Abstract
Aim: To assess concordance between HER2 status measured by traditional methods and ERBB2 amplification measured by next-generation sequencing and its association with first-line trastuzumab clinical benefit in patients with advanced esophagogastric cancer. Methods: Retrospective analysis of HER2/ERBB2 concordance using a deidentified USA-based clinicogenomic database. Clinical outcomes were assessed for patients with HER2+ advanced esophagogastric cancer who received first-line trastuzumab. Results: Overall HER2/ERBB2 concordance was 87.5%. Among patients who received first-line trastuzumab, concordant HER2/ERBB2 was associated with longer time to treatment discontinuation (adjusted hazard ratio [aHR]: 0.63; 95% CI: 0.43–0.90) and overall survival (aHR: 0.51; 95% CI: 0.33–0.79). ERBB2 copy number ≥25 (median) was associated with longer time to treatment discontinuation (aHR: 0.56; 95% CI: 0.35–0.88) and overall survival (aHR: 0.52; 95% CI: 0.30–0.91). Conclusion: HER2/ERBB2 concordance and higher ERBB2 copy number predicted clinical benefit from trastuzumab.
Lay abstract
Trastuzumab is a drug that has been shown to prolong survival in some patients with advanced esophagogastric cancer whose tumor expresses a protein biomarker called HER2. There are different methods for assessing whether a patient’s tumor expresses HER2, including but not limited to traditional methods such as immunohistochemistry and in situ hybridization and novel methods such as next-generation sequencing, which detects alterations in the gene (ERBB2) that encodes the HER2 protein. In our study, we assessed concordance between HER2 status (HER2-positive or HER2-negative) measured by traditional methods and ERBB2 amplification measured by next-generation sequencing, to determine whether there was an association between concordance and clinical benefit in patients with advanced esophagogastric cancer treated with trastuzumab. Our results suggest that, when HER2 positivity is detected through traditional methods, both ERBB2 concordance (i.e., agreement that a patient’s tumor had the biomarker) and a higher ERBB2 copy number (the amount of the ERBB2 gene expressed by the tumor) were associated with longer time to treatment discontinuation and overall survival in patients with advanced esophagogastric cancer treated with first-line trastuzumab.
Globally, esophagogastric cancers (EGCs) account for almost 9% of all newly diagnosed cancer cases and are the third (gastric) and sixth (esophageal) leading causes of cancer-related deaths [Citation1]. More than half of patients with EGC have evidence of spread to regional or distant sites at initial diagnosis [Citation2–4]. For patients with advanced disease, therapeutic options are limited and 5-year survival is less than 6% in the USA [Citation5–7].
The growth factor HER2 is encoded by the ERBB2 proto-oncogene [Citation8,Citation9]. ERBB2 amplification results in HER2 protein overexpression in 15–30% of EGC, leading to uncontrolled activation of downstream signaling pathways that may drive subsequent carcinogenesis [Citation5,Citation8–14]. For patients with HER2+ advanced gastric or gastroesophageal junction (EGJ) cancer, first-line treatment with the anti-HER2 therapy trastuzumab in combination with chemotherapy was shown to improve survival in the Trastuzumab for Gastric Cancer (ToGA) study [Citation12,Citation15].
Both National Comprehensive Cancer Network (NCCN) and joint College of American Pathologists, American Society for Clinical Pathology and American Society for Clinical Oncology (CAP-ASCP-ASCO) guidelines recommend testing for HER2 overexpression in patients with advanced EGC (advEGC) who are candidates for trastuzumab [Citation5,Citation16,Citation17]. The CAP-ASCP-ASCO guidelines recommend initial HER2 protein expression evaluation on tumor tissue by immunohistochemistry (IHC), followed by in situ hybridization (ISH) or FISH for cases with equivocal (2+) IHC staining. The guidelines do not recommend for or against genomic testing for ERBB2 amplification, citing insufficient evidence [Citation5].
Current NCCN guidelines recommend ascertaining HER2, PD-L1, microsatellite instability (or mismatch repair) and NTRK gene fusion status in patients with advEGC to identify candidates for approved targeted therapies [Citation16,Citation17]. For patients with limited tumor tissue available for testing, NCCN suggests use of comprehensive genomic profiling (CGP) via next-generation sequencing (NGS), with the caveat that traditional test methods are preferred [Citation16,Citation17]. NGS can also identify molecular alterations that may co-occur with ERBB2 amplifications and have been associated with lack of benefit from trastuzumab [Citation9,Citation18–21]. In addition, prior studies have demonstrated an association between higher ERBB2 copy number (CN) and increased clinical benefit from trastuzumab, and NGS can also provide this important information [Citation18,Citation22,Citation23].
Although limited information exists regarding the reliability of evaluating HER2 status in EGC via NGS compared with traditional IHC and ISH methods, overall concordance between these methods appears to be high [Citation8,Citation18]. Additional data from patients with advEGC managed in the community setting are needed to assess the utility of NGS to measure HER2 status and to predict clinical benefit from anti-HER2 therapy. To address this evidence gap, we sought to evaluate the association of concordance between HER2 status (assessed with IHC ± ISH) and ERBB2 amplification (assessed with CGP) with clinical benefit from first-line trastuzumab for patients with advEGC treated in routine oncology practice. We also examined the frequency of co-occurring genomic alterations associated with lack of trastuzumab benefit and explored associations between quantitative ERBB2 CN and clinical benefit for patients treated with trastuzumab.
