Abstract
Aims: This study describes real-world outcomes of pretreated EGFR T790M-positive (T790M+) advanced non-small-cell lung cancer patients progressing after first- or second-generation tyrosine kinase inhibitors and receiving osimertinib, compared with T790M-negative (T790M-) patients. We have also described progression patterns and treatment sequences. Patients & methods: This is a retrospective multicenter Italian observational study including consecutive Caucasian patients referred between 2014 and 2018. Results: 167 patients were included. Median progression-free survival was 9.8 months (95% CI: 8.3–13.3) for T790M+ and 6.0 months (95% CI: 4.9–7.2) for T790M- patients, respectively. Median overall survival was 20.7 months (95% CI: 18.9–28.4) for T790M+ and 10.6 months (95% CI: 8.6–23.6) for T790M- patients, respectively. The T790M mutation correlated with absence of new sites of disease. After progression, most T790M+ patients continued osimertinib, whereas most T790M- patients received a different treatment line. Conclusion: Better outcomes were shown in patients receiving osimertinib. A more limited progression pattern for T790M+ was suggested.
Lay abstract
Osimertinib is an oral drug that inhibits the growth of non-small-cell lung cancer (NSCLC) tumors with a specific mutation in EGFR. Osimertinib is given to patients with advanced EGFR-mutant NSCLC as initial therapy or after the failure of prior first- or second-generation tyrosine kinase inhibitors in patients who develop the EGFR T790M resistance mutation. Real-world data about the efficacy of EGFR-mutant NSCLC patients receiving osimertinib are needed to confirm the findings of large randomized clinical trials. Most real-world studies have investigated outcomes in Asian populations. This study aims to describe outcomes in EGFR T790M-positive patients receiving osimertinib after the failure of first- or second-generation tyrosine kinase inhibitors, compared with T790M-negative patients receiving a systemic treatment, in a Caucasian population. In addition, the study aims to describe how the disease spreads once it starts progressing again and any subsequent treatment lines. 167 patients were included. The results of this study suggest that EGFR T790M-positive patients receiving osimertinib as second- or further-line treatment had better outcomes and a more limited progression compared with T790M-negative cases.
Lung cancer is the leading cause of cancer-related death [Citation1]. In the Caucasian population, EGFR activating mutations are found in about 15% of advanced non-small-cell lung cancer (aNSCLC) patients [Citation2, Citation3]. The most frequent are exon 19 in-frame deletions (50%) and exon 21 L858R point mutation (40%) [Citation4, Citation5]. Several Phase III clinical trials have demonstrated the superior efficacy of first- and second-generation EGFR tyrosine kinase inhibitors (1–2gen TKIs) compared with first-line platinum-based chemotherapy in this subpopulation [Citation6–12].
After about 9–15 months of 1–2gen TKI treatment, acquired resistance occurs, causing disease progression. The most common mechanism of acquired resistance is the EGFR exon 20 T790M mutation, detectable in about 50% of rebiopsy samples [Citation13, Citation14].
Osimertinib, a third-generation EGFR TKI, targets activating mutations as well as the T790M mutation. This compound irreversibly binds to the C797 residue in the EGFR mutant protein ATP-binding pocket, sparing wild-type EGFR. The Phase III AURA3 trial compared osimertinib with platinum–pemetrexed chemotherapy in previously treated, EGFR acquired T790M mutation-positive (T790M+) aNSCLC patients, showing a superior median progression-free survival (mPFS) compared with chemotherapy (10.1 vs 4.4 months; hazard ratio [HR]: 0.30, 95% CI: 0.23–0.41; p < 0.001), as well as a higher overall response rate (ORR) for osimertinib (71% vs 31%) [Citation15]. Osimertinib was associated with a better safety profile and lower rate of adverse events leading to permanent discontinuation than platinum–pemetrexed [Citation15]. CNS ORR was 70% with osimertinib and 31% with chemotherapy [Citation16]. These results led to the US FDA and the EMA approval of osimertinib for acquired EGFR T790M+ aNSCLC patients progressing on or after 1–2gen TKIs. In the AURA3 trial, median overall survival (mOS) was not significantly different between the osimertinib arm and chemotherapy (26.8 vs 22.5 months), likely due to a high crossover rate (71%) [Citation17].
