Abstract
Aim: Define changes in clinical management resulting from the use of the prognostic 31-gene expression profile (31-GEP) test for cutaneous melanoma in a surgical oncology practice. Patients & methods: Management plans for 112 consecutively tested patients with stage I–III melanoma were evaluated for duration and number of clinical visits, blood work and imaging. Results: 31-GEP high-risk (class 2; n = 46) patients received increased management compared with low-risk (class 1; n = 66) patients. Test results were most closely associated with follow-up and imaging. Of class 1 patients, 65% received surveillance intensity within guidelines for stage I–IIA patients; 98% of class 2 patients received surveillance intensity equal to stage IIB–IV patients. Conclusion: We suggest clinical follow-up and metastatic screening be adjusted according to 31-GEP test results.
Recent advances in genomic medicine have facilitated individualized oncology care based on an assessment of tumor biology and associated risk. Molecular tests to inform clinical-decision making have been developed in breast [Citation1–5], prostate [Citation6–12] and thyroid cancers [Citation13]. These tests can inform clinical decision-making by serving as objective tools to optimize management decisions in the context of traditional clinical and pathologic disease features.
An example of the practice-changing impact of molecular testing in oncology is the 15-gene expression profile (GEP) test for estimating metastatic risk in uveal melanoma [Citation14–19]. Given the limited accuracy of clinicopathologic staging and the relatively high rate of metastasis (up to 50% within 5 years) in uveal melanoma, most patients traditionally received intense surveillance and follow-up out of caution. The use of GEP, which uses 15 differentially expressed genes, identifies tumors with low, intermediate and high risk of metastasis, and has allowed physicians to focus frequent and more intensive surveillance on high-risk patients, while patients with a low likelihood of metastasis are spared intensive metastatic screening [Citation20,Citation21]. This clinical utility of GEP to inform risk-appropriate surveillance in uveal melanoma has been recently recognized by the National Comprehensive Cancer Network (NCCN) [Citation22].
Similarly, a 31-GEP test has been developed for predicting the risk of metastasis in cutaneous melanoma, a disease in which standard clinicopathologic staging can miss early-stage patients with aggressive disease [Citation23]. The 31-GEP uses the expression of 31 genes to assign a tumor to one of four prognostic classes with increasing 5-year metastatic risk: class 1A or 1B (class 1: low risk) and class 2A or 2B (class 2: high risk) [Citation23]. The test has been previously validated to predict metastatic risk in several retrospective and prospective studies totaling nearly 1400 patients [Citation23–27], and was therefore not a primary outcome measured in this study. Clinical use of the 31-GEP test has expanded during the last several years. Single- and multicenter studies have now demonstrated physicians use of the 31-GEP test result to adjust the frequency of office visits, frequency and intensity of surveillance imaging and adjuvant therapy recommendations in accordance with GEP-predicted risk [Citation28–30]. Physician-based surveys and expert consensus have also supported the use of the GEP test in managing melanoma patients [Citation31–33].
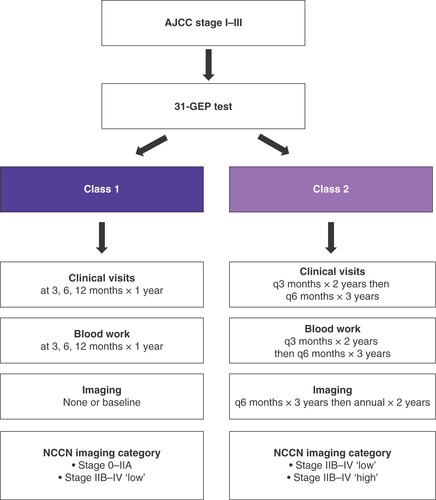
Our center began using the 31-GEP test for cutaneous melanoma in 2015. In this study, we examined planned management regimens for patients after receipt of their 31-GEP test results, including clinical follow-up visits, surveillance imaging and bloodwork. We evaluated these management plans in the context of NCCN guideline recommendations and compared the relative impact of the American Joint Committee on Cancer (AJCC) stage and GEP class on the prescribed management plans. AJCC staging (AJCC 7th edition) is broadly categorized as low risk (stage I–IIA: tumors less than 1.0 mm thick with or without ulceration or mitotic rate and no metastasis, or tumors less than 2.0 mm thick with no ulceration, mitotic rate or metastasis), and high risk (stage IIB–IV: tumors less than 2.0 mm thick with ulceration or mitotic rate, or tumors that have nodal disease or metastasis). NCCN recommends reduced blood work, clinical visits and imaging surveillance for low-risk patients compared with high-risk patients. Based on our experience with the 31-GEP test, we hypothesized that patients with a class 2 result received increased melanoma management compared with class 1 patients and propose an algorithm of melanoma patient care that incorporates the 31-GEP test results.