Materials & methods
Data source
This study used the nationwide (USA-based) deidentified Flatiron Health-–Foundation Medicine (FMI) clinicogenomic database (CGDB) of patients with EGC. The deidentified data originated from approximately 280 US cancer clinics (∼800 sites of care) with data recency through 31 December 2019. Genomic alterations were identified via CGP of >300 cancer-related genes on FMI’s NGS-based FoundationOne® panel [Citation24]; gene panel coverage data can be found in Supplementary Table 1A–C. Retrospective, longitudinal, patient-level structured and unstructured clinical data were obtained via technology-enabled abstraction of electronic health records (EHR) and linked to genomic data derived from FMI CGP tests by deidentified, deterministic matching [Citation25,Citation26]. All data were processed and harmonized centrally and stored in a secure manner compliant with the Health Insurance Portability and Accountability Act, as previously described [Citation27]. Institutional Review Board approval was obtained prior to study conduct and included a waiver of informed consent.
Study population
The study population included patients diagnosed with advanced gastric, esophageal or gastroesophageal junction cancer (advEGC) between 1 January 2011 and 30 September 2019 who received CGP testing on a solid tissue specimen collected any time from 30 days before to 14 days after first-line systemic therapy initiation. advEGC was defined as stage IV disease at diagnosis, unresectable disease, or locoregional or distant recurrence following surgery. Patients were required to have a clinically documented negative or positive HER2 test result (IHC, ISH, or method not otherwise specified [NOS] in the EHR data). Detailed selection criteria are provided in Supplementary Table 2 & Supplementary Figure 1.
Clinical data, genomics, & outcomes
Clinical HER2 status was based on abstracted test results for IHC, ISH or method NOS, documented up to 30 days before EGC diagnosis date and no more than 14 days after first-line therapy start date. Definitions were as follows. HER2+: any IHC 3+ or ISH positive or HER2-positive test result with method NOS; HER2−: IHC 0–1+ only, ISH negative or HER2-negative test result with method NOS. Patients with only IHC 2+ results and no documented confirmatory ISH testing were excluded because a HER2 status could not be assigned. If a patient had the same result on more than one test type, the source of the patient’s HER2 status was assigned in the following order: FISH/ISH, IHC, NOS. ERBB2 amplification was measured by CGP up to 30 days before to any time on or after EGC diagnosis date. For this analysis, ERBB2 amplification positivity (Amp+) was defined as gene CN ≥+3 of the tumors’ base ploidy (e.g., CN = 6 in triploid, CN = 7 in tetraploid) and gene CN of ≥6, otherwise the result was considered ERBB2 Amp− [Citation24]. CN was defined as the highest estimated CN of the ERBB2 gene in the tumor portion of the tested sample.
Dates of death were based on a composite mortality variable comprising structured and unstructured EHR data linked to commercial mortality data and the Social Security Administration Death Master File. These data have been successfully validated via comparison to the US National Death Index database [Citation28].
Clinical benefit from trastuzumab was assessed by time to treatment discontinuation (TTD) and overall survival (OS). TTD was defined as time from initial to last first-line trastuzumab administration (regardless of whether other therapies given in combination with trastuzumab were substituted or discontinued) within a defined line of therapy, or death [Citation29]. If the last trastuzumab administration was within 3 weeks of the patient’s last structured activity date (e.g., non-cancelled medication orders, medication administrations or clinic visits with vital signs measured), or the data cut-off date (31 December 2019), and the patient had not died in that window, the patient was considered censored at their last structured activity date [Citation29]. This 3-week window was chosen to correspond to the standard cycle length for trastuzumab administrations in gastric cancer [Citation15]. If the last administration was more than 3 weeks before the last structured activity date, then this was counted as an event in the TTD analysis. OS was defined as time from the start of first-line trastuzumab to date of death from any cause; patients who did not die were censored at the later of their last structured activity date or CGP report date.
Statistical analyses
Demographic, clinical, genomic and testing characteristics were summarized and compared, stratified by highest abstracted clinical HER2 status and by ERBB2 amplification status. Test result concordance based on percentage agreement (overall, positive and negative) was calculated for HER2 status measured by IHC or ISH compared with ERBB2 amplification status measured by CGP. Patients were stratified into four groups based on their test results: HER2+, ERBB2 Amp+ (HER2+ concordant); HER2−, ERBB2 Amp− (HER2− concordant); HER2+, ERBB2 Amp− (HER2+ discordant) and HER2−, ERBB2 Amp+ (HER2− discordant).
For agreement comparisons, no ‘gold-standard’ was assumed between CGP and non-CGP testing modalities; concordance was simply assessed between the two testing methods [Citation30]. A sensitivity analysis of concordance was conducted where CGP specimens with a ‘qualified’ quality control status were excluded, leaving only specimens with ‘pass’ quality control status for the analysis. In addition, differences in HER2/ERBB2 Amp concordance were assessed based on ISH/IHC HER2 test type. Categorical variables are presented as proportions and compared across groups using Chi-squared or Fisher’s exact test. Continuous variables are presented as medians and interquartile ranges (IQR) and compared across groups with the Kruskal–Wallis test.