More recently, the Phase III FLAURA trial compared standard first-line EGFR TKIs (gefitinib or erlotinib) with osimertinib in previously untreated aNSCLC patients with EGFR exon 19 deletion or L858R mutation. FLAURA reported prolonged mPFS in patients treated with first-line osimertinib (18.9 vs 10.2 months; HR: 0.46; 95% CI: 0.37–0.57; p < 0.001) compared with those receiving gefitinib or erlotinib [Citation18]. Osimertinib was also associated with a lower rate of grade ≥3 adverse events compared with gefitinib or erlotinib (34% vs 45%) [Citation18]. Thus osimertinib received approval as first-line therapy for patients with EGFR-mutant aNSCLC, regardless of T790M status. CNS ORR in FLAURA was superior for patients treated with osimertinib compared with those receiving gefitinib or erlotinib (91% vs 68%) [Citation19]. FLAURA overall survival (OS) results were finally published, confirming the superior efficacy of first-line osimertinib compared with gefitinib or erlotinib (mOS 38.6 vs 31.8 months; HR: 0.80; 95% CI: 0.64–1.00; p = 0.046), with a crossover rate of 31% [Citation20].
Platinum-based chemotherapy is currently the standard of care in patients progressing to 1–2gen TKIs in T790M-negative (T790M-) patients and in patients experiencing osimertinib failure [Citation21]. The use of osimertinib as front-line therapy is supported by the FLAURA OS data [Citation20], the real-life observation that up to 30–40% of patients are not able to receive subsequent therapies after first-line 1–2gen TKIs due to poor performance status (PS) or death [Citation22–24] and finally the lower toxicity rate produced by osimertinib [Citation20]. Nevertheless, other authors still claim a role for sequential use of early-generation TKIs followed by osimertinib [Citation25]. Once available, the APPLE trial result may help to clarify this clinical question [Citation26].
Previous analysis of the pattern of recurrence with early-generation TKIs reported a more aggressive tumor behavior in T790M+ patients, characterized by more new sites of disease compared with negative cases [Citation27]. Several studies investigated the efficacy and safety of osimertinib in the real world in T790M+ patients among Asian [Citation28–33], Caucasian [Citation34–38] and both populations [Citation39–41]. However, few data about progression patterns with osimertinib are available in T790M+ patients, and previous studies did not include a T790M- control group [Citation30, Citation33, Citation34, Citation36, Citation37]. Real-life data on progression pattern and on further treatments after the failure of post-TKI treatment could help measure osimertinib impact on the natural history of EGFR-mutant aNSCLC and improve clinical decision-making.
This study describes the diagnostic–therapeutic pathway of pretreated EGFR-mutant aNSCLC patients receiving osimertinib or chemotherapy after the failure of 1–2gen TKIs, based on T790M status and according to clinical practice. The primary end point was to describe treatment outcomes in terms of ORR, PFS and OS; as secondary aims, we explored progression patterns and the sequence of further systemic treatments.
Patients & methods
Study design & patients
This is a retrospective observational study collecting a consecutive series of patients referred to six Italian centers between 2014 and 2018. The main inclusion criteria were: histological diagnosis of aNSCLC (stages IIIB and IV), presence of EGFR activating mutation, radiological or clinical progression to previous systemic treatment with 1–2gen EGFR TKI (erlotinib, gefitinib or afatinib at standard doses) and the presence of at least one liquid biopsy or tissue rebiopsy before study entry as confirmation of T790M status.
This study was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki. An informed consent form was submitted to living patients, according to Regulation (EU) 2016/679 of the European Parliament and of the Council on personal data protection. For patients who were dead or lost to follow-up at the time of study enrollment, we used the Italian Data Protection Authority Authorization 9/2016 on ‘privacy protective rules for recording clinical data for research and study purposes’.
Baseline patient data collected included: gender, age, smoking status, Charlson comorbidity index, histology, type of EGFR activating mutation, stage at diagnosis according to the TNM Classification of Malignant Tumors (eighth edition), baseline metastatic sites, disease-related symptoms, Eastern Co-operative Oncology Group PS, type of previous 1–2gen TKI and best response to 1–2gen TKI.
Disease progression (PD) was defined as the date of progression upon therapy with osimertinib (for T790M+ patients) or systemic therapy (for T790M- patients). Data recorded at PD were: progression type, type and number of metastatic sites, disease-related symptoms, subsequent systemic or locoregional treatment, subsequent rebiopsy, date of treatment discontinuation and date of death or last follow-up.
Patients received second-line treatment with osimertinib (T790M+), 80 mg orally daily, or best investigator chemotherapy choice (T790M-). In case of baseline T790M negative results, a liquid or tissue biopsy was subsequently performed after second-line chemotherapy and, in case of T790M positivity, osimertinib was prescribed in third or further lines. All patients receiving osimertinib in second or further lines are included in the outcome analysis within the same T790M+ group.
