Abstract
Aim: To compare symptoms and blood test results prior to cancer diagnosis in individuals who developed lung cancer and those who did not. Patients&methods: Nested case–control study, lung cancer patients were matched to up four controls with no record of cancer. Differences in symptoms and blood test results were investigated in the 2-year period prior to diagnosis. Results: 26,379 lung cancer patients were matched to 92,125 controls. Elevated C-reactive protein (CRP) was independently predictive of lung cancer at every 2-month interval 12 months prior to diagnosis. Elevated CRP in conjunction with at least one symptom was associated with greater than fourfold higher odds of lung cancer. Conclusion: CRP may be a prediagnostic marker for lung cancer, and when present with other symptoms could facilitate the investigation of high-risk individuals.
Keywords::
Lung cancer is the third most common cancer in the United Kingdom (UK), accounting for 13% (∼46,000 patients) of all new cancer cases [Citation1]. Lung cancer is most commonly diagnosed at an advanced stage, when the disease is rapidly fatal, and 5-year survival estimates are only 10% [Citation2]. Earlier diagnosis of lung cancer improves patient outcomes and is a focus of the UK National Health Service (NHS) long-term plan for improving cancer care [Citation3].
The majority of lung cancer cases are diagnosed symptomatically when patients present to primary care with cancer ‘alarm’ symptoms [Citation4]. Cancer alarm symptoms are those symptoms at the clinical presentation of a patient that suggest serious or malignant disease, and have been widely publicized as part of public health campaigns [Citation5]. Despite this, cancer symptoms present a challenge for primary care physicians, since these can be common and nonspecific, making it difficult to reliably distinguish patients who need further investigation from those who can be reassured [Citation6]. Earlier diagnosis of lung cancer could be improved by facilitating the prompt investigation and referral of high-risk symptomatic individuals who present to primary care [Citation6]. Importantly, previous studies have shown that blood markers of inflammation are elevated among patients with cancer, and may also represent prediagnostic markers of cancer, particularly in patients who develop lung cancer [Citation7,Citation8].
This study aimed to assess suspected cancer symptoms and blood test results captured by primary care physicians from patients who developed lung cancer up to 2 years prior to their cancer diagnosis to help identify those at the highest risk for further investigation or referral. We matched data from patients to those from controls within the UK Clinical Practice Research Datalink (CPRD) [Citation9] as it is representative of the population for whom earlier identification is likely to be the most helpful.
Methods
Study population
The UK CPRD is a government-managed research service, which from 1987 has collected data from electronic health records used in routine primary care clinical practice in the UK (https://www.cprd.com/home/) [Citation9]. CPRD contains prospectively collected demographic and clinical information that include diagnoses, symptoms, medications and tests. Validation studies have demonstrated the reliability and validity of CPRD data and CPRD-coded diagnoses [Citation9]. The study was approved by the Independent Scientific Advisory Committee of the CPRD.
Study design
This was a nested case–control study in which data collected within the CPRD were used to compare symptoms and blood test results between cases (i.e., individuals who later received a diagnosis of cancer) and controls (i.e., individuals with no cancer record). The initial extraction population included all patients recorded in CPRD GOLD at December 2018. Inclusion criteria for cases were: a read code indicating a diagnosis of any lung cancer (see Supplementary Table 1 for code lists) occurring within the study period (1 January 1987–30 June 2018); a defined gender (male and female only); and a minimum of 3 years of useable (CPRD defined high quality) data prior to first cancer diagnostic code. Cases were matched to up to four controls with no record of cancer by sex, age at index date, general practitioner (GP) practice and year of registration. The index date was defined as the date of first lung cancer diagnosis for the cases and a matched index date for the controls.
Symptoms of interest
Symptoms were identified from the UK National Institute for Health and Care Excellence guidelines that include recommendations on the symptoms and signs that warrant investigation and referral for suspected cancer [Citation10]. We used read codes to identify symptoms of lung cancer such as cough, shortness of breath, chest pain, haemoptysis, fatigue and weight loss reported in both cases and controls in each 2-month interval in the 2-year period prior to first lung cancer diagnosis (or matched index date for controls). See Supplementary Tables 2–7 for read codes.
Blood tests of interest
Available test results for the level of basophils, eosinophils, lymphocytes, monocytes, neutrophils, platelets and C-reactive protein (CRP) in each 2-month interval of the 2-year period prior to first lung cancer diagnosis (or matched index date for controls) were investigated.