Patients & methods
Patient cohort & data collection
This single-center study protocol was reviewed and approved by the centralized Western Institutional Review Board. A total of 112 consecutive patients with cutaneous melanoma at Desert Surgical Oncology (CA, USA) between 2015–2017 were included in this study. All patients were prospectively tested with the 31-GEP test (DecisionDx®-Melanoma, Castle Biosciences, Inc., TX, USA) as part of their clinical assessment. The test evaluates the expression of 31 genes (28 prognostic and three control genes) within primary melanoma tumor tissue and applies a machine-learning algorithm to compare gene expression of a clinically tested tumor to a training set of 164 melanomas with known outcomes. The test classifies patients as low risk (class 1A or 1B) or high risk (2A or 2B) for metastasis within 5 years of diagnosis, as has been previously described [Citation23]. The 31-GEP test result, routine clinical data and recommended/planned follow-up, including bloodwork, surveillance imaging and clinical visit schedule, were collected for each patient. All clinical data were monitored for accuracy. The current study was approved by the centralized Western Institutional Review Board. The study protocol was granted a waiver of informed consent because this was a retrospective data review without direct impact on participant care or outcome, suggesting minimal potential risk to patients in this noninterventional study. This approach also avoided bias toward surviving patients.
Statistical analysis
Statistical analysis was performed in R version 3.5.0 (2018-04-23). Variables were compared using either Pearson's Chi-square tests, Wilcoxon F-tests, or generalized linear models for multivariate comparisons where appropriate and as indicated in the results, tables and figures. p-Values of less than 0.05 were considered significant. Bloodwork, imaging and clinical visits were examined in terms of both duration of surveillance (i.e., monitoring for 1 year vs 5 years) and number of events, (i.e., baseline imaging event versus eight distinct imaging events). Unless otherwise indicated, AJCC stage IA and GEP class 1 were selected as the baseline groupings for generalized linear model comparisons.
Results
Study cohort clinical & molecular characteristics
The clinical characteristics and 31-GEP results of the 112 patients included in this study are described in . Patients were AJCC 7th edition (the version in use at the time of diagnosis) stage I–III, with 22.3% (25/112) stage IA, 26.8% (30/112) stage IB, 19.6% (22/112) stage IIA, 19.6% (22/112) stage IIB, 3.6% (4/112) stage IIC and 7.1% (8/112) stage III. One patient could not be staged due to missing pathologic data (0.9%). All patients were recommended to have a sentinel lymph node biopsy (SLNB) and most patients (107/112; 96%) underwent the procedure. Eight (7.5%) patients had a positive result. Sixty-six (59%) patients were class 1 and 46 (41%) were class 2. Class 2 tumors had significantly increased presence of ulceration and were thicker than class 1 tumors (p < 0.001 for both variables), but did not differ in patient age, gender, or nodal status as assessed by SLNB.
Table 1. Clinical characteristics of study cohort.
31-GEP test result impacts duration & cumulative number of follow-up & surveillance events
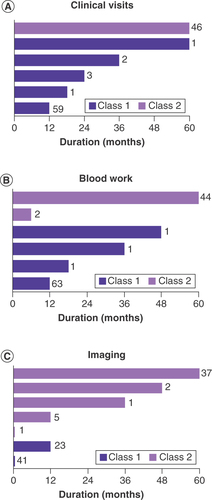
All recommended plans for follow-up and surveillance were made after receiving the 31-GEP test results and were made in conjunction with current clinicopathological features. To determine if class 1 and 2 results were associated with differential management plans, the recommended follow-up and surveillance regimens, including clinical visits, bloodwork and imaging, were evaluated and compared by GEP class and AJCC stage. We first evaluated total duration of clinical follow-up and surveillance. As shown in & Supplementary Table 1, recommended durations for clinical visit follow-up and surveillance with bloodwork and imaging were significantly longer for class 2 patients compared with class 1 patients (p < 0.001, by a generalized linear model that includes AJCC stage as a covariate for all management methods). This impact of GEP class on management was independent of AJCC stage (Supplementary Table 1 & Supplementary Figures 1–3). AJCC stage did not significantly impact surveillance imaging or clinical visits but was significantly associated with changes in recommended blood work comparing stage IA to IIC (Supplementary Table 1).