Tumor assessment was performed through chest and abdomen CT scan with iodine contrast. Brain CT scan with iodine contrast or nuclear magnetic resonance with gadolinium contrast were performed at baseline and then when clinically indicated (baseline brain metastases or clinical suspicion of CNS disease spread). Assessment was performed every 2–3 months as per clinical practice. Response to treatment was classified according to Response Evaluation Criteria in Solid Tumors version 1.1. Three different progression patterns were defined: solitary (appearance or growth of one lesion), oligo (progression in up to three lesions in two organs) and systemic progression (progression in more than three lesions).
PFS was measured as the time between the beginning of osimertinib or second-line chemotherapy and the radiological and/or clinical evidence of progression, or death; time to treatment failure (TTF) was measured from the start of osimertinib therapy to treatment cessation for any reason. OS was defined as the time between the beginning of osimertinib or other systemic therapy until death from any cause.
EGFR mutational analysis
At the time of diagnosis, EGFR mutations were identified in exons 18–21. The analysis was performed on tumor tissue according to standard clinical practice. At the time of 1–2gen TKI progression, plasma samples were collected for liquid biopsy to detect the EGFR T790M mutation in circulating tumor DNA. For T790M- patients only, a second tissue biopsy was achieved if feasible and accepted by the patient.
For analyses on tissue sample, tumor DNA was extracted from formalin-fixed, paraffin-embedded tumor slices using the QIAamp® DNA Micro kit or QIAamp® DNA FFPE kit (Qiagen, Hilden, Germany); DNA sequencing was carried out with Sanger sequencing, pyrosequencing and PCR-based methods (Easy® EGFR kit, Diatech Pharmacogenetics, Jesi, Italy; cobas® EGFR Mutation Test v2, Roche, Basel, Switzerland), mass spectrometry-based methods (Sequenom Mass Array, Diatech Pharmacogenetics), or next-generation sequencing (Ion Torrent, Oncomine™ panel, Thermo Fisher Scientific, MA, USA). For liquid biopsy, cell-free DNA was isolated from 2 ml of plasma using the cobas® cf-DNA Sample Preparation kit (Roche) and analyzed with the techniques described above.
Statistical analysis
Statistical analyses were performed using R statistical software (The R Foundation for Statistical Computing, Vienna, Austria). The chi-square, Mann–Whitney or Fisher exact test and multiple logistic regression were applied to correlate clinical features with T790M status and the Kaplan–Meier estimator was used to evaluate mPFS, median TTF (mTTF) and median OS (mOS). The log-rank test and Cox regression model were applied. Statistical significance was set at p = 0.05.
Results
Study population
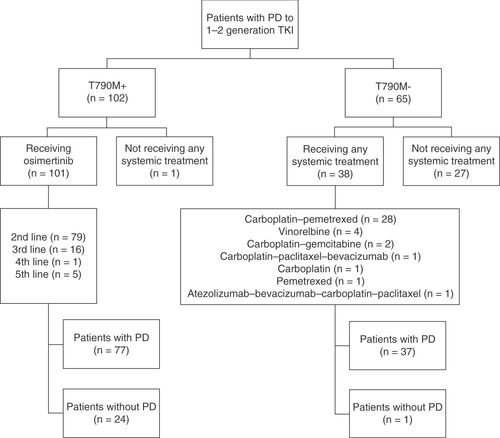
One hundred and sixty seven patients were consecutively included across six cancer centers in Italy. Of these, 102 were included in the T790M+ group and 65 in the T790M- group. Among the T790M+ patients, 101 (99%) received osimertinib, while one (1%) did not receive any further treatment. Among the T790M- patients, 38 (58.5%) received a further line of treatment, while 27 (41.5%) did not (p < 0.001). Median follow-up from the start of postprogression treatment was 14.1 months (range: 1.0–59.7). depicts patients’ flow and study design.
Baseline characteristics
Baseline characteristics were similar between the two subgroups defined according to T790M status. Most patients were female, had never smoked, had stage IV adenocarcinoma and Eastern Co-operative Oncology Group PS 0–1 at diagnosis. EGFR exon 19 deletion (p = 0.025), response to 1–2gen EGFR TKI (p = 0.013) and bone metastases at diagnosis (p = 0.019) were significantly more frequent in T790M+ patients. Multivariate analysis confirmed that response to 1–2gen EGFR TKI was associated with T790M mutation (p = 0.046) ().
Table 1. Baseline clinical features according to EGFR T790M mutation status.