Covariates
Covariates were identified in the preindex period (minimum 3-year period defined by the inclusion criteria) and included smoking status, BMI and Charlson comorbidity index (CCI) [Citation11]. Information on smoking status and BMI closest to index date was used, within a maximum time period of 12 months prior to index date.
Statistical analysis
Descriptive analyses were performed on patient characteristics, blood test results and symptoms. Categorical variables were reported as counts (n) and frequencies (%), and continuous variables as mean, standard deviation. Standardized differences were calculated to compare baseline characteristics between cases and controls. Multivariable logistic regression was used to assess the association between blood test results or symptoms, and lung cancer diagnosis. For the regression model, blood test results were classified as high or low using standard thresholds (Supplementary Table 2).
Results
Demographic characteristics of cases&controls
A total of 26,379 patients and 92,125 matched controls meeting the inclusion criteria were included in the analyses. Characteristics of both cases and controls are shown in . Lung cancer patients and matched controls had similar age and sex distributions, as expected given the matching process. Smoking status and BMI appeared to differ between the two groups and had the largest standardized differences, although a high proportion of data was missing for these variables (>30%). CCI scores were similar between lung cancer patients and controls.
Table 1. Clinical and demographic characteristics of cases and controls.
Differences between cases and controls in the frequency of symptoms reported and mean laboratory test values for each 2-month interval (for individuals having a test in that period) are shown in –D and Supplementary Figures 1–9. There were differences in mean laboratory test values between lung cancer patients and controls from at least 6 months prior to cancer diagnosis for CRP, neutrophil, monocyte and platelet tests, and therefore these variables were carried forward into the multivariable models. Differences were not observed for basophil, eosinophil or lymphocyte count. For all symptoms, differences were observed between cases and controls in the frequency of symptoms reported at least 6 months prior to cancer diagnosis (cough, shortness of breath, chest pain, hemoptysis, fatigue and weight loss).
(A) CRP laboratory test trends for cases and controls over 2 years prior to diagnosis of lung cancer. Figure shows mean laboratory test results and standard error at each 2-month interval in the 2-year period prior to diagnosis. Number of patients with available test data at each time point are provided. *Normal range: 3–10 mg/l. (B) Monocyte lab test trends for over 2 years prior to diagnosis of lung cancer. Figure shows mean lab test result and standard error at each 2-month interval in the 2-year period prior to diagnosis. Number of patients with available test data at each time point are provided. *Normal range: 0.2–0.8 × 109/l. (C) Neutrophil lab test trends for over 2 years prior to diagnosis of lung cancer. Figure shows mean lab test result and standard error at each 2-month interval in the 2-year period prior to diagnosis. Number of patients with available test data at each time point are provided. *Normal range: 2.5–7.5 × 109/l. (D) Platelet lab test trends for over 2 years prior to diagnosis of lung cancer. Figure shows mean lab test result and standard error at each 2-month interval in the 2-year period prior to diagnosis. Number of patients with available test data at each time point are provided. *Normal range: 150–450 × 109/l.
CRP: C-reactive protein.
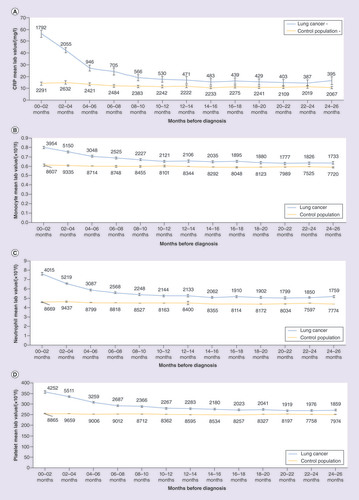
To investigate the independent effect of CRP, neutrophil, monocyte and platelet test results on lung cancer diagnosis, multivariable regression modeling was performed. At 6 months prior to diagnosis, high CRP and platelet counts were independently predictive of lung cancer, after adjusting for other blood tests, symptoms and covariates ( for results 4–6 months prior to diagnosis). High CRP was also independently predictive of cancer diagnosis at every time point up to at least 12 months before diagnosis (Supplementary Table 3) for results for other time points).
Table 2. Multivariable regression results for symptom and laboratory test association with lung cancer at 4–6 months prior to cancer diagnosis.
Multivariable logistic regression modeling was also used to further explore the impact of having reported any cancer symptom and/or a high CRP test in the period 4–6 months prior to first lung cancer diagnosis, when differences in these parameters began to emerge between cases and controls. After adjustment for smoking status and CCI score, having at least one symptom had an odds ratio of 1.99 (1.6–2.5) for lung cancer and having a high CRP test in conjunction with any other symptom yielded an increased odds ratio of 4.62 (95% CI: 3.32–6.42) for lung cancer ().