For first-line therapy regimens containing trastuzumab, TTD and OS from first-line start date were stratified by HER2/ERBB2 agreement and median ERBB2 CN and estimated with unadjusted Kaplan–Meier analysis and adjusted hazard ratios (aHR) from Cox proportional hazards models. Adjusted models were controlled for age at advEGC diagnosis, sex and practice type (community vs academic). Given that patients in this study could have received CGP testing either before or after starting first-line therapy, the study cohort may be affected by left truncation, as inclusion in the CGDB requires a CGP report for an EGC and at least two visits in the Flatiron Health network. To account for this, patients were treated as being at risk for an OS event only after the later of these two events, using risk set adjustment. Additionally, tests for dependent left truncation were used to see whether hazards changed with later entry into the cohort. Supplementary analyses were also conducted to examine differential survival outcomes for co-alterations that were strongly associated with ERBB2/HER2+ concordance, as well as survival outcomes for those with equivocal HER2 IHC (2+) testing that was later confirmed as HER2+ by ISH. R version 3.6.3 was used for all analyses.
Results
Patient demographic & clinical characteristics
Out of 2031 patients with EGC included in the CGDB, 752 with advEGC were selected for the analysis cohort ( & Supplementary Figure 1). Among these 752 patients, 194 (25.8%) had HER2+ tumors at first-line therapy start. Median age at advEGC diagnosis was 64.0 years (IQR: 55.0–71.0), and 566 patients (75.3%) were male. There were 724 patients (96.3%) with adenocarcinoma; all 16 patients with squamous cell carcinoma were HER2- concordant.
Table 1. Patient demographics and clinical characteristics.
HER2 & ERBB2 testing patterns & results
Testing patterns and results are reported in . Stomach tumors were more common among patients who were HER2− concordant (39.9%) versus HER2+ concordant (19.3%). Median ERBB2 CN among patients who were ERBB2 Amp+ was 22.0 (IQR: 7–79), 39 (IQR: 12–106) in patients whose tumors were HER2+ by IHC and 14 (IQR: 8–71.5) in patients whose tumors were HER2+ by ISH. Among nine patients who were clinically HER2− and had an identified ERBB2 CN amplification, median CN was 7 (IQR: 6–8). There were 27 patients with unknown or indeterminate HER2 status who were excluded from the analysis; three were ERBB2 Amp+ and 24 were ERBB2 Amp− (Supplementary Table 3).
Treatment patterns
Among patients in the HER2+ concordant and HER2+ discordant groups, 82/109 (75.2%) and 60/85 (70.6%) received first-line trastuzumab-based regimens, respectively (). The most common regimen for both groups was trastuzumab plus fluorouracil and oxaliplatin (FOLFOX; Supplementary Table 4). Overall, 91% of patients whose tumors were HER2+ concordant and 87% whose tumors were HER2+ discordant received first-line trastuzumab with a fluoropyrimidine (e.g., fluorouracil or capecitabine) backbone. Among patients who were HER2− concordant, 475/549 (86.5%) received first-line combination chemotherapy ().
HER2 status & ERBB2 amplification concordance
Concordance between clinical HER2 status measured by IHC or ISH (reference standard) and ERBB2 amplification measured by CGP is presented in . HER2 status/ERBB2 amplification concordance was 87.5% (658/752) overall and was 89.9% by IHC and 84.4% by ISH. HER2/ERBB2 positive percentage agreement among patients who were HER2+ was 56.2% for all patients (109/194; 56.3 and 56.7% for patients who were HER2+ by IHC and ISH, respectively).
HER2/ERBB2-negative percentage agreement among patients who were HER2− was 98.4% for all patients (549/558; 98.5 and 98.0% for patients who were HER2− by IHC and ISH, respectively).
Table 2. Concordance between HER2 status measured by immunohistochemistry or in situ hybridization and ERBB2 amplification status measured by comprehensive genomic profiling.
Clinical outcomes for first-line trastuzumab in patients with HER2+ tumors
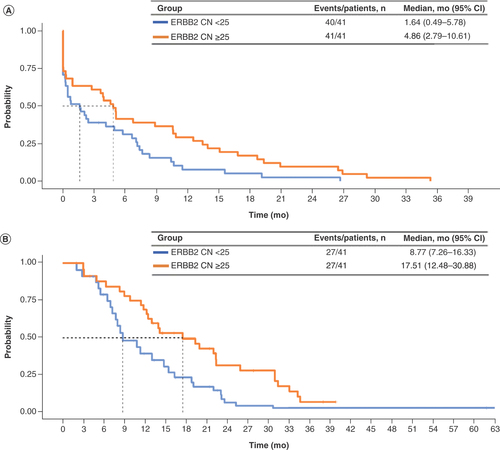
Of 194 patients with tumors documented as clinically HER2+, 82 with HER2+ concordant tumors and 60 with HER2+ discordant tumors had a documented first-line therapy regimen containing trastuzumab. Demographic and clinical characteristics for these patients were similar to characteristics for the overall patient cohort (Supplementary Table 5). Among these patients, HER2+ concordant versus HER2+ discordant results between CGP and traditional HER2 tests were associated with significantly longer TTD (3.22 vs 0.95 months; aHR: 0.63; 95% CI: 0.43–0.90) and significantly longer OS (13.04 vs 7.52 months; aHR: 0.51; 95% CI: 0.33–0.79) ( & ). Only two patients whose results were HER2− discordant had documented first-line trastuzumab treatment, and thus conclusions could not be drawn about clinical outcomes for these patients. When stratified by ERBB2 CN, ERBB2 CN ≥25 versus <25 (median) was also associated with significantly longer TTD (4.86 vs 1.64 months; aHR: 0.56; 95% CI: 0.35–0.88) and OS (17.51 vs 8.77 months; aHR: 0.52: 95% CI: 0.30–0.91) ( & ). An additional analysis looked at patients who had equivocal IHC (2+) HER2 testing followed by an ISH HER2+ result (Supplementary Figure 2 & Supplementary Tables 7 & 8). Patients with either negative (0–1+) or equivocal (2+) IHC results appeared to have shorter TTD than those with a positive IHC (3+) result, but the small patient population in the study with these results limits the conclusions we can draw from this additional analysis. Less evidence of an effect of IHC was seen on OS.