Treatment outcomes
The 101 T790M+ patients received osimertinib, 79 (78.2%) of them as a second-line treatment and the remaining 22 (21.8%) after one or more lines of systemic treatment because of delayed T790M acquisition (). Conversely, the 38 T790M- patients received chemotherapy, of whom 32 (84.2%) were given a platinum combination and 6 (15.8%) a single-agent chemotherapy ().
ORR was higher for T790M+ patients treated with osimertinib compared with patients in the T790M- group (62.4 vs 47.4%), even though the difference did not attain statistical significance, whereas the disease control rate (DCR) was similar between the two groups (80.2 vs 76.3%; Supplementary Table 1).
At data cutoff (17 May 2020), 77 of 101 (76.2%) T790M+ patients and 37 of 38 (97.4%) T790M- patients had a progression event.
The mPFS was 9.8 months (95% CI: 8.3–13.3) for T790M+ and 6.0 months (95% CI: 4.9–7.2) for T790M- patients (log-rank test p = 0.00017; HR: 0.47; 95% CI: 0.32–0.70; p = 0.0004) (). The mPFS was similar between patients receiving osimertinib as a second-line therapy or as a further-line treatment (9.4 vs 9.7 months) (Supplementary Figure 1A). At univariate analysis, T790M- status, baseline brain metastases, stable disease (SD)/progressive disease (PD) as best response to previous TKI and SD/PD as best response to treatment were associated with a higher risk of progression. Multivariate analysis confirmed that T790M- status, baseline brain metastases and SD/PD as best response to treatment were significant covariates associated with a higher risk of progression (Supplementary Table 2).
(A) Progression-free survival. (B) Time to osimertinib treatment failure. (C) Overall survival.
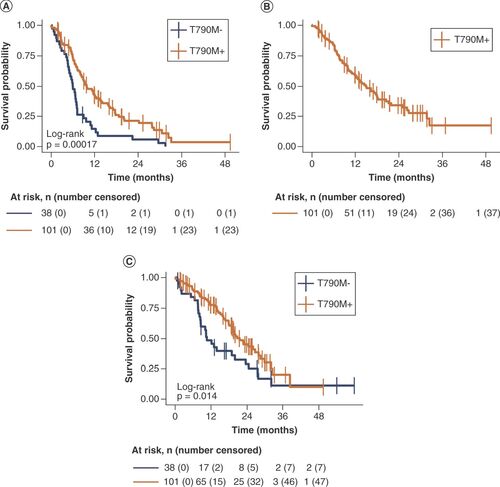
Osimertinib mTTF was 14.9 months (95% CI: 11.8–20.5) (). For T790M- patients receiving chemotherapy, TTF was not calculated because treatment beyond progression was not an option.
At data cutoff, 53 of 101 (52.5%) T790M+ patients and 29 of 38 (76.3%) T790M- patients had died.
The mOS was significantly higher in the T790M+ group (20.7 months; 95% CI: 18.9–28.4) compared with the T790M- group (10.6 months; 95% CI: 8.6–23.6; log-rank test p = 0.014; HR: 0.57; 95% CI: 0.36–0.90; p = 0.02) ().
The mOS was similar between patients receiving osimertinib as a second-line therapy or as a further-line treatment (20.7 vs 18.0 months; Supplementary Figure 1B).
At univariate analysis, EGFR T790M mutation and baseline EGFR exon 19 deletion were associated with a lower risk of death, whereas baseline bone metastases, baseline brain metastases and SD/PD as best response to treatment were significantly associated with a higher risk of death. Multivariate analysis confirmed EGFR T790M mutation as a positive prognostic factor, with baseline bone metastases, baseline brain metastases and SD/PD as best response to treatment as significant covariates associated with a higher risk of death (Supplementary Table 3).
Progression pattern with osimertinib compared with chemotherapy
We explored the progression pattern with osimertinib in T790M+ compared with chemotherapy in T790M- patients. In ten cases, complete information about progression pattern was not available.
At univariate analysis, when information on clinical and radiological progression was available (n = 104; T790M+, n = 71; T790M-, n = 33), the T790M mutation was significantly correlated with the absence of new sites of disease (p = 0.048). There was a trend toward more frequent liver and bone progression in T790M+ patients and toward more systemic progression (compared with oligo or isolated progression), thoracic, distant nodes, pleura, brain, adrenal, soft tissue, peritoneum and pericardium progression in T790M- patients, although these trends did not reach statistical significance. The correlation between T790M mutation and the presence of new sites of disease was not confirmed at multivariate analysis ( & Supplementary Figure 2).
Table 2. Progression pattern with osimertinib or chemotherapy according to EGFR T790M mutation status.