Table 3. Multivariable regression results for having at least one symptom or one symptom and a high C-reactive protein test on lung cancer diagnosis at 4–6 months prior to diagnosis.
Discussion
We show here that CRP test results are independently predictive of a subsequent lung cancer diagnosis up to at least 12 months prior to first diagnosis. CRP test results are not currently included in UK guidelines for cancer referral in primary care; the current findings suggest that high CRP, particularly when observed in conjunction with at least one cancer ‘alarm’ symptom may reflect an important prediagnostic marker of lung cancer, and help primary care physicians to consider whether or not to refer a patient for further investigation.
Despite significant advances in the treatment landscape in oncology, achieving early diagnosis remains critical to improving outcomes for patients. However, this remains challenging, particularly in lung cancer where many early symptoms (such as a persistent cough) can be common and nonspecific [Citation12]. GPs, as the gatekeepers to secondary care in the UK, have an important role to play in identifying at-risk patients who require further investigation, however, GPs have reported they often require high levels of suspicion to refer patients on to specialist respiratory consultations [Citation13]. Therefore, potential markers that could help GPs to identify individuals at the highest risk of developing cancer is of significant value.
Our findings are broadly comparable with the results of others who have assessed CRP levels on risk of lung cancer [Citation8,Citation14–16]. For example, one recent study that showed elevated CRP levels were associated with a 3.5% incidence of cancer at 1 year, twice the risk of those with a normal test result in the same period [Citation16]. The results of the present study add to this existing literature to show that elevated CRP particularly when present with one or more cancer ‘alarm’ symptom could be clinically helpful, and potentially used by primary care physicians to facilitate earlier diagnosis.
The study has limitations that should be considered when interpreting the results. First, the dataset was limited to patients recorded in CPRD, and as such some cancer diagnoses may have been missed or the date of first diagnosis may not have been accurately recorded; linkage to secondary care data would allow more complete and accurate case ascertainment, and should be explored in future studies [Citation17]. Furthermore, not all patients had complete information on blood tests within any given period, the proportion of people-reporting symptoms prior to diagnosis was relatively small, and there were more data available on lung cancer patients than controls particularly at time points closer to first diagnosis, which may have generated bias. We also cannot rule out the possibility that elevated CRP levels among lung cancer patients reflect a surrogate for poorly measured or unmeasured risk factors, and although we adjusted for a number of important potentially confounding factors including smoking status, BMI and co-morbidities, we also observed a high proportion of missing values for these variables. It is also important to note that the period of time before the patient’s first diagnosis (in which laboratory test was evaluated) is not equivalent to the period of the time in which the patient was cancer-free. As such, this study cannot help to establish causality in the relationship between CRP and lung cancer, but rather suggests that CRP may be a clinically helpful biomarker, particular among patients who present symptomatically to primary care. In light of the limitations discussed, our results should be regarded as speculative rather than definitive: they represent results from what can be done using very large-scale, routinely collected administrative data. They need replication using different study designs to confirm or refute the findings.
Conclusion
This exploratory study into blood test results and symptom reporting prior to lung cancer diagnosis suggests that CRP levels may be an important prediagnostic marker of the disease. Prospective studies are, however, needed to determine the predictive value of CRP for lung cancer in conjunction with other symptoms.
Future perspective
Future studies should continue to explore the nature and extent of the association between CRP and lung cancer to better understand how this marker could be leveraged to facilitate earlier diagnosis. While CRP is unlikely to reflect a definitive test that could be used to prospectively discriminate those with and without lung cancer, it is possible that the marker could be effectively integrated into risk determination tools that improve the selection of patients who present to primary care and require further investigation. Potential pathways underlying the observed association as well as the extent to which the results generalize to different histological subtypes or indeed to other cancers should continue to be investigated.
Earlier diagnosis of lung cancer is a priority, and could be improved by facilitating the investigation and referral of high-risk symptomatic individuals who present to their general practitioner.
This was a nested case–control study using primary care data from the United Kingdom’s Clinical Practice Research Datalink; lung cancer patients (n = 26,379) were matched to up four controls with no record of cancer (n = 92,125) and differences in suspected cancer symptoms as well as blood test results were investigated in the 2-year period prior to diagnosis.
Results demonstrated that were differences in mean laboratory test values between lung cancer patients and controls from at least 6 months prior to cancer diagnosis for C-reactive protein (CRP), neutrophil, monocyte and platelet tests, and differences were also identified for symptom reporting during this period.