Table 3. Cox proportional hazard clinical outcomes for first-line trastuzumab in patients whose tumors were clinically HER2+ stratified by HER2/ERBB2 concordance (n = 142).
(A) Time to treatment discontinuation. (B) Overall survival.
Amp: Amplification; mo: Months.
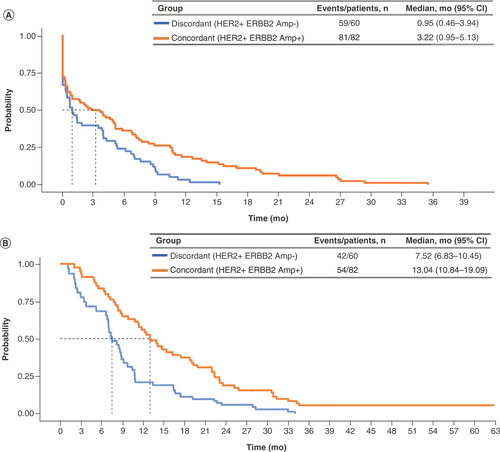
Table 4. Cox proportional hazard clinical outcomes for first-line trastuzumab in patients whose tumors were ERBB2 amp stratified by median ERBB2 copy number (n = 82).
Concurrent genomic alterations
Among patients whose tumors were HER2+ concordant (n = 109), the most frequently (>10%) observed concurrent alterations were CCNE1 alteration (23%), MYC amplification (20%), SMAD4 alteration (16%), CDK6 alteration (14%) and PIK3CA mutation (11%) (Supplementary Table 6). In the subset of HER2+ concordant patients who received first-line trastuzumab, notable differences in alteration frequency between patients with ERBB2 CN ≥25 versus <25 (median) were observed for CCNE1 alterations (14 vs 33%), SMAD4 alterations (19 vs 9.5%), CDK6 alterations (24 vs 2.4%) and PIK3CA mutations (7.1 vs 21%) (data not shown). Among patients whose tumors were HER2+ discordant (n = 85), the most frequently (>10%) observed concurrent alterations were KRAS alterations (26%), CCNE1 alteration (18%), SMAD4 alteration (12%) and MYC amplification (13%). KRAS alteration prevalence was also high (23%) among patients whose tumors were HER2− concordant. In light of these findings, we examined whether KRAS status was predictive of OS or TTD in first-line trastuzumab-treated patients beyond its association with concordance; we did not see strong evidence of an independent effect (Supplementary Figure 3 & Supplementary Table 9).
Discussion
In this large real-world clinicogenomic dataset, overall HER2/ERBB2 concordance was 87.5% among HER2-tested patients with advEGC; this was driven by negative agreement, which was >98% in all groups. This study supports previous findings of high overall concordance between NGS and traditional IHC and ISH methods for assessing HER2 status in EGC, with positive agreement lower than negative agreement in all studies [Citation8,Citation18], and adds to previously reported evidence in breast cancer [Citation31,Citation32].
The positive agreement in this real-world study was 56.2%, which was considerably lower than agreement in studies from academic institutions. This lower agreement could be related to heterogeneity of HER2 expression in EGC. In prior studies that used the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets assay, intratumoral heterogeneity in ERBB2 amplification and low tumor content in test specimens both limited NGS detection capability [Citation8,Citation18]. Other studies have shown that intratumoral heterogeneity can lead to discordant test results between IHC and FISH testing methods as well as between primary and metastatic lesions in EGC [Citation33,Citation34]. Blood-based testing of cell-free DNA and routine testing of metastatic lesions have been suggested as ways to provide more accurate representation of alteration status for EGCs [Citation35,Citation36]. Personalized treatment approaches that match targeted therapies to tumor mutational profiles and reassess alteration status when disease progression occurs have been demonstrated to improve OS in recent clinical studies. This approach to treatment incorporates the evolving understanding of molecular heterogeneity and its impact on patient outcomes [Citation37].
Lower agreement could also be related to the use of different specimens for IHC/ISH versus CGP testing. We were not able to determine whether the same specimens were used for both tests, and did not know if specimens were derived from endoscopic biopsies or surgical resections of the primary tumor, or from metastatic lesions. Another source of low agreement could be related to differences in IHC and ISH testing procedures between pathology labs. Studies comparing HER2 status assessed at local versus central pathology labs consistently demonstrate discordance between results [Citation38,Citation39]. The IHC and ISH tests included in this study were performed in many different pathology labs as a part of routine clinical practice, and as such the assay performance and interpretation of test results may differ between labs and impact assessment of concordance.
