Tweetable abstract
Tumor-associated macrophages might promote the distant metastasis of tumor cells by semi-phagocytosis. The authors propose that this newly discovered process occurs in tumor-associated macrophages and may lead to a novel approach for blocking cancer metastasis.
“Macrophage-mediated phagocytosis is defined as the uptake of macromolecules and larger particles into the intracellular space by membrane protrusions”
Immunotherapies targeting tumor-associated macrophages (TAMs) have been used for many years, especially in highly metastatic cancers, and a greater understanding of the molecular mechanisms involved in antitumor immunotherapies has recently led to increased efficacy of currently available therapies [Citation1]. TAMs, which are important infiltrating immune cells found in various tumors, have been demonstrated to promote tumor metastasis by affecting the primary site and distant metastatic niche [Citation1,Citation2]. Previous studies have shown that TAMs, along with cancer-associated endothelium and cancer cells, could assemble into a specific microanatomical structure known as ‘tumor microenvironment of metastasis’ to facilitate the escape of breast cancer cells for metastasis [Citation3–5]. Furthermore, in hepatocellular carcinoma, TAMs could transfer αMβ2 integrin to cancer cells to facilitate metastasis by activating the matrix metalloproteinase 9 pathway and causing degradation of the extracellular matrix [Citation6]. Subsequently, TAMs located close to blood vessels would cause increased tumor cell infiltration of the perivascular area as part of the premetastatic process [Citation6]. These pieces of evidence have suggested that TAMs in the primary site ‘indirectly’ exhibit a promotive role in tumor metastases, especially in chemotherapy-induced metastases, and any therapeutic antitumor approaches should consider suppressing or eliminating this effect. For distant metastases, TAMs would secrete several cytokines, chemokines and proteins, including TNF-α, TGF-β and VEGF, and these bioactive molecules could be loaded into extracellular vehicles to exert key functions in forming premetastatic niches [Citation2]. Mechanistically, a distinct population of macrophages in metastatic sites, such as F4/80+/CD11c+ macrophages, would be domesticated as metastasis-associated macrophages by tumor-derived stimulating factors, and these specific macrophages could secrete chemokines to alter the microenvironment of the premetastatic niche and promote colonization of cancer cells [Citation2,Citation7]. In summary, this evidence has shown that TAMs indirectly promote cancer metastasis by acting as a ‘guide’ for tumor cells; however, whether TAMs combine with tumor cells directly to promote distant metastasis remains largely unknown.
Currently, accumulating evidence has supported the concept that TAMs located upstream of T-cell responses can combine with T cells directly through PD-1 and PD-L1 to induce T-cell exhaustion and dysfunction as well as facilitate the escape of circulating tumor cells from immune surveillance, subsequently leading to distant metastasis of breast and lung cancers, among others [Citation8]. Therefore, some molecular inhibitors targeting TAMs have been designed to enhance their clinical therapeutic efficacy in breast and liver cancers by additionally restoring T-cell functions, and their clinical prognosis is encouraging [Citation3]. Intriguingly, in a mouse model of oral cancer, it was found that the ablation of macrophages inhibits tumor growth independent of T cells [Citation9]. In addition, no obvious effect on lung metastasis was observed, indicating that TAMs promote cancer metastasis via T-cell-mediated immune responses that are tumor- or cell-type specific. Hence, whether distant metastasis would be eliminated by targeting macrophages remains to be properly investigated, and the potential mechanism needs to be elucidated to develop individuated treatment programs in the future. Furthermore, in ligand–receptor binding, CSF1R expressed on TAMs can recognize and combine with CSF1 in tumor cells. Recently, Yin et al. reported that NHWD-870, a targeted inhibitor of bromodomain and extraterminal domain family proteins, inhibits the expression of CSF1 and blocks the interaction between macrophages and cancer cells [Citation10], indicating that targeting the complexes of TAMs and cancer cells by blocking ligand–receptor binding has substantial therapeutic applications in clinical settings. Alternatively, CD47, which acts as a ‘don't-eat-me’ signal and inhibits macrophage phagocytosis for immune evasion in most tumors, has been viewed as a novel potent immunotherapy target [Citation11]; however, whether promoting macrophage phagocytosis by targeting CD47 would enhance the long-term clinical benefit in patients, especially in highly metastatic cancers, remains unknown.