A trend toward new distant nodes, brain, soft tissue, lung, adrenal, pleura and kidney progression was shown for T790M+ patients, whereas new bone, peritoneum, liver and pericardium progression were more frequent in T790M- patients, though the difference did not reach statistical significance ().
Further treatments & rebiopsy rate
Of the 77 T790M+ patients whose disease progressed with osimertinib given as second- or later-line therapy, 33 (42.8%) continued the drug beyond progression, 28 patients (36.4%) switched to a new systemic therapy and 16 (20.8%) did not receive any further treatment ().
Table 3. Further systemic and locoregional therapies after osimertinib or chemotherapy according to EGFR T790M mutation status.
Among patients with progression with osimertinib given as second-line treatment (n = 58), 24 (41.4%) continued osimertinib beyond progression, 23 (39.7%) switched to a new systemic therapy and 11 patients (18.9%) did not receive any further treatment. Among patients with progression with osimertinib given as third- or further-line treatment (n = 19), nine (47.4%) continued osimertinib beyond progression, five (26.3%) switched to a new systemic therapy and five patients (26.3%) did not receive any further treatment. The proportion of patients receiving no further treatments was similar between the two groups (p = 0.523).
After progression to chemotherapy, 25 of 37 (67.6%) T790M- patients received a further systemic therapy line, whereas 12 patients (32.4%) did not. Twenty-one of 28 (75%) T790M+ patients received a platinum combination therapy, while 12 of 25 (48%) T790M- patients received docetaxel ().
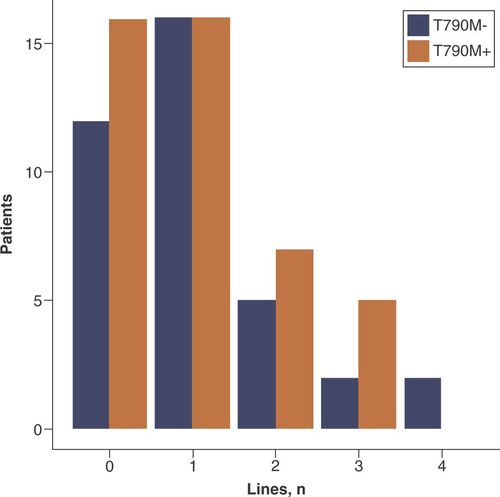
The median number of further systemic treatment lines was one in both groups (Mann–Whitney test; p = 0.794; ).
A similar proportion of patients (30 of 77 T790M+ patients [39.0%] vs 11 of 37 T790M- patients [29.7%]; p = 0.451) received local ablative therapy after progression ().
Data on rebiopsy after progression with osimertinib or chemotherapy was available in 40 out of 77 T790M+ patients and in 22 out of 37 T790M- patients.
A significantly higher proportion of T790M- patients underwent rebiopsy (9 of 40 [22.5%] T790M+ and 15 of 22 [68.2%] T790M-; p = 0.001). Liquid biopsy was performed in four of nine (44.4%) T790M+ patients and in eight of 15 (53.4%) T790M- patients. Tissue biopsy was performed in four of nine (44.4%) T790M+ patients and in two of 15 (13.3%) T790M- patients. One of nine (11.2%) patients in the T790M+ group and five of 15 (33.3%) in the T790M- group underwent both liquid and tissue rebiopsy.
Discussion
This real-world, multicenter study has shown treatment outcomes and progression pattern of pretreated patients with EGFR mutation-positive NSCLC receiving osimertinib or chemotherapy as second- or further-line systemic therapy.
The activity of osimertinib in our Caucasian T790M+ population is consistent with the pooled analysis of two Phase II studies, AURA extension and AURA2, and the Phase III AURA3 trial data (ORR 66–71% and disease control rate 91–93%) [Citation15, Citation42].
Reported mPFS was 9.9 months in the AURA extension and AURA2 studies pooled analysis, and 10.1 months in AURA3 for patients treated with osimertinib [Citation15, Citation42]. Similarly, we reported a mPFS of 9.8 months for patients treated with osimertinib. Conversely, mOS in patients receiving osimertinib in our study (20.7 months) was slightly shorter than in the experimental arm of AURA3 (26.8 months); this could be related to the higher selection of patients eligible for the clinical trial, potentially impacting on subsequent treatment lines [Citation15]. The main differences in patients’ clinical characteristics were related to ethnicity (predominantly Asian in AURA trials) and the proportion of patients treated with afatinib (31.7% in our population vs 7% in AURA3 and 18% in AURA extension and AURA2 pooled analysis) [Citation15, Citation42].