We also evaluated the association between HER2/ERBB2 concordance and clinical benefit assessed by TTD and OS. Among patients treated with first-line trastuzumab, HER2+ concordant results between CGP and traditional HER2 tests were associated with significantly longer TTD and OS compared with HER2+ discordant results. We did not assess clinical outcomes for patients whose tumors were HER2− discordant who received HER2-targeted therapy due to the small patient population (n = 2). However, the results of this analysis are consistent with subgroup analysis data from the ToGA study, in which patients with discordant IHC and FISH results tended to have worse OS compared with patients with concordant IHC and FISH results, and did not derive a clinical benefit from trastuzumab compared with chemotherapy [Citation12]. These results are also consistent with those of a limited analysis of the association between IHC/ISH and NGS concordance with clinical benefit from first-line trastuzumab in 50 patients with EGC whose tumors were HER2+, which showed that progression-free survival was significantly shorter in patients whose test results were discordant compared with patients whose tumors were ERBB2-amplified by NGS [Citation18].
The TTD estimates reported in this study are consistent with duration-of-therapy estimates described for other real-world EGC populations. Median duration of first-line therapy was 2.2 months for patients with advanced EGC in a descriptive study of US-based treatment patterns [Citation40]. Median TTD was 2.8 versus 1.9 months in a study of patients with EGC who received ramucirumab in combination with chemotherapy versus ramucirumab monotherapy [Citation41].
The median OS estimates described in this study are consistent with estimates reported by other real-world studies of patients with metastatic EGC in Canada, Germany, Italy and The Netherlands who received first-line trastuzumab therapy. In these studies, median OS ranged from 9.3 to 14.1 months [Citation42–45]. In a study of 364 patients in Germany, median OS was 14.1 months in patients with HER2 status confirmed by IHC 3+ or IHC2+ confirmed by ISH and 9.1 months in patients with marginal HER2 status (defined as IHC2+ without confirmation by ISH) [Citation42]. This pattern is consistent with the difference in OS that we observed for patients who were HER2/ERBB2 concordant (13.02 months) versus HER2/ERBB2 discordant (7.52 months). Although these real-world OS estimates are lower than what has been reported from clinical trials [Citation46], this difference is expected given the known gap in efficacy as measured in clinical trials compared with real-world effectiveness for cancer therapies. Clinical trial eligibility criteria typically limit participation for patients with significant comorbidities, and most trial populations are younger and healthier than real-world patients.
Additional stratified analyses found that ERBB2 CN ≥25 in patients whose tumors were HER2+ was associated with significantly longer TTD and OS compared with ERBB2 CN <25. Significantly longer progression-free survival was observed in a study of patients with EGC with ERBB2 CN in the highest quartile who were treated with first-line trastuzumab [Citation18]. In addition, a post hoc exploratory analysis in the ToGA study found that patients with high HER2 expression (e.g., IHC 3+ or IHC2+ and FISH positive) had significantly longer OS when treated with trastuzumab plus chemotherapy compared with patients treated with chemotherapy alone [Citation12]. A possible explanation for this finding is that a higher ERBB2 CN may confer increased sensitivity to trastuzumab, as the cancer cell is inherently more ‘addicted’ to overexpressed HER2 protein, with the assumption that gene CN correlates to increased copies at the protein level, broadly consistent with the data presented here [Citation22]. This finding is consistent with prior studies that reported longer OS in trastuzumab-treated patients with advanced gastric cancer with higher levels of ERBB2 amplification measured by ISH [Citation23].
Molecular alterations co-occurring with ERBB2 amplification that have been associated with lack of benefit from trastuzumab include alterations in genes involved in receptor tyrosine kinase signaling pathways, the PI3K/mTOR signaling pathway (PIK3CA), the MAP kinase signaling pathway (KRAS) and the cell cycle control pathway (CCNE1, CDK6, CCND1) [Citation9,Citation18–21]. In this study, KRAS alterations were more common among patients who did not have ERBB2 amplification than in patients with ERBB2 amplification. This finding is consistent with results from a study of patients with metastatic colorectal cancer which reported a lower frequency of ERBB2 amplifications in patients with KRAS alterations [Citation47]. A study of biomarkers associated with lack of trastuzumab benefit in patients with HER2+ metastatic gastric cancer also reported a higher frequency of both KRAS and PI3K alterations in patients who derived less benefit from trastuzumab [Citation48]. In our study, PIK3CA alterations were less common among trastuzumab-treated patients with an ERBB2 CN ≥25 compared with those with CN <25. This analysis provides additional evidence of differences in the frequency of co-occurring alterations between patients with and without ERBB2 amplification. More research that includes a larger number of patients is needed to assess the relationship between ERBB2 amplification, co-occurring alterations and clinical benefit from trastuzumab.
The results of this study are subject to several limitations. First, as the clinical data for this real-world study were derived from EHRs, tests, treatments, events and outcomes occurring outside of the Flatiron Health network or not documented in the EHR may be missing, and certain prognostic factors (such as site of metastasis) were not available in the data model. Second, patients were required to have CGP testing by FMI for study entry, which may introduce a selection bias for patients who received care from physicians with distinct practice patterns. Third, exposure to neoadjuvant/adjuvant chemotherapy for potentially resectable EGC may have impacted first-line chemotherapy choice or timing of CGP testing, which could not be evaluated in this study. Fourth, this study is subject to left truncation, as a CGP report (which corresponds with study cohort entry, and thus a patient’s ability to be at risk for death) may have been issued after first-line therapy start. Risk set adjustment was used to account for left truncation, and while dependence between entry time and mortality may invalidate survival estimates using this method, statistical tests (using entry time as a covariate in a Cox model) did not reveal evidence of dependence.