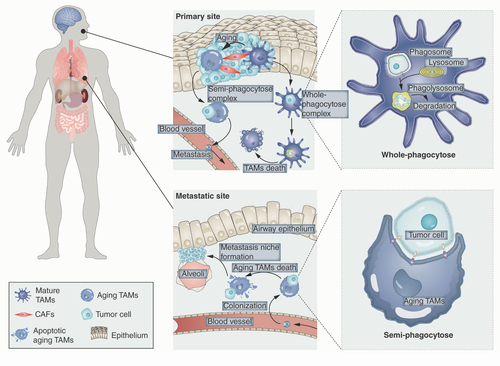
Macrophage-mediated phagocytosis is defined as the uptake of macromolecules and larger particles into the intracellular space by membrane protrusions; these absorbed particles are then enzymatically degraded by the endosomal–lysosomal system [Citation12]. Tumor-derived exosomes containing various bioactive molecules, including nucleic acids (DNA, mRNA and noncoding RNA), proteins, lipids and cytokines, can be phagocytized and delivered by TAMs to distant organs to establish premetastatic niches for tumor metastasis [Citation13]. However, the authors have recently concluded that macrophage-derived exosomes are heterogeneous in different cancers and play paradoxical roles in tumor progression and metastasis mainly through post-transcriptional control and protein phosphorylation within cancer cells [Citation14]. Moreover, Sharma et al. have reported that in the tumor microenvironment, TAMs might engulf live microbiota in specific tumor tissues and then extrude them within tumor niches to suppress tumor progression by regulating the depolarization of M2 macrophages (pro-tumor) into M1 macrophages (antitumor) [Citation15]. However, interestingly, in a mouse model of breast cancer, Su et al. have provided evidence that TAMs can engulf the tumor cell completely, allowing the tumor cell to escape immunosurveillance for distant metastasis, and that the effect of antibody-dependent cellular phagocytosis-induced immunosuppression caused by TAMs could be blocked by an anti-HER2 antibody [Citation16]. These pieces of evidence indicate that TAM-mediated phagocytosis exhibits tumor heterogeneity specifically orchestrated by the crosstalk between TAMs and the microbiota or cancer cells. In fact, most, if not all, of the engulfed cell fragments and/or cells would be degraded by membrane-bound vesicles of the endosomal–lysosomal system rather than engaged in protecting tumor cells [Citation12], indicating that other types of TAM-mediated phagocytosis of tumor cells are involved in metastasis. To avoid degradation by lysosomes, the authors propose a new concept, ‘semi-phagocytosis,’ which is mediated by macrophages in the process of cancer metastasis. In this regard, hypothesizing that TAMs at the primary tumor site phagocytize tumor cells or tumor stem cells via semi-phagocytosis to evade elimination by the immune system and avoid degradation by the endosomal–lysosomal system, followed by specific TAMs anchoring target organs or tissues by ligand–receptor binding and continuing apoptosis in a distant metastatic site, which maintains the potential tumorigenicity of cancer cells and triggers distant metastasis, is highly conceivable.
The authors believe that the semi-phagocytosis of tumor cells by TAMs is a special phase occurring during the process of engulfment, starting from the cytoplasm, which is partly phagocytized by TAMs, to when tumor cells are nearly engulfed by phagosomes. This process does not extend to the endosomal–lysosomal system because of the stagnation of phagosome maturation and/or effects of the special characteristics of tumor cells. In the presence of TAMs, since macrophage-mediated phagocytosis consists of recognition, budding of the plasma membrane, envelopment, internalization and involution of membrane budding [Citation17], semi-phagocytosis would occur in a certain stage of the entire process. This occurs especially during internalization when the cytoplasm of tumor cells is not completely engulfed. Moreover, the biological behaviors of macrophages would change from maturation to aging in the cellular life cycle [Citation18], and aging macrophages can cause changes in various biological behaviors, including the decline of numbers, antigen presentation and phagocytosis, which can promote carcinogenesis and development of metastatic carcinoma [Citation19]. Thus, the authors suspect that semi-phagocytosis occurs in aging macrophages with a decreased phagocytosis ability that does not allow them to completely engulf a cell or particle. Moreover, since cellular processes, including phagocytosis, require substantial energy [Citation18] and aging macrophages exhibit attenuated mitochondrial function, semi-phagocytosis might be triggered by an insufficient energy supply, especially in aging TAMs. The tumor cell volume would be too large to be completely engulfed by the phagosome, with subsequent transfer to the endosomal–lysosomal system; however, in that case, TAMs would only partially phagocytize tumor cells. In addition, the uptake of unsaturated fatty acids from the microenvironment into the macrophage membrane could enhance phagocytic activity by modulating membrane fluidity. Furthermore, the decrease in the unsaturated-to-saturated fatty acid ratio of lipid rafts (membrane microdomains enriched in signal transduction proteins) in macrophages can block receptor associations and prevent downstream signaling cascades to attenuate phagocytic activity [Citation17]. Thus, changes in the unsaturated-to-saturated fatty acid ratio in the macrophage membrane might be involved in semi-phagocytosis in TAMs.
Because of their effects on metastasis-associated macrophages, chemokines are known to play a promoting role in cancer metastasis, and phagocytosis of tumor cells by macrophages has been demonstrated as well [Citation16,Citation20]; however, the role of TAM semi-phagocytosis in metastasis is not as well defined. Although TAMs in metastatic cancers have been widely studied, the authors hypothesize that aging TAMs in the primary site phagocytize tumor cells via semi-phagocytosis to form the semi-phagocytose complex to evade immune elimination and enter the blood circulation to promote distant metastasis. When colonizing the metastatic site, tumor cells will be released to form the metastasis niche after the apoptosis of aging TAMs (). To support this proposed hypothesis, the authors suggest a clinical blood-based study to isolate the semi-phagocytose complex of TAMs and cancer cells as well as a single-cell study to detect its tumorigenesis in vivo. Herein the authors propose that semi-phagocytosis in TAMs represents a novel therapeutic avenue for blocking metastasis in cancers.