Moreover, treatment regimens after disease progression in real life compared with those in randomized trials may impact survival data. In our analysis, 42.8% of T790M+ patients continued osimertinib beyond progression, 36.4% switched to a new systemic therapy and 20.8% did not receive any further treatment. Likewise, continuing osimertinib beyond progression was the most common treatment strategy in pivotal trials (73% of patients in the pooled AURA Phase II analysis and 64% in AURA3) [Citation15, Citation42]. A higher proportion of patients receiving subsequent anticancer therapy after osimertinib failure in the Phase II AURA pooled analysis (69%) and AURA3 (59.2%) was reported compared with our study (36.4%) [Citation15, Citation42]. Again, this could be related to more selective inclusion criteria in clinical trials.
Other real-world studies have recently reported outcomes and response rates in line with pivotal trials, even though the added value of our study is the inclusion of a T790M- group [Citation28–32, Citation34–36, Citation38–41, Citation43]. In real-world studies, a variable rate of patients received a subsequent treatment after osimertinib failure [Citation29, Citation34–37]. The study by Auliac et al. included only patients aged >80 years and this could explain the lower proportion of patients receiving subsequent therapies after osimertinib [Citation35], whereas the higher rate reported by Le et al. may be explained by selection bias [Citation40]. As in our experience, after osimertinib, most patients received chemotherapy [Citation34–36], according to guidelines [Citation21]. One study reported immune checkpoint inhibitors as the most frequent therapy after osimertinib, but included patients from 2011 [Citation40].
Our study showed no statistically significant difference in treatment outcome when osimertinib was prescribed in different treatment lines. Consistent with our findings, a similar mPFS and mOS was reported for patients receiving osimertinib as a second, third or further line of treatment in the AURA extension and AURA2 pooled analysis [Citation42]. Three real-world studies reported no statistically significant difference between outcomes for osimertinib administered as a second or as a further line of treatment [Citation29, Citation34, Citation35]. On the basis of these observations, it is tempting to suggest a role of tissue rebiopsy or repeated liquid biopsy in T790M- patients at every disease progression event in order to detect T790M mutation.
Furthermore, regarding progression pattern, in the pooled analysis of AURA extension and AURA2 trials, 41% of patients had disease progression with new lesions, whereas in AURA3 this figure was 26% (compared with 45% receiving chemotherapy) [Citation15, Citation42]. Consistent with these findings, we reported that 34% of patients treated with osimertinib had new sites of disease, defining a population with better disease control and a lower risk of disease spread with systemic treatment compared with T790M- patients (58.8%). In our records, at univariate analysis we revealed a significant correlation between T790M mutation and the absence of new sites of disease compared with the T790M- group.
In several studies investigating progression patterns with osimertinib, progression in T790M+ patients was characterized by local and oligoprogression compared to systemic progression [Citation30, Citation33, Citation36]. Oligoprogression, although defined with different criteria, occurred in 23–72% of the patients, while systemic progression occurred in 23–32% of the patients [Citation30, Citation33, Citation36]. Accordingly, we reported 22.5% isolated progression, 22.5% oligoprogression and 55% systemic progression with osimertinib. Two studies showed that systemic progression was associated with a worse outcome [Citation30, Citation33], whereas in another study mOS was not statistically different between the oligoprogression and systemic progression groups [Citation36].
Some papers focused on disease progression sites. However, all these studies lacked a control T790M- group; therefore an association with the T790M mutation and, similarly, with osimertinib treatment, could not be statistically established. One study reported lung, CNS, bone and liver as the most frequent disease progression sites in T790M+ patients and CNS, bone and liver as the most frequent metastatic sites [Citation34], while another study confirmed that the main sites of progression were lung, bone, lymph nodes, brain, pleura and liver [Citation36].
Moreover, in our case series, 39.0% of patients received a local ablative treatment after osimertinib progression and 29.7% after post-TKI systemic chemotherapy in the T790M- group. Similarly, in published real-world data, 36.8–62% of the patients receiving osimertinib continued the drug beyond disease progression, in association with a local ablative therapy in 14–69% of patients [Citation34–37, Citation40, Citation41].
Finally, we observed a significantly lower rebiopsy rate for T790M+ patients compared with T790M- patients after progression on post-TKI systemic therapy. Osimertinib resistance mechanisms have been described for AURA3 and FLAURA patients [Citation44, Citation45]. Consequently, ongoing trials are exploring the application of a personalized therapy approach even after osimertinib progression [Citation46–49]. This should prompt clinicians to rebiopsy patients even in the T790M+ setting.