Fifth, as patients with only IHC2+ (equivocal) results without documented confirmatory ISH testing were not included in this study, and patients who may have positive or negative ISH testing after the start of their first-line therapy might be eligible for inclusion, the cohort is theoretically subject to immortal time bias. However, no patients in the first-line trastuzumab-treated cohort had a confirmatory ISH test after their CGP report, so no misattribution of time at risk was made due to reflexive ISH testing. Sixth, specimen quality was hypothesized as a predictor of concordance because this could impact assay sensitivity; however, no correlation was noted between these two factors. Seventh, IHC and ISH tests were performed in many different pathology labs as a part of routine clinical practice, and as such the assay performance and interpretation of test results may differ between labs and impact assessment of concordance. Finally, the broader EGC cohort included all EGC sub-types (e.g., adenocarcinoma and squamous cell carcinoma), and it is important to note that squamous cell patients are not considered candidates for trastuzumab [Citation15]. However, no patients with squamous cell carcinoma were eligible for inclusion in the first-line trastuzumab or ERBB2 amplification/HER2+ analytic cohorts.
Conclusion
In summary, concordance between HER2 status measured by IHC and/or ISH and ERBB2 measured by CGP and higher ERBB2 CN in patients with advEGC were associated with significantly longer TTD and OS in response to first-line trastuzumab. As NGS testing continues to be more widely employed, its role in identifying patients who may benefit from HER2 directed therapy should be prospectively examined. In addition, as other HER2 directed therapies are studied in advEGC, it will be important to determine which testing strategy most accurately predicts clinical benefit.
Future perspective
Use of NGS has increased in the community setting in recent years [Citation49,Citation50]. As NGS testing continues to grow, it is increasingly important to determine which tests are sufficient to support treatment decisions and which are redundant, especially when more than one molecular target must be assessed. In the future, broad molecular profiling may become a more practical approach in routine clinical practice and as a way to identify patients who are eligible for molecularly driven clinical trials, especially as criteria that cross-validate assessment of alteration status across different test methods are developed [Citation51].
The results in this study should be viewed as hypothesis generating and do not suggest that clinicians change their current approach for assessing HER2 status in patients with advEGC. Further research is needed to explore associations between HER2/ERBB2 concordance and clinical outcomes in these patients and to assess the role of ERBB2 CN as measured by CGP in selecting patients who may benefit from first-line trastuzumab. Our results suggest there is a relationship between ERBB2 CN level and clinical benefit from trastuzumab. Given our limited cohort, we investigated this by examining outcomes above and below median ERBB2 CN. Future, larger studies could examine the relationship of ERBB2 CN with outcomes in a more precise manner, such as examining different cut points or evaluating ERBB2 CN as a continuous biomarker. Additionally, the role of concurrent alterations in association with ERBB2 amplification and clinical benefit from trastuzumab could be further explored. Future studies could also evaluate these associations in other tumor types, such as breast or colorectal cancers, as innate differences in tumor biology and concurrent genomic alterations may be important factors. Finally, economic evaluations could examine the cost–effectiveness of NGS testing in EGC and the potential for cost and time savings associated with NGS as compared with running multiple tests for single biomarkers, as has been shown recently in non-small-cell lung cancer [Citation52].
Summary points
As the number of targeted therapies for advanced esophagogastric cancer (advEGC) increases, next-generation sequencing (NGS) is becoming an attractive alternative to single biomarker tests, particularly for patients with limited tissue available for testing.
It is important to understand whether NGS can support treatment selection as consistently as conventional single-biomarker tests.
We used a retrospective, linked clinicogenomic database to assess whether concordance between immunohistochemistry or in situ hybridization-based HER2 status and NGS-based ERBB2 amplification was associated with first-line trastuzumab clinical benefit in real-world patients with advEGC.
Overall HER2/ERBB2 concordance was 87.5% among HER2-tested patients with advEGC; this was driven by negative agreement.
We found that patients with concordant (HER2+/ERBB2 amplified) results had significantly longer time to treatment discontinuation and overall survival versus patients with discordant (HER2+/ERBB2 not amplified) results.
We also observed that higher ERBB2 copy number may predict trastuzumab benefit.
These findings suggest that NGS may have clinical value as a complement to traditional HER2 testing and warrant further exploration.
As other HER2-directed therapies are studied in advEGC, it will be important to determine which testing strategy most accurately predicts clinical benefit.
Author contributions
Study design (literature, background search): J Snider, M McCusker, S Ali, E Castellanos, S Stein. Data collection: Flatiron Health, Inc. and Foundation Medicine, Inc. Data analysis (and figures as applicable): A Schrock, J Snider, M McCusker, S Stein, R Miksad. All authors contributed to data interpretation and to writing and review of the manuscript.
Data sharing statement
The data that support the findings of this study have been originated by Flatiron Health, Inc. and Foundation Medicine, Inc. These deidentified data may be made available upon request, and are subject to a license agreement with Flatiron Health and Foundation Medicine; interested researchers should contact [email protected] to determine licensing terms.
Ethical conduct of research
Institutional Review Board approval was obtained prior to study conduct, and included a waiver of informed consent.