In multivariable models, elevated CRP was independently predictive of lung cancer at every 2-month interval up to 12 months before first diagnosis, but associations with other blood test results and subsequent lung cancer diagnosis were not consistently observed.
When observed in conjunction with at least one suspected cancer symptom, elevated CRP was associated with a greater than fourfold higher odds of lung cancer, after adjusting for confounding factors.
CRP test results are not currently included in UK guidelines for cancer referral in primary care, but the current findings suggest that high CRP, particularly when observed in conjunction with at least one cancer ‘alarm’ symptom may help primary care physicians to consider whether or not to refer a patient for further investigation.
This is an exploratory study that adds to the literature on CRP and lung cancer, prospective studies are needed to determine the predictive value of CRP for lung cancer in conjunction with other symptoms.
Author contributions
SV Ramagopalan is the guarantor of the study, conceived and designed it. A Harish led the analysis. All contributed to the analysis and interpretation of the data. SV Ramagopalan wrote the first draft and all contributed to subsequent drafts and the final paper.
Ethical conduct of research
This study was approved by the Independent Scientific Advisory Committee (ISAC) with CPRD number 19_155.
Supplemental table 1
Download MS Word (255.6 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2019-0442
Financial&competing interests disclosure
This study was funded by Bristol-Myers Squibb. L McDonald, R Carroll, N Tanna, F Mehmud and SV Ramagopalan are employees of Bristol-Myers Squibb. R Alikhan received financial support for this work from Bristol-Myers Squibb. A Harish is an employee of Mu Sigma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
CPRD data were provided under a license that does not permit sharing. Data are, however, obtainable directly from CPRD under their standard conditions.
Additional information
Funding
References
- Cancer Research UK . Lung cancer statistics (2016). www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer
- Walters S , MaringeC, ColemanMPet al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax68, 551–564 (2013).
- NHS England . Long term plan implementation. www.england.nhs.uk/cancer/strategy/
- Hamilton W . The CAPER studies: five case–control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br. J. Cancer101, S80–S86 (2009).
- Jones R , LatinovicR, CharltonJ, GullifordMC. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ334, 1040 (2007).
- Hippisley-Cox J , CouplandC. Identifying patients with suspected lung cancer in primary care: derivation and validation of an algorithm. Br. J. Gen. Pract.61, e715–e723 (2011).
- Allin KH , BojesenSE, NordestgaardBG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J. Clin. Oncol.27, 2217–2224 (2009).
- Shiels MS , PfeifferRM, HildesheimAet al. Circulating inflammation markers and prospective risk for lung cancer. J. Natl. Cancer Inst.105, 1871–1880 (2013).
- Herrett E , GallagherAM, BhaskaranKet al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int. J. Epidemiol.44, 827–836 (2015).
- NICE Guidance . Suspected cancer: recognition and referral. Recommendations organised by symptom and findings of primary care investigations. (2015). www.nice.org.uk/guidance/ng12/chapter/Recommendations-organised-by-symptom-and-findings-of-primary-care-investigations
- Khan NF , PereraR, HarperS, RosePW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam. Pract.11, 1 (2010).
- Bradley SH , KennedyMPT, NealRD. Recognising lung cancer in primary care. Adv. Ther.36, 19–30 (2019).
- Wagland R , BrindleL, JamesE, MooreM, EsquedaA, CornerJ. Facilitating early diagnosis of lung cancer amongst primary care patients: the views of GPs. Eur. J. Cancer Care.26, e12704 (2017).
- Shiels MS , KatkiHA, HildesheimAet al. Circulating inflammation markers, risk of lung cancer, and utility for risk stratification. J. Natl. Cancer Inst.107(10), pii: djv199 (2015).
- Muller DC , LaroseTL, HodgeAet al. Circulating high sensitivity C-reactive protein concentrations and risk of lung cancer: nested case-control study within Lung Cancer Cohort Consortium. BMJ364, k4981 (2019).
- Brown D , ZingoneA, YuYet al. Relationship between circulating inflammation proteins and lung cancer diagnosis in the national lung screening trial. Cancer Epidemiol. Biomarkers Prev.28, 110–118 (2019).
- Arhi CS , BottleA, BurnsEMet al. Comparison of cancer diagnosis recording between the Clinical Practice Research Datalink, Cancer Registry and Hospital Episodes Statistics. Cancer Epidemiol.57, 148–157 (2018).