Duration of recommended routine clinical follow-up (A), blood work (B) and imaging (C) according to gene expression profile class. Two class 1 patients did not have a planned imaging protocol specified and are not depicted in C. In all graphs, dark purple bars indicate class 1 and light purple bars indicate class 2. The number of patients having each duration is indicated next to the bars.
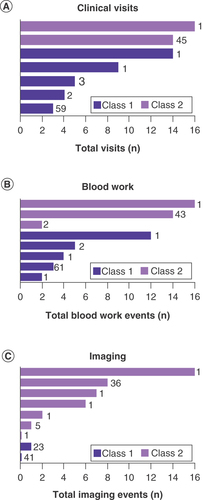
We also evaluated the association of GEP class with the total number of times patients would have clinical visits, bloodwork and imaging. Planned number of blood work, imaging and clinical visit events were significantly higher for patients with a class 2 result compared with patients with a class 1 result ( and Supplementary Table 2, p < 0.001 by generalized linear model including AJCC stage as a covariate for all management methods). The impact of GEP class was independent of AJCC stage (Supplementary Table 2 & Supplementary Figures 4–6). AJCC stage did not significantly impact treatment planning for imaging or clinical visits but was significantly associated with changes in treatment for blood work comparing stage IA to IIC (Supplementary Table 2).
Total number of recommended routine clinical visits (A), blood work events (B) and imaging events (C) according to gene expression profile class. Two class 1 patients did not have a planned imaging protocol specified and are not depicted in C. In all graphs, dark purple bars indicate class 1 and light purple bars indicate class 2. The number of patients having each cumulative total is indicated next to the bars.
31-GEP test result helps define surveillance imaging regimens in the context of NCCN guideline recommendations
The NCCN provides guidelines for recommended imaging protocols of patients with melanoma for the purpose of metastatic screening following definitive treatment with no evidence of remaining disease. These guidelines are assigned to AJCC staging groups. Asymptomatic AJCC stage 0–IIA patients are not recommended to having routine imaging; stage IIB–IV patients are recommended to undergo imaging every 3–12 months for 3–5 years. From these guidelines, we established three groups in which to evaluate this cohort: stage 0–IIA, no imaging; stage IIB—IV ‘low,’ imaging every 6–12 months for less than 3 years; and stage IIB–IV ‘high,’ imaging at intervals less than 6 months or for longer than 3 years. Thus, stage IIB-IV ‘low’ and ‘high’ represent less and more intense surveillance regimens, respectively, within the broader recommendation for this group of patients.
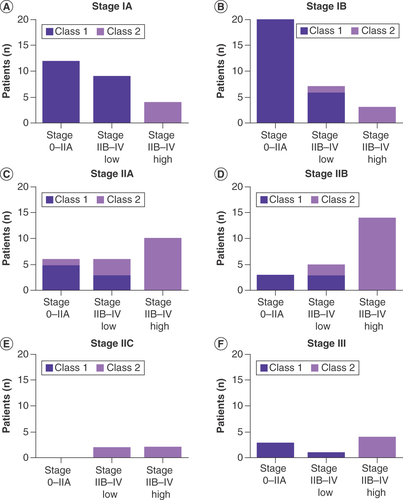
Using these three surveillance imaging bins, 31-GEP class was the only significant predictor of NCCN-recommended imaging protocol when considering 31-GEP class and AJCC stage together (p < 0.05 for 31-GEP and p > 0.05 for all AJCC stages, ). The majority (43/66, 65%) of class 1 patients had NCCN stage I–IIA imaging intensity, while 45/46 (98%) of class 2 patients had comparatively higher intensity imaging, within the NCCN stage IIB–IV category. Four stage IA–IIA patients were upstaged by a class 2 result to have imaging plans aligned with the NCCN stage IIB–IV ‘low’ category (), and 17 stage IA–IIA cases were upstaged to follow the more intense stage IIB–IV ‘high’ imaging schedule following a class 2 result (–C). Conversely, six-stage IIB or III patients had reduced intensity planned surveillance schedules (NCCN recommended for stage IA–IIA) following a class 1 result ( & F). In this cohort, the impact of 31-GEP class on NCCN imaging strategy was greater than that of AJCC stage, even though AJCC stage is the basis of the guideline cut-points.