Supplemental Figures
Download MS Word (753.7 KB)Supplemental Materials
Download MS Word (54.3 KB)Acknowledgments
The authors would like to thank C Patton (Flatiron Health, Inc) for medical writing and publication management support. This study was sponsored by Flatiron Health, Inc., which is an independent subsidiary of the Roche Group.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2021-0203
Financial & competing interests disclosure
This study was sponsored by Flatiron Health, Inc., which is an independent subsidiary of the Roche Group. During the study period, J Snider, M McCusker, R Miksad, E Castellanos and A Swaminathan report employment at Flatiron Health, Inc., which is an independent subsidiary of the Roche Group. B Alexander, A Schrock, R Madison, S Ali and J Venstrom report employment at Foundation Medicine Inc., a wholly owned subsidiary of Roche. J Snider, M McCusker, R Miksad, E Castellanos, R Madison, B Alexander, A Schrock and A Swaminathan report stock ownership in Roche. J Snider, R Miksad and E Castellanos report equity ownership in Flatiron Health, Inc. In addition, S Ali reports employment at EQRx, advisory positions at Incysus and Elevation Oncology, and patent interests. S Stein reports con sulting/advisory roles in Genentech/Roche, Eisai, QED, Exelixis and Merck. R Miksad reports a consulting/advisory role in the De Luca Foundation. B Alexander reports consulting/advisory roles in Abbvie and Precision Health Economics, and research funding from Eli Lilly, Puma and Celgene. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance was utilized in the production of this manuscript. Identify funding for such assistance.
Additional information
Funding
References
- Bray F , FerlayJ, SoerjomataramI, SiegelRL, TorreLA, JemalA. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.68(6), 394–424 (2018).
- Arnold M , LaversanneM, BrownLM, DevesaSS, BrayF. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am. J. Gastroenterol.112(8), 1247–1255 (2017).
- Karimi P , IslamiF, AnandasabapathyS, FreedmanND, KamangarF. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomarkers Prev.23(5), 700–713 (2014).
- Howlader N , NooneAM, KrapchoMet al. SEER cancer statistics review (CSR) 1975–2017. https://seer.cancer.gov/csr/1975_2017/
- Bartley AN , WashingtonMK, ColasaccoCet al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol.35(4), 446–464 (2017).
- National Cancer Institute Surveillance, Epidemiology, and End Results Program . Cancer stat facts: stomach cancer. https://seer.cancer.gov/statfacts/html/stomach.html
- National Cancer Institute Surveillance, Epidemiology, and End Results Program . Cancer stat facts: esophagus cancer. https://seer.cancer.gov/statfacts/html/esoph.html
- Ross DS , ZehirA, ChengDTet al. Next-generation assessment of human epidermal growth factor receptor 2 (ERBB2) amplification status: clinical validation in the context of a hybrid capture-based, comprehensive solid tumor genomic profiling assay. J. Mol. Diagn.19(2), 244–254 (2017).
- Battaglin F , NaseemM, PucciniA, LenzH. Molecular biomarkers in gastro-esophageal cancer: recent developments, current trends and future directions. Cancer Cell. Int.18, 99 (2018).
- Van Cutsem E , BangY, Feng-YiFet al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer18(3), 476–484 (2015).
- Ieni A , BarresiV, GiuffrèGet al. HER2 status in advanced gastric carcinoma: a retrospective multicentric analysis from Sicily. Oncol. Lett.6(6), 1591–1594 (2013).
- Bang Y , Van CutsemE, FeyereislovaAet al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase 3, open-label, randomised controlled trial. Lancet376(9742), 687–697 (2010).
- Tafe LJ , JanjigianYY, ZaidinskiMet al. Human epidermal growth factor receptor 2 testing in gastroesophageal cancer: correlation between immunohistochemistry and fluorescence in situ hybridization. Arch. Pathol. Lab. Med.135(11), 1460–1465 (2011).
- Tanner M , HollménM, JunttilaTTet al. Amplification of HER-2 in gastric carcinoma: association with topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol.16(2), 273–278 (2005).
- HERCEPTIN® (trastuzumab) [package insert]. Genentech, Inc, CA, USA (2018).
- National Comprehensive Cancer Network . Gastric cancer version 4.2020. www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- National Comprehensive Cancer Network . Esophageal and esophagogastric junction cancers version 5.2020. www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Janjigian YY , Sanchez-VegaF, JonssonPet al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov.8(1), 49–58 (2018).
- Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature513(7517), 202–209 (2014).
- Kim J , BowlbyR, MungallAJet al. Integrated genomic characterization of oesophageal carcinoma. Nature541(7636), 169–175 (2017).
- Klempner SJ , ChaoJ, BaileyMet al. Genomic alterations (GA) predicted to confer lack of benefit from trastuzumab in advanced esophagogastric cancers (EGC): analysis of 527 HER2-amplified (HER2amp) cases. J. Clin. Oncol.36(Suppl. 4), 44–44 (2018).
- Ellegård S , VeenstraC, Pérez-TenorioGet al. ERBB2 and PTPN2 gene copy numbers as prognostic factors in HER2-positive metastatic breast cancer treated with trastuzumab. Oncol. Lett.17(3), 3371 (2019).
- Gomez-Martin C , PlazaJC, Pazo-CidRet al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J. Clin. Oncol.31(35), 4445–4452 (2013).
- Frampton GM , FichtenholtzA, OttoGAet al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol.31(11), 1023–1031 (2013).
- Birnbaum B , NussbaumN, Seidl-RathkopfKet al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research (2020). ( Epub ahead of print). https://arxiv.org/abs/2001.09765
- Ma X , LongL, MoonS, AdamsonBJS, BaxiSS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron health, SEER, and NPCR (2020). ( Epub ahead of print). www.medrxiv.org/content/10.1101/2020.03.16.20037143v2
- Singal G , MillerPG, AgarwalaVet al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database. JAMA321(14), 1391–1399 (2019).