Conclusion
This real-life study confirms the efficacy of osimertinib in the real world when used as a second or further line of treatment. Thus serial rebiopsy across treatment lines in T790M- patients should be considered. A more limited progression pattern to post-TKI progression treatment was suggested, although further validation is needed. The availability and feasibility of postprogression treatment in T790M+ and T790M- patients may impact on OS.
Real-world data on outcomes of EGFR-mutant Caucasian patients with non-small-cell lung cancer receiving systemic therapy after failure of early-generation EGFR tyrosine kinase inhibitors are needed.
This study confirmed the activity and efficacy of osimertinib in the real world in second and further treatment lines, suggesting the relevance of serial liquid rebiopsy at every disease progression in order to detect EGFR T790M mutation.
A lower risk for disease spread was suggested for osimertinib compared with chemotherapy, although further validation is needed.
A higher risk of death was reported for patients with T790M negative status, baseline brain and bone metastases and stable disease or progressive disease as best response to treatment.
After progression with osimertinib, most T790M-positive patients continued the drug, whereas after progression with chemotherapy, most T790M-negative patients received a further systemic therapy line.
The median number of further systemic treatment lines was one in both T790M-positive and T790M-negative patients.
A significantly higher proportion of T790M-negative patients underwent rebiopsy compared with T790M-positive patients.
Different therapeutic pathways in T790M-positive and T790M-negative patients may impact on overall survival.
Author contributions
A Dal Maso, M Lorenzi, A Ferro: conception and design; data acquisition, analysis and interpretation; manuscript draft, critical revision; final approval; agreement to be accountable for all aspects of the work. S Pilotto, F Cecere, A Follador, V Polo, A Del Conte, G Sartori, M Giavarra, D Scattolin, G De Maglio, J Menis: data acquisition; manuscript draft, critical revision; final approval; agreement to be accountable for all aspects of the work. S Indraccolo, S Frega, L Bonanno, F Calabrese: data acquisition, analysis and interpretation; manuscript draft, critical revision; final approval; agreement to be accountable for all aspects of the work. V Guarneri, PF Conte: manuscript draft, critical revision; final approval; agreement to be accountable for all aspects of the work. G Pasello: conception and design; data acquisition, analysis and interpretation; manuscript draft, critical revision; final approval; agreement to be accountable for all aspects of the work.
Additional file 1:
Download PDF (233.7 KB)Additional file 1:
Download PDF (85.1 KB)Supplemental table 1. Treatment response in T790M positive and T790M negative patients
Download MS Word (13.1 KB)Supplemental table 2. Cox regression univariate and multivariate analysis including all covariates potentially impacting on progression free survival
Download MS Word (16 KB)Supplemental table 3. Cox regression univariate and multivariate analysis including all covariates potentially impacting on overall survival
Download MS Word (14.8 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2021-0356
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Bray F , FerlayJ, SoerjomataramI, SiegelRL, TorreLA, JemalA. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.68(6), 394–424 (2018).
- Douillard J-Y , OstorosG, CoboMet al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a Phase-IV, open-label, single-arm study. Br. J. Cancer110(1), 55–62 (2014).
- Rosell R , MoranT, QueraltCet al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med.361(10), 958–967 (2009).
- Shigematsu H , LinL, TakahashiTet al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl Cancer Inst.97(5), 339–346 (2005).
- Wu J-Y , WuS-G, YangC-Het al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin. Cancer Res.14(15), 4877–4882 (2008).
- Rosell R , CarcerenyE, GervaisRet al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised Phase 3 trial. Lancet Oncol.13(3), 239–246 (2012).
- Mitsudomi T , MoritaS, YatabeYet al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised Phase 3 trial. Lancet Oncol.11(2), 121–128 (2010).
- Zhou C , WuY-L, ChenGet al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, Phase 3 study. Lancet Oncol.12(8), 735–742 (2011).
- Sequist LV , YangJC-H, YamamotoNet al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol.31(27), 3327–3334 (2013).
- Wu Y-L , ZhouC, HuC-Pet al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised Phase 3 trial. Lancet Oncol.15(2), 213–222 (2014).
- Mok TS , WuY-L, ThongprasertSet al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med.361(10), 947–957 (2009).
- Maemondo M , InoueA, KobayashiKet al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N. Engl. J. Med.362(25), 2380–2388 (2010).
- Sequist LV , WaltmanBA, Dias-SantagataDet al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med.3(75), 75ra26–75ra26 (2011).
- Yun C-H , MengwasserKE, TomsAVet al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl Acad. Sci. USA105(6), 2070–2075 (2008).