Number of stage IA (A), IB (B), IIA (C), IIB (D), IIC (E), III (F) patients with recommended imaging schedules that fell into each NCCN-based category, according to gene expression profile class. NCCN stage I–IIA is no imaging recommended; NCCN stage IIB–IV ‘low’ is imaging recommended every 6–12 months and less than 3 years; and NCCN stage IIB–IV ‘high’ is imaging more frequently than every 6 months or for more than 5 years. American Joint Committee on Cancer stage was unknown for one class 1 patient; that patient is not depicted in this figure. In all graphs, dark purple bars indicate class 1 and light purple bars indicate class 2.
NCCN: National Comprehensive Cancer Network.
Proposed management workflow that incorporates 31-GEP testing
In evaluating the most common follow-up and surveillance regimens according to GEP class, the majority of class 1 patients had clinical visits and blood work at 3, 6 and 12 months (89 and 93%, respectively), which constitutes three of each event over the duration of 1 year ( & B). In contrast, most class 2 patients had clinical visits and blood work every 3 months for 2 years and then every 6 months for 3 years (96 and 94%, respectively), which would result in 14 of each event over the duration of 5 years. Imaging was not recommended for 62% of class 1 patients and 35% had only baseline imaging recommended. In contrast, only one (2%) class 2 patient did not have a recommended imaging plan and 10% had only baseline imaging recommended (). Instead, 78% of class 2 patients had imaging recommended every 6 months for 3 years and then annually for 2 years (eight events over the duration of 5 years). Based on these results, we propose a melanoma patient care algorithm that incorporates 31-GEP testing to adjust frequency and duration of clinical visits, blood work and surveillance imaging according to metastatic risk ().
Discussion
Genomic tests, primarily based on gene expression profiling, can enhance diagnosis, prognosis and prediction of treatment response, and complement traditional clinicopathologic methodologies to focus resources in a risk-appropriate manner. The current study was designed to determine the effect of 31-GEP risk assessment on low (stage I–IIA) and high-risk (stage IIB–III) patient management and to develop an algorithm of melanoma patient care that incorporates the 31-GEP test results. We showed that clinicians familiar with the prognostic accuracy of the 31-GEP test alter patient management in a risk-appropriate direction. Further, we propose that patients classified by the GEP test as low risk (class 1) receive clinical visits and blood work done at 3, 6 and 12 months for 1-year, and baseline or no imaging, and that high-risk patients (class 2) receive clinical visits and blood work done every 3 months for 2 years then every 6 months for 3 years, and imaging done every 3 months for 3-years, then annually for 2-years.
Historically in our practice, we did not perform routine surveillance imaging for early-stage melanoma patients, except for baseline scanning following a positive SLNB result. This was largely due to the lack of effective melanoma treatment options and to the relatively lengthy window of risk. However, given the existence of effective therapeutic options now available for resected and metastatic disease, our protocols for clinical follow-up, blood work and imaging have evolved to promote early detection of metastases.
The primary utility of the 31-GEP test is to add objective prognostic information, based on intrinsic tumor biology, which may be missed by traditional staging factors, to develop appropriate risk-tailored management strategies. With a median time to recurrence for patients with class 2 tumors at less than 2 years [Citation34], the most intensive surveillance can be focused during this period. Because all patients were recommended for an SLNB, this patient cohort is at a perceived higher risk for adverse events, based on clinicopathological features, than patients who do not receive SLNB recommendations. However, the low SLN positivity rate of 7.5% suggests improved prognostic measures can help to determine high and low-risk patients. In addition, studies have shown that class 1 patients who do have a future adverse event have high overall survival compared with class 2 patients, and therefore are likely not harmed by reduction in surveillance intensity [Citation24,Citation35]. Therefore, because these patients were determined to be high risk at diagnosis, management intensity for class 1, stage I–IIA patients would be expected to be higher than guideline recommendations for stage I–IIA patients at baseline. For some patients where there is more divergence between clinicopathologic features and the 31-GEP test result, a hybrid approach to patient management is used, combining traditional staging and molecular profiling, to develop an informed, risk-appropriate plan for the patient. Other reasons to deviate from a GEP-directed follow-up regimen include the monitoring of individuals at increased risk for developing new melanoma or other malignancy based on their global clinical assessment.