- Curtis MD , GriffithSD, TuckerMet al. Development and validation of a high-quality composite real-world mortality endpoint. Health Serv. Res.53(6), 4460–4476 (2018).
- Blumenthal GM , GongY, KehlKet al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann. Oncol.30(5), 830–838 (2019).
- US Food and Drug Administration . Statistical guidance on reporting results from studies evaluating diagnostic tests - guidance for industry and FDA staff. www.fda.gov/regulatory-information/search-fda-guidance-documents/statistical-guidance-reporting-results-studies-evaluating-diagnostic-tests-guidance-industry-and-fda
- Yip W , SkoletskyJ, MaPet al. Abstract 1607: an ERBB2 follow-on companion diagnostic for clinical care of patients with breast cancer. Cancer Res.78(Suppl. 13), 1607 (2018).
- US Food and Drug Administration . PMA P170019/S006: FDA summary of safety and effectiveness data. www.accessdata.fda.gov/cdrh_docs/pdf17/P170019S006B.pdf
- Yang J , LuoH, LiYet al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem. Biophys.62(1), 221–228 (2012).
- Kim MA , LeeH, YangH, BangY, KimWH. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology59(5), 822–831 (2011).
- Pectasides E , StachlerMD, DerksSet al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov.8(1), 37–48 (2018).
- Maron SB , ChaseLM, LomnickiSet al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin. Cancer Res.25(23), 7098 (2019).
- Catenacci DVT , MoyaS, LomnickiSet al. Personalized antibodies for gastroesophageal adenocarcinoma (PANGEA): a Phase II study evaluating an individualized treatment strategy for metastatic disease. Cancer Discov.11(2), 308–325 (2021).
- Huang D , LuN, FanQet al. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS ONE8(11), e80290 (2013).
- Huemer F , WeissL, RegitnigPet al. Local and central evaluation of HER2 positivity and clinical outcome in advanced gastric and gastroesophageal cancer-results from the AGMT GASTRIC-5 registry. J. Clin. Med.9(4), 935 (2020).
- Le DT , OttPA, KorytowskyBet al. Real-world treatment patterns and clinical outcomes across lines of therapy in patients with advanced/metastatic gastric or gastroesophageal junction cancer. Clin. Colorectal Cancer19(1), 32–38.e3 (2020).
- Paulson AS , HessLM, LiepaAMet al. Ramucirumab for the treatment of patients with gastric or gastroesophageal junction cancer in community oncology practices. Gastric Cancer21(5), 831–844 (2018).
- Al-Batran SE , MoorahrendE, MaintzCet al. Clinical practice observation of trastuzumab in patients with human epidermal growth receptor 2-positive metastatic adenocarcinoma of the stomach or gastroesophageal junction. Oncologist25(8), e1181–e1187 (2020).
- Dijksterhuis WPM , VerhoevenRHA, MeijerSLet al. Increased assessment of HER2 in metastatic gastroesophageal cancer patients: a nationwide population-based cohort study. Gastric Cancer23(4), 579–590 (2020).
- Franchi M , TrittoR, TorroniL, RenoC, LaVecchia C, CorraoG. Effectiveness and healthcare cost of adding trastuzumab to standard chemotherapy for first-line treatment of metastatic gastric cancer: a population-based cohort study. Cancers12(6), 1691 (2020).
- Merchant SJ , KongW, GyawaliBet al. Effectiveness of trastuzumab in routine clinical practice: a population-based study of patients with HER-2-positive oesophageal, gastroesophageal and gastric cancer. Clin. Oncol. (R. Coll. Radiol.)33(3), 202–207 (2021).
- Phillips CM , ParmarA, GuoHet al. Assessing the efficacy–effectiveness gap for cancer therapies: a comparison of overall survival and toxicity between clinical trial and population-based, real-world data for contemporary parenteral cancer therapeutics. Cancer126(8), 1717–1726 (2020).
- Nam SK , YunS, KohJet al. BRAF, PIK3CA, and HER2 oncogenic alterations according to KRAS mutation status in advanced colorectal cancers with distant metastasis. PLoS ONE11(3), e0151865 (2016).
- Pietrantonio F , FucàG, MoranoFet al. Biomarkers of primary resistance to trastuzumab in HER2-positive metastatic gastric cancer patients: The AMNESIA case-control study. Clin. Cancer Res.24(5), 1082–1089 (2018).
- McKenzie A , SchlauchD, SharmaYet al. Adoption and utilization of NGS-based molecular profiling in community-based oncology practices: insights from sarah cannon. J. Clin. Oncol.37(Suppl. 15), e18064 (2019).
- Presley CJ , TangD, SoulosPRet al. Association of broad-based genomic sequencing with survival among patients with advanced non-small cell lung cancer in the community oncology setting. JAMA320(5), 469–477 (2018).
- Fujii S , MaglioccoAM, KimJet al. International harmonization of provisional diagnostic criteria for ERBB2-amplified metastatic colorectal cancer allowing for screening by next-generation sequencing panel. JCO Precis. Oncol.4, 6–19 (2020).
- Pennell NA , MutebiA, ZhouZet al. Economic impact of next-generation sequencing versus single-gene testing to detect genomic alterations in metastatic non–small-cell lung cancer using a decision analytic model. JCO Precis. Oncol.3, 1–9 (2020).