Abstract
Although complete omentectomy is traditionally performed in patients with gastric cancer as part of radical gastrectomy to ensure the elimination of micrometastases, the prognostic value of omentectomy during gastrectomy remains unclear. Retrospective studies have shown that the incidence of metastases in the greater omentum is very low in T1–T3 gastric cancer. Thus radical gastrectomy with D2 lymphadenectomy and preservation of the greater omentum may be a proper curative treatment for gastric cancer patients with T1–T3 tumors. The aim of this article is to describe the design and rationale for this prospective, randomized controlled DRAGON-05 trial, conducted to evaluate the prognostic value of omentum-preserving gastrectomy for patients with T1–T3 gastric cancer.
Clinical trial registration: ChiCTR2000040045 (ClinicalTrials.gov)
The omentum is a mesenteric tissue apron that hangs down from the stomach, providing a protective cushion and reducing intestinal adhesion [Citation1]. Traditionally, complete omentectomy is only performed in patients with gastric cancer as part of radical gastrectomy to ensure the elimination of micrometastases [Citation2]. However, the prognostic value of omentectomy during gastrectomy remains unclear. The Japanese, American and European guidelines for the treatment of gastric cancer have not reached consensus with respect to omentectomy. The Japanese gastric cancer treatment guidelines suggest that the omentum can be preserved for T1 or T2 tumors, whereas the National Comprehensive Cancer Network guidelines advise resecting both greater and lesser omentum, regardless of tumor stage [Citation3]. Retrospective studies have shown that omental lymph node or tumor deposits are present in about 5–10% of cancer patients undergoing gastrectomy, but the incidence of metastases in greater omentum is very low in T1–T3 gastric cancer [Citation4,Citation5]. It was also found that only 10% of early gastric cancer patients have metastatic lymph nodes, which are primarily located in the perigastric area (first-level lymph nodes) [Citation6]. Thus radical gastrectomy with D2 lymphadenectomy and preservation of the greater omentum at >3 cm from the gastroepiploic arcade may be a proper curative treatment for gastric cancer patients with T1–T3 tumors.
DRAGON-05 study
Herein we describe the design and rationale for this prospective, randomized controlled DRAGON-05 trial (ClinicalTrials.gov; ChiCTR2000040045), conducted to evaluate the prognostic value of omentum-preserving gastrectomy for patients with T1–T3 gastric cancer.
Background & rationale
Recently, the survival benefit of bursectomy versus omentectomy alone was denied by the JCOG1001 trial for resectable gastric cancer [Citation7]. Nevertheless, many physicians have been interested in more limited surgeries for treating early gastric cancer. Given that there has so far been no evidence showing a definitive improvement in survival after gastrectomy with omentectomy, even for cases of advanced gastric cancer, the Japanese gastric cancer treatment guidelines (5th edition) edited by the Japanese Gastric Cancer Association state that ‘removal of the greater omentum is usually integrated in the standard gastrectomy for T3 (SS) or deeper tumors. For T1/2 tumors, the omentum more than 3 cm away from the gastroepiploic arcade may be preserved’ [Citation3]. Alternatively, the American guidelines edited by the National Comprehensive Cancer Network advise resection of both greater and lesser omentum, whereas the European guidelines edited by the European Society for Medical Oncology do not give any advice regarding omentectomy [Citation8]. The inconsistency between guidelines on this topic reflects the lack of evidence evaluating the prognostic benefits of omentectomy during curative surgery for gastric cancer. The Japanese Clinical Oncology Group has conducted a randomized controlled Phase III trial (JCOG1711) to evaluate omentum-preserving gastrectomy for patients with advanced gastric cancer. Notably, high-quality evidence or clinical trials evaluating the survival benefit of complete omentectomy are still lacking for T1–T3 gastric cancer patients.
The advantage of complete omentectomy is in accordance with the current understanding of the lymphatic drainage of the omentum by means of milky spots, which are located throughout the entire greater omentum and act as a gate through the abdominal cavity into the subperitoneum, which contributes to the peritoneal seeding of cancer cells [Citation9]. Thus complete resection of the greater omentum has been believed to be essential to eliminate tumor cells during surgery for gastric cancer. However, the disadvantage of this procedure is also considerable. Complete omentectomy increases the operating time (especially for laparoscopic procedures) and is associated with greater blood loss and a higher risk of complications such as abdominal abscesses, ascites, anastomotic leakage, ileus, wound infections and colonic and mesocolonic injuries [Citation5,Citation10].