National guidelines provide general recommendations for metastatic screening, focusing on patients presenting with stage IIB–IV melanoma [Citation36]. However, the recommendations for stage IIB–IV are broad with imaging recommended anywhere from 3–12 months for 3–5 years. In this study, we compared our recommended surveillance imaging plans to NCCN-recommended plans. Out of 111 patients with known AJCC stage, 66 patients (59%) had planned imaging that aligned with their AJCC stage, while 45 (41%) had surveillance imaging that was adjusted to either a reduced or intensified NCCN-based schedule. Of these 45 patients, 27 (60%) had reduced or intensified plans that corresponded with class 1 or 2 results, respectively. Additionally, when evaluating the spectrum of frequency and duration recommended by the NCCN for stage IIB–IV patients, all the patients who had planned management at higher intensity or longer duration (imaging frequency >6 months; duration >3 years) were class 2, including 20 patients who had AJCC stage IIB–IV disease. This demonstrates the impact of the 31-GEP test result in applying NCCN recommendations, even within the same clinicopathologic risk group. It is important to note that some patients had imaging within an elevated NCCN-based category, which was not related to AJCC stage or 31-GEP test result. These patients were primarily stage I–IIA patients with a class 1 result who had a baseline scan, which would be considered in the stage IIB–IV ‘low’ category. No stage I–IIA class 1 patients were recommended to have more than one scan. Taken together, even though NCCN guidelines are currently based on AJCC stage, in our cohort, the 31-GEP test result was the only significant predictor of NCCN-based surveillance imaging category.
A limitation of this study is the absence of clinical outcomes data associated with the 31-GEP class and an analysis of the completed follow-up and surveillance regimens. Evaluation of these end points was limited by the relatively short follow-up of this cohort. However, the clinical accuracy of the 31-GEP test to predict patient outcomes has been extensively reported, including two recent systematic reviews and meta-analyses [Citation23,Citation24,Citation34,Citation37,Citation38], including in prospective cohorts [Citation26,Citation27]. Longer follow-up of our cohort will permit an evaluation of the combination of the test result and completed management plans to identify recurrence. However, the results presented in this study are consistent with other investigations in which the 31-GEP test substantially influenced patient management recommendations for class 1 and 2 patients, as a function of their disparate biological risk [Citation28–30]. A second limitation is that the cohort includes a higher-risk group in which all patients had an SLNB performed. Thus, the majority of management changes reflect upstaging of patients with stage I–IIA tumors and a class 2 result to more intensive surveillance. Therefore, extrapolation of results to impact on healthcare costs would likely be overestimated compared with a more diverse melanoma population as was reported by Vetto et al. [Citation39] who showed that class 1A patients over the age of 65 years old had <5% risk of SLN positivity and could therefore forego SLNB and may offset costs incurred by increased surveillance in high risk patients.
Diagnostic, prognostic and predictive tools for assessment of cutaneous melanoma have provided the opportunity to precisely guide patient management on an individualized basis. Improved diagnosis with non-invasive techniques such as optical coherence tomography suggest that the incidence of melanoma will continue to increase, raising the need for effective and accurate prognostic tools. The best validated molecular test for melanoma is the 31-GEP, which has demonstrated accuracy of prognosis in prospective and retrospective studies and in two meta-analyses. Clinical impact of the 31-GEP was the focus of the current study, which showed that results of the test lead to changes in patient management that are primarily associated with surveillance procedures, and that are within recommendations of national guidelines for low- and high-risk cutaneous melanoma patients.
Conclusion
Based on this study, the 31-GEP impacted management decisions in a surgical oncology practice resulting in recommendations for class 1 patients that include shorter durations and frequencies of clinical visits, blood work and imaging surveillance compared with class 2 patients.
A prognostic 31-gene expression profile (GEP) test predicts metastatic risk in cutaneous melanoma, a disease in which standard clinicopathologic staging can miss aggressive disease in early-stage patients.
