risk neuroblastoma. BMC Cancer16, 97 (2016).
- Lamers F , SchildL, den HartogIJMet al. Targeted BCL-2 inhibition effectively inhibits neuroblastoma tumour growth. Eur. J. Cancer48(16), 3093–3103 (2012).
- Teh TC , NguyenNY, MoujalledDMet al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia32(2), 303–312 (2017).
- Lin TL , StricklandSA, FiedlerWet al. Phase Ib/II study of venetoclax with low-dose cytarabine in treatment-naive patients aged ≥65 years with acute myelogenous leukemia. J. Clin. Oncol.34(Suppl. 15), Abstract 7007 (2016).
- Potluri J , XuT, HongWJ, MabryMH. Phase III, randomized, double-blind, placebo-controlled study of venetoclax combined with azacitidine versus azacitidine in treatment-naive patients with acute myeloid leukemia. J. Clin. Oncol.35(15 Suppl.), Abstract TPS7069 (2017).
- Zinzani PL , ToppMS, YuenSLSet al. Phase II study of venetoclax plus rituximab or randomized ven plus bendamustine+rituximab (BR) versus BR in patients with relapsed/refractory follicular lymphoma: interim data. Blood128(22), Abstract 617 (2016).
- Zelenetz AD , SallesGA, MasonKDet al. Results of a Phase Ib study of venetoclax plus R- or G-CHOP in patients with B-cell non-Hodgkin lymphoma. Blood128(22), Abstract 3032 (2016).
- Roberts AW , DavidsMS, PagelJMet al. Targeting BCL-2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med.374(4), 311–322 (2016).
- Chiney MS , MenonRM, BuenoOF, TongB, SalemAH. Clinical evaluation of P-glycoprotein inhibition by venetoclax: a drug interaction study with digoxin. Xenobiotica48(9), 904–910 (2018).
- Agarwal SK , HuB, ChienD, WongSL, SalemAH. Evaluation of rifampin’s transporter inhibitory and CYP3a inductive effects on the pharmacokinetics of venetoclax, a BCL-2 inhibitor: results of a single- and multiple-dose study. J. Clin. Pharmacol.56(11), 1335–1343 (2016).
- Salem AH , HuB, FreiseKJ, AgarwalSK, SidhuDS, WongSL. Evaluation of the pharmacokinetic interaction between venetoclax, a selective BCL-2 inhibitor, and warfarin in healthy volunteers. Clin. Drug Investig.37(3), 303–309 (2017).
- Agarwal SK , DiNardoCD, PotluriJet al. Management of venetoclax–posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin. Ther.39(2), 359–367 (2017).
- Fischer U , ForsterM, RinaldiAet al. Genomics and drug profiling of fatal TCF3–HLF-positive acute lymphoblastic leukemia identifies recurrent mutation patterns and therapeutic options. Nat. Genet.47(9), 1020–1029 (2015).
- Frismantas V , DobayMP, RinaldiAet al. Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia. Blood129(11), e26–e37 (2017).
- O’Hara K , WrightIM, SchneiderJJ, JonesAL, MartinJH. Pharmacokinetics in neonatal prescribing: evidence base, paradigms and the future. Br. J. Clin. Pharmacol.80(6), 1281–1288 (2015).
- Mirrakhimov AE , AliAM, KhanM, BarbaryanA. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors6(2), 5389 (2014).
- Liu S , YuanY. Bayesian optimal interval designs for Phase I clinical trials. J. R. Stat. Soc. Ser. C Appl. Stat.64(3), 507–523 (2015).
- Yin G . Clinical trial design: Bayesian and frequentist adaptive methods. John Wiley & Sons, Hoboken, NJ, USA, 173–175 (2012).
- Hay AE , RaeC, FraserGAet al. Accrual of adolescents and young adults with cancer to clinical trials. Curr. Oncol.23(2), e81–e85 (2016).
- National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) v4.0 (2017).http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- Salem AH , AgarwalSK, DunbarMet al. Effect of low- and high-fat meals on the pharmacokinetics of venetoclax, a selective first-in-class BCL-2 inhibitor. J. Clin. Pharmacol.56(11), 1355–1361 (2016).
- Salem AH , DunbarM, AgarwalSK. Pharmacokinetics of venetoclax in patients with 17p deletion chronic lymphocytic leukemia. Anticancer Drugs28(8), 911–914 (2017).
- Cheson BD , FisherRI, BarringtonSFet al. Recommendations for initial evaluation, staging and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol.32(27), 3059–3068 (2014).
- Park JR , BagatellR, CohnSLet al. Revisions to the International Neuroblastoma Response Criteria: a consensus statement from the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol.35(22), 2580–2587 (2017).
- Eisenhauer EA , TherasseP, BogaertsJet al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer45(2), 228–247 (2009).
- Leverson JD , SampathD, SouersAJet al. Found in translation: how preclinical research is guiding the clinical development of the BCL2-selective inhibitor venetoclax. Cancer Discov.7(12), 1376–1393 (2017).
- Vandenberg CJ , CoryS. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood121(12), 2285–2288 (2013).
- Del Gaizo Moore V , LetaiA. BH3 profiling – measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett.332(2), 202–205 (2013).
- Vassal G , RousseauR, BlancPet al. Creating a unique, multistakeholder Paediatric Oncology Platform to improve drug development for children and adolescents with cancer. Eur. J. Cancer51(2), 218–224 (2015).
- Seymour JF , MaS, BranderDMet al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a Phase Ib study. Lancet Oncol.18(2), 230–240 (2017).