Based on previous results, we planned a prospective randomized controlled Phase III trial (DRAGON-05) to evaluate the efficacy and prognostic value of omentum-preserving gastrectomy for patients with T1–T3 gastric cancer. It should be noted that patients treated by preoperative therapy would be excluded in this study, because treatment strategies for gastric cancer in our hospital are more similar to those described in the Japanese guideline, which states that neoadjuvant chemotherapy is conditionally recommended for a patient with extensive lymph node metastasis [Citation3]. Although Western guidelines suggest that patients with T2 or higher tumors should preferentially receive perioperative chemotherapy [Citation8], we believe that this trial could also provide a valuable evidence for Western populations if the prognostic value of omentum-preserving gastrectomy is confirmed. The Ruijin Hospital Ethics Committee approved this study in September 2020, and this study was activated in November 2020. This trial was registered in the Chinese Clinical Trial Registry as ChiCTR2000040045.
Study design
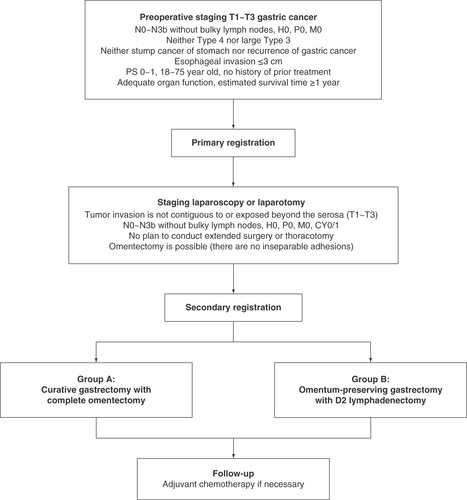
DRAGON-05 is a prospective, randomized controlled, single-institution, Phase III trial (). A total of 565 patients are planned to be enrolled in 4 years. Based on past data in our hospital, T1 tumors accounted for 46.6%, T2 tumors accounted for 19.9% and T3 tumors accounted for 33.5% of cases; thus the estimated numbers of T1, T2 and T3 tumors in this study may be 263, 113 and 189.
Preoperative staging
Patients are evaluated consecutively by the following examinations to check the feasibility of the surgery and to estimate whether tumor invasion is exposed beyond the serosa:
Complete blood count and comprehensive chemistry profile;
Tumor markers (α-FP, CEA, CA125, CA19-9, CA724);
Contrast-enhanced thoracic, abdominal and pelvic CT scan;
Endoscopic ultrasound if T1–T3 versus T4 needs to be determined;
PET/CT scan if no evidence of distant metastasis and if clinically indicated.
Registration
Patients who meet the eligibility criteria are eligible for primary registration. Staging laparoscopy or laparotomy will be performed before secondary registration to judge the depth of invasion, the extent of lymph node metastasis and the presence of liver or peritoneal dissemination. It is also judged that R0 surgery with total omentectomy is possible (there are no inseparable adhesions) and that there is no need to conduct extended surgery or thoracotomy. If the tumor is located on the posterior surface, a part of the gastrocolic ligament should be opened. A randomization sequence was pre-generated and stored on a web server. Patients who are eligible for secondary registration will be randomly assigned to group A (curative gastrectomy with complete omentectomy) or group B (omentum-preserving gastrectomy with D2 lymphadenectomy) according to the randomization sequence. The randomization is ethically justified, because evidence or clinical trial evaluating the survival benefit of complete omentectomy is still lacking for T1–T3 gastric cancer patients.
Surgery
Both group A and group B patients will receive curative gastrectomy with D2 lymphadenectomy according to the Japanese gastric cancer guidelines (5th edition) [Citation3]. For patients assigned to group A, complete omentectomy is performed as follows: the transverse colon and the greater omentum are divided at the thin membrane beside the colon, and the omentum is completely resected. For patients assigned to group B, omentum-preserving gastrectomy is performed as follows: the greater omentum is dissected 3 cm away from the gastroepiploic arcade, and the greater omentum on the transverse colon side is preserved.
Both open and laparoscopic surgery are allowed. Subtotal gastrectomy is undertaken if there is a tumor-free distance of at least 3 cm to the proximal resection margin for Type 1 and 2 tumors, and at least 5 cm for Type 3 and 4 tumors; all other patients will undergo total gastrectomy. D2 lymphadenectomy associated with total gastrectomy contains lymph node stations 1–7, 8a, 9, 11p, 11d and 12a, whereas in conjunction with subtotal gastrectomy it includes lymph node stations 1, 3, 4sb, 4d, 5–7, 8a, 9, 11p and 12a. Reconstruction is performed by the Roux-en-Y method in patients undergoing total gastrectomy, and by Billroth I, Billroth II with Braun enteroenterostomy, or uncut Roux-en-Y method, in those undergoing distal gastrectomy, with end-to-side or side-to-side anastomosis using circular or linear staplers.