Physician-based surveys and expert consensus have supported the use of GEP testing in managing melanoma patients; the test has been validated in retrospective and prospective studies totaling over 3000 patients.
Patients classified as high risk (class 2) by the 31-GEP test had longer duration and more frequent follow-up compared with low-risk (class 1) patients.
31-GEP testing is an objective prognostic tool to complement current guidelines for melanoma patient care.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval from the centralized Western Institutional Review board. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Data sharing statement
The authors certify that this manuscript reports original data. No data will be made available publicly.
Supplementary Figure 1
Download JPEG Image (162.2 KB)Supplementary Figure 2
Download JPEG Image (157.8 KB)Supplementary Figure 3
Download JPEG Image (156.4 KB)Supplementary Figure 4
Download JPEG Image (149.5 KB)Supplementary Figure 5
Download JPEG Image (164.6 KB)Supplementary Figure 6
Download JPEG Image (154.9 KB)Supplementary Table 1
Download MS Word (14.9 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2020-0827
Financial & competing interests disclosure
Support for this study (data collection) was provided by Castle Biosciences, Inc. DM Hyams is a member of the speakers’ bureau of Castle Biosciences, Inc. KR Covington, CE Johnson, KM Plasseraud and RW Cook are employees and options holders at Castle Biosciences, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by Brian Martin, Ph.D. (employee and options holder at Castle Biosciences, Inc.) and was funded by Castle Biosciences, Inc.
Additional information
Funding
References
- Paik S , ShakS, TangGet al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. New Eng. J. Med.351(27), 2817–26 (2004).
- Filipits M , RudasM, JakeszRet al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin. Cancer Res.17(18), 6012–20 (2011).
- Hyams DM , SchuurE, AngelAristizabal Jet al. Selecting postoperative adjuvant systemic therapy for early stage breast cancer: a critical assessment of commercially available gene expression assays. J. Surg. Oncol.115(6), 647–662 (2017).
- Wallden B , StorhoffJ, NielsenTet al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med. Genomics8(1), 54 (2015).
- van de Vijver MJ , HeYD, van'tVeer LJet al. A gene-expression signature as a predictor of survival in breast cancer. New Eng. J. Med.347(25), 1999–2009 (2002).
- Cooperberg MR , SimkoJP, CowanJEet al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J. Clin. Oncol.31(11), 1428–1434 (2013).
- Cuzick J , SwansonGP, FisherGet al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol.12(3), 245–255 (2011).
- Cullen J , RosnerIL, BrandTCet al. a biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer. Eur. Urol.68(1), 123–131 (2015).
- Klein EA , HaddadZ, YousefiKet al. Decipher Genomic classifier measured on prostate biopsy predicts metastasis risk. Urology90, 148–152 (2016).
- Karnes RJ , BergstralhEJ, DavicioniEet al. Validation of a genomic classifier that predicts metastasis following radical prostatecomy in an at risk patient population. J. Urol.190(6), 2047–2053 (2013).
- Erho N , CrisanA, VergaraIAet al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE8(6), e66855 (2013).
- Alford AV , BritoJM, YadavKK, YadavSS, TewariAK, RenzulliJ. The use of biomarkers in prostate cancer screening and treatment. Rev. Urol.19(4), 221–234 (2017).
- Chudova D , WildeJI, WangETet al. Molecular classification of thyroid nodules using high-dimensionality genomic data. J. Clin. Endocrinol. Metab.95(12), 5296–5304 (2010).
- Onken MD , WorleyLA, CharDHet al. Collaborative ocular oncology group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology119(8), 1596–1603 (2012).
- Onken MD , WorleyLA, TuscanMD, HarbourJW. An accurate clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J. Mol. Diagn.12(4), 461–468 (2010).
- Chappell MC , CharDH, ColeTBet al. Uveal melanoma: molecular pattern, clinical features, and radiation response. Am. J. Ophthalmol.154(2), 227–232.e2 (2012).
- Correa ZM , AugsburgerJJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications. Graefes Arch. Clin. Exp. Ophthalmol.252(1), 131–135 (2014).
- Corrêa ZM , AugsburgerJJ. Independent prognostic significance of gene expression profile class and largest basal diameter of posterior uveal melanomas. Am. J. Ophthalmol.162, 20–27.e1 (2016).