Aging TAMs in the primary site (e.g. head and neck etc.) semi-phagocytize tumor cells and transfer into blood circulation, then colonize in the metastasis site (e.g. lung etc.), in which the released cancer cells would trigger carcinogenesis in the distant organ/tissue. Mature TAMs could engulf tumor cells completely by whole-phagocytosis to form the phagosome and the absorbed tumor cells will be enzymatically degraded. However, aging TAMs might not engulf the whole cancer cells by semi-phagocytosis and would carry them for distant metastasis.
CAF: Cancer-associated fibroblast; TAM: Tumor-associated macrophage.
Author contributions
H Zhou and F Wu contributed to the study conception and design. T Ye and J Liu were co-first authors and wrote the manuscript. Related literature was searched by W Zhao, S Gao and S Wang. All authors read and approved the final manuscript.
Financial & competing interests disclosure
This work was funded by the Key Research and Development Program of Sichuan Province (no. 2019YFS0361), the National Natural Science Foundation of China (no. 82002884, 81772898 and 82071124) and the Science and Technology Program of Chengdu City (no. 2019YF0501151SN). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The manuscript was edited for English language, grammar, punctuation and spelling by Enago, the editing brand of Crimson Interactive (Beijing) Consulting Co. Ltd., under Advance Editing B2C. This assistance was supported by funding received by the authors as detailed in the financial and competing interests disclosure.
Additional information
Funding
References
- Lin Y , XuJ, LanH. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol.12(1), 76 (2019).
- Genard G , LucasS, MichielsC. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front. Immunol.8, 828 (2017).
- Karagiannis GS , CondeelisJS, OktayMH. Chemotherapy-induced metastasis: molecular mechanisms, clinical manifestations, therapeutic interventions. Cancer Res.79(18), 4567–4576 (2019).
- Coste A , KaragiannisGS, WangYet al. Hematogenous dissemination of breast cancer cells from lymph nodes is mediated by tumor microenvironment of metastasis doorways. Front. Oncol.10, 57110 (2020).
- Ginter PS , KaragiannisGS, EntenbergDet al. Tumor microenvironment of metastasis (TMEM) doorways are restricted to the blood vessel endothelium in both primary breast cancers and their lymph node metastases. Cancers (Basel)11(10), 1507 (2019).
- Wu J , GaoW, TangQet al. M2 macrophage-derived exosomes facilitate hepatocarcinoma metastasis by transferring αMβ2 integrin to tumor cells. Hepatologydoi:10.1002/hep.31432 (2020) ( Epub ahead of print).
- Zhao S , MiY, GuanBet al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol.13(1), 156 (2020).
- Flerin NC , PiniotiS, MengaA, CastegnaA, MazzoneM. Impact of immunometabolism on cancer metastasis: a focus on T cells and macrophages. Cold Spring Harb. Perspect. Med.10(9), a037044 (2020).
- Wu FL , NolanK, StraitAAet al. Macrophages promote growth of squamous cancer independent of T cells. J. Dent. Res.98(8), 896–903 (2019).
- Yin M , GuoY, HuRet al. Potent BRD4 inhibitor suppresses cancer cell-macrophage interaction. Nat. Commun.11(1), 1833 (2020).
- Tong B , WangM. CD47 is a novel potent immunotherapy target in human malignancies: current studies and future promises. Future Oncol.14(21), 2179–2188 (2019).
- Hartenstein V , MartinezP. Phagocytosis in cellular defense and nutrition: a food-centered approach to the evolution of macrophages. Cell Tissue Res.377(3), 527–547 (2019).
- Plebanek MP , AngeloniNL, VinokourEet al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat. Commun.8(1), 1319 (2017).
- Liu J , WuF, ZhouH. Macrophage-derived exosomes in cancers: biogenesis, functions and therapeutic applications. Immunol. Lett.227, 102–108 (2020).
- Sharma NK , SarodeSC, SarodeGS, PatilS. Vomocytosis by macrophages: a crucial event in the local niche of tumors. Future Oncol.15(14), 1545–1550 (2019).
- Su S , ZhaoJ, XingYet al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell175(2), 442–457 (2018).
- Schumann J . It is all about fluidity: fatty acids and macrophage phagocytosis. Eur. J. Pharmacol.785, 18–23 (2016).
- Stahl EC , HaschakMJ, PopovicB, BrownBW. Macrophages in the aging liver and age-related liver disease. Front. Immunol.9, 2795 (2018).
- Lian J , YueY, YuW, ZhangY. Immunosenescence: a key player in cancer development. J. Hematol. Oncol.13(1), 151 (2020).
- Kitamura T , QianBZ, SoongDet al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med.212(7), 1043–1059 (2015).