Postoperative chemotherapy
All patients with disease greater than pathological stage IA should receive adjuvant chemotherapy unless their condition does not allow. Postoperative chemotherapy is to be started within 42 days after surgery. For patients with stage IB or stage II, a 3-week course consisting of 2 weeks’ administration of daily oral S-1 80 mg/m2/day and 1 week withdrawal is repeated during the first year after surgery. For patients with stage III, six cycles of SOX regimen (oxaliplatin 130 mg/m2 day 1 + S-1 80 mg/m2 days 1–14, repeated every 3 weeks) are conducted, and oral S-1 (3-week course) is maintained until 1 year after surgery.
Follow-up
Patients are followed up on a fixed schedule for 5 years after surgery. Physical and blood examinations are done every 3 months for the first 3 years and every 6 months thereafter. If recurrence is not suspected, contrast-enhanced thoracic, abdominal and pelvic CT scans are done every 6 months. Upper gastrointestinal endoscopy is done once every year for patients who underwent subtotal gastrectomy.
Eligibility criteria
The eligibility criteria of this study are as follows:
Histologically proven gastric adenocarcinoma;
Preoperative staging indicates that the serosa is not invaded (T1–T3);
Neither bulky lymph node (≥3 × 1 cm or ≥1.5 × 2 cm) nor para-aortic lymph node (No.16a2 / 16b1) metastasis diagnosed by enhanced abdominal CT;
No distant metastasis confirmed by enhanced thoracic/abdominal/pelvic CT (≤5 mm slice thickness) and clinically no sign of distant metastasis;
Neither Borrmann Type 4 nor large (≥8 cm) Type 3;
Length of esophageal invasion ≤3 cm;
Neither stump cancer of stomach nor recurrence of gastric cancer;
Age between 18 and 75 years at registration;
Performance status Eastern Co-operative Oncology Group score 0 or 1 and American Association of Anesthesiologists grades 1–3;
No sign of peritoneal metastasis;
No plan to conduct total gastrectomy with thoracotomy, gastrectomy with combined resection (except combined cholecystectomy), or Appleby’s operation;
No prior chemotherapy, radiation therapy, targeted therapy or immunotherapy;
No prior surgery for stomach except for endoscopic resection;
Adequate organ function, such as:
Normal bone marrow function (neutrophils ≥1.5 × 109/l, hemoglobin ≥8.0 g/dl, platelets ≥100 × 109/l);
Normal liver function (total bilirubin ≤1.5 mg/dl, aspartate aminotransferase ≤100 IU/l, alanine aminotransferase ≤100 IU/l);
Normal kidney function (creatinine clearance ≥50/ml/min/body);
Estimated survival time ≥1 year;
Written informed consent from patient.
Exclusion criteria
The exclusion criteria of this study are as follows:
Synchronous or metachronous (within 5 years) malignancies;
Infectious disease requiring systemic treatment;
Emergency surgery;
During pregnancy, within 28 days post-parturition, or during lactation;
Severe mental disease;
Receiving continuous systemic corticosteroid or immunosuppressant treatment;
Poorly controlled valve disease, dilated or hypertrophic cardiomyopathy;
Poorly controlled diabetes or hypertension;
Other conditions that may definitely interfere with the research process.
End points
The primary end point is the 5-year overall survival. The secondary end points are disease-free survival and peritoneal metastasis rate in patients with R0 resection, the proportion of postoperative complications, and perioperative parameters such as type of anastomosis, blood loss, duration of operation, pathology results and nutritional status after surgery. The Clavien–Dindo classification is used to describe postoperative complications [Citation11]. Nutritional status is evaluated by body weight. Detailed background data including comorbidity, past medical history, pathology results, surgery information and postoperative examinations will be collected during this trial. Two interim analyses which report the baseline data, primary efficacy and safety of omentum-preserving gastrectomy are scheduled after enrollment of 250 and 500 patients.