- Walter SD , ChaoDL, FeuerW, SchiffmanJ, CharDH, HarbourJW. Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmol.134(7), 734–740 (2016).
- Aaberg TM , CookRW, OelschlagerK, MaetzoldD, RaoPK, MasonJO. Current clinical practice: differential management of uveal melanoma in the era of molecular tumor analyses. Clin. Ophthalmol.8, 2449–60 (2014).
- Plasseraud KM , CookRW, TsaiTet al. Clinical performance and management outcomes with the decisiondx-um gene expression profile test in a prospective multicenter study. J. Oncol. 2020, 5325762 (2016).
- Coit D , ThompsonJA, AlbertiniMet al. Uveal melanoma NCCN Guidelines National Comprehensive Cancer Network, v1.2019. (2019).
- Gerami P , CookRW, WilkinsonJet al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin. Cancer Res.21(1), 175–183 (2015).
- Gastman BR , GeramiP, KurleySJ, CookRW, LeachmanS, VettoJT. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J. Am. Acad. Dermatol.80(1), 149–157.e4 (2019).
- Gerami P , CookRW, RussellMCet al. Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. J. Am. Acad. Dermatol.72(5), 780–785.e3 (2015).
- Greenhaw BN . Estimation of prognosis in invasive melanoma using a gene expression profile test. Dermatol. Surg.44(12), 1494- 1500 (2016).
- Hsueh EC , DeBloomJR, LeeJet al. Interim analysis of survival in a prospective, multi-center registry cohort of cutaneous melanoma tested with a prognostic 31-gene expression profile test. J. Hematol. Oncol.10(1), 152 (2017).
- Berger AC , DavidsonRS, PoitrasJKet al. Clinical impact of a 31-gene expression profile test for cutaneous melanoma in 156 prospectively and consecutively tested patients. Curr. Med. Res. Opin.32(9), 1599–1604 (2016).
- Dillon LD , GadziaJE, DavidsonRSet al. Prospective, multicenter clinical impact evaluation of a 31-gene expression profile test for management of melanoma patients. SKIN J. Cutaneous Med.2(2), 111–121 (2018).
- Schuitevoerder D , HeathM, CookRWet al. Impact of gene expression profiling on decision-making in clinically node negative melanoma patients after surgical staging. J. Drugs Dermatol.17(2), 196–199 (2018).
- Svoboda RM , GlazerAM, FarbergAS, RigelDS. Factors affecting dermatologists’ use of a 31-gene expression profiling test as an adjunct for predicting metastatic risk in cutaneous melanoma. J. Drugs Dermatol.17(5), 4 (2018).
- Farberg AS , GlazerAM, WinkelmannRR, RigelDS. Assessing genetic expression profiles in melanoma prognosis. Dermatol. Clin.35(4), 545–550 (2017).
- Winkelmann RR , FarbergAS, GlazerAMet al. Integrating skin cancer-related technologies into clinical practice. Dermatol. Clin.35(4), 565–576 (2017).
- Greenhaw BN , CovingtonKR, KurleySJet al. Molecular risk prediction in cutaneous melanoma: a meta-analysis of the 31-gene expression profile prognostic test in 1,479 patients. J. Am. Acad. f. Dermatol.83(3), 745 –753 (2020).
- Keller J , SchwartzTL, LizalekJMet al. Prospective validation of the prognostic 31‐gene expression profiling test in primary cutaneous melanoma. Cancer Med.8(5), 2205–2212 (2019).
- Coit DG , ThompsonJA, AlbertiniMRet al. Cutaneous melanoma, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw.17(4), 367–402 (2019).
- Zager JS . Performance of a 31-gene expression profile in a previously unreported cohort of 334 cutaneous melanoma patients. J. Clin. Oncol.34(Suppl. 15), 9581 (2016).
- Litchman GH , PradoG, TeplitzRW, RigelD. A systematic review and meta-analysis of gene expression profiling for primary cutaneous melanoma prognosis. SKIN J. Cutaneous Med.4(3), 221–237 (2020).
- Vetto JT , MonzonFA, CookRW, JohnsonC, CovingtonKR, LeachmanS. Clinical utility of a 31-gene expression profile test to determine eligibility for sentinel lymph node biopsy in melanoma patients >65 years of age. 37(Suppl. 15), 6630–6630 (2018).