Statistics
In this trial, a sample size of 565 patients provides a power (1-β) of 80% and a threshold value of 10% (corresponding to a hazard ratio of 1.65) for the primary end point using one-side testing (α) at a 5% significance level. The relatively wide noninferiority margin was deemed acceptable following that of the CLASS-01 trial [Citation12]. The hazard ratio is calculated corresponding to the 5-year overall survival rate which was 81.87% at our hospital. For primary and secondary end points, survival rates are analyzed using survival curves calculated by the Kaplan–Meier method; prognostic factors are analyzed using Cox regression; clinicopathological variables are analyzed using Chi-squared test for discrete variables and t-test for continuous variables; p-values < 0.05 are considered significant.
Conclusion
We described the design and rationale for the single-institution, prospective, randomized controlled, Phase III trial (DRAGON-05) to evaluate the efficacy of omentum-preserving gastrectomy for patients with T1–T3 gastric cancer. If the survival rate of omentum-preserving gastrectomy is noninferior to that of gastrectomy with complete omentectomy, this trial can provide favorable evidence for clinical guidelines when considering the prognostic value of omentum-preserving gastrectomy.
Background
The prognostic value of omentectomy during gastrectomy remains unclear.
Retrospective studies showed that the incidence of metastases in the greater omentum is very low in stage T1–T3 gastric cancer.
DRAGON-05 study design & eligibility criteria
DRAGON-05 is a prospective, randomized controlled, single-institutional, Phase III trial to evaluate the efficacy and prognostic value of omentum-preserving gastrectomy for patients with stage T1–T3 gastric cancer.
Patients are eligible if preoperative staging indicates that the serosa is not invaded (T1–T3), and there is no bulky lymph node and no distant metastasis.
Outcome measures/end points
The primary end point is the 5-year overall survival.
The secondary end points are disease-free survival and peritoneal metastasis rate in patients with R0 resection, the proportion of postoperative complications, and perioperative parameters.
Conclusion
If the survival rate of omentum-preserving gastrectomy is noninferior to that of gastrectomy with complete omentectomy, this trial can provide favorable evidence for clinical guidelines when considering the prognostic value of omentum-preserving gastrectomy.
Author contributions
C Yan, M Yan and Z-G Zhu contributed to the conception of the study. S Lu and Z-Y Yang contributed significantly to preparing the trial and manuscript. W-T Liu, Z-T Ni, X-X Yao, Z-C Hua, R-H Feng, Y-N Zheng, Z-Q Wang, B Kumar Sah, M-M Chen, Z-L Zhu, C-Y He and C Li contributed to performing the surgery.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Data sharing statement
Individual participant data that underlie the results reported by our articles will be shared with researchers who provide a methodologically sound proposal, beginning 3 months and ending 1 year following article publication.
Financial & competing interests disclosure
This study was funded in part by the National Natural Science Foundation of China (no. 81772518). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Platell C , CooperD, PapadimitriouJM, HallJC. The omentum. World J. Gastroenterol.6(2), 169–176 (2000).
- Hagiwara A , SawaiK, SakakuraCet al. Complete omentectomy and extensive lymphadenectomy with gastrectomy improves the survival of gastric cancer patients with metastases in the adjacent peritoneum. Hepato-gastroenterol.45(23), 1922–1929 (1998).
- Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer24(1), 1–21 (2020).
- Haverkamp L , BrenkmanHJ, RuurdaJP, TenKate FJ, van HillegersbergR. The oncological value of omentectomy in gastrectomy for cancer. J. Gastrointest. Surg.20(5), 885–890 (2016).
- Jongerius EJ , BoermaD, SeldenrijkKAet al. Role of omentectomy as part of radical surgery for gastric cancer. Br. J. Surg.103(11), 1497–1503 (2016).
- Noh SH , HyungWJ, CheongJ. Minimally invasive treatment for gastric cancer: approaches and selection process. J. Surg. Oncol.90(3), 188–193 (2005).
- Kurokawa Y , DokiY, MizusawaJet al. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): a Phase 3, open-label, randomised controlled trial. Lancet Gastroenterol. Hepatol.3(7), 460–468 (2018).
- National Comprehensive Cancer Network. (NCCN) . Clinical Practice Guidelines in Oncology. Gastric Cancer, Version 2 (2021). https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- Hagiwara A , TakahashiT, SawaiKet al. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res.53(3), 687–692 (1993).
- Kim M-C , KimK-H, JungG, RattnerDW. Comparative study of complete and partial omentectomy in radical subtotal gastrectomy for early gastric cancer. Yonsei Med. J.52(6), 961–966 (2011).
- Dindo D , DemartinesN, ClavienP-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg.240(2), 205–213 (2004).
- Yu J , HuangC, SunYet al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer. JAMA321(20), 1983–1992 (2019).