- Mok TS , WuY-L, AhnM-Jet al. Osimertinib or platinum–pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med.376(7), 629–640 (2017).
- Wu YL , AhnMJ, GarassinoMCet al. CNS efficacy of osimertinib in patients with T790M-positive advanced non–small-cell lung cancer: data from a randomized Phase III trial (Aura3). J. Clin. Oncol.36(26), 2702–2709 (2018).
- Wu Y-L , MokTSK, HanJ-Yet al. Overall survival (OS) from the AURA3 Phase III study: osimertinib vs platinum-pemetrexed (plt-pem) in patients (pts) with EGFR T790M advanced non-small cell lung cancer (NSCLC) and progression on a prior EGFR-tyrosine kinase inhibitor (TKI). Ann. Oncol.30, ix158 (2019).
- Soria J-C , OheY, VansteenkisteJet al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N. Engl. J. Med.378(2), 113–125 (2018).
- Reungwetwattana T , NakagawaK, ChoBCet al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J. Clin. Oncol.36(33), 3290–3297 (2018).
- Ramalingam SS , VansteenkisteJ, PlanchardDet al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med.382(1), 41–50 (2020).
- Planchard D , PopatS, KerrKet al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.29(Suppl. 4), iv192–iv237 (2018).
- Roeper J , FalkM, TiemannMet al. Risk of not receiving 2nd line therapy is high in EGFR mt+ pts: real world data of certified lung cancer centers on treatment sequence in EGFR mt+ pts. J. Clin. Oncol. 36(Suppl. 15), e21220–e21220 (2018).
- Chiang A , FernandesA, PavilackMet al. Real world biomarker testing and treatment patterns in patients with advanced NSCLC receiving EGFR-TKIs. J. Thorac. Oncol.13(10), S410–S411 (2018).
- Gray JE , ThakrarB, SunP, MaclachlanS, ChehabN, PotterD. Treatment (tx) patterns in patients (pts) with lung cancer starting 1st or 2nd generation (1G/2G) EGFR-TKI: a US insurance claims database analysis. Ann. Oncol.29, ix156–ix157 (2018).
- Hochmair MJ , MorabitoA, HaoDet al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Future Oncol.15(25), 2905–2914 (2019).
- Remon J , MenisJ, HasanBet al. The APPLE trial: feasibility and activity of AZD9291 (osimertinib) treatment on positive plasma T790M in EGFR-mutant NSCLC patients. EORTC 1613. Clin. Lung Cancer18(5), 583–588 (2017).
- Dal Maso A , LorenziM, RocaEet al. Clinical features and progression pattern of acquired T790M-positive compared with T790M-negative EGFR mutant non-small-cell lung cancer: catching tumor and clinical heterogeneity over time through liquid biopsy. Clin. Lung Cancer21(1), 1-14.e3 (2020).
- Cao Y , QiuX, XiaoG, HuH, LinT. Effectiveness and safety of osimertinib in patients with metastatic EGFR T790M-positive NSCLC: an observational real-world study. PLoS ONE14(8), e0221575 (2019).
- Mu Y , XingP, HaoX, WangY, LiJ. Real-world data of osimertinib in patients with pretreated non-small cell lung cancer: a retrospective study. Cancer Manag. Res.11, 9243–9251 (2019).
- Mu Y , HaoX, YangKet al. Clinical modality of resistance and subsequent management of patients with advanced non-small cell lung cancer failing treatment with osimertinib. Target. Oncol.14(3), 335–342 (2019).
- Oh DK , JiWJ, KimWSet al. Efficacy, safety, and resistance profile of osimertinib in T790M mutation-positive non-small cell lung cancer in real-world practice. PLoS ONE14(1), e0210225 (2019).
- Igawa S , OnoT, KasajimaMet al. Impact of EGFR genotype on the efficacy of osimertinib in EGFR tyrosine kinase inhibitor-resistant patients with non-small cell lung cancer: a prospective observational study. Cancer Manag. Res.11, 4883–4892 (2019).
- Li H , YangH, WangK, WuX, KongF, LvD. P2.13-23 osimertinib treatment result of plasma T790M positive in different clinical failure modes after first-line EGFR TKI for EGFR mutant NSCLC. J. Thorac. Oncol.13(10), S807 (2018).
- Auliac JB , PérolM, PlanchardDet al. Real-life efficacy of osimertinib in pretreated patients with advanced non-small cell lung cancer harboring EGFR T790M mutation. Lung Cancer127, 96–102 (2019).
- Auliac JB , SaboundjiK, AndreMet al. Real-life efficacy of osimertinib in pretreated octogenarian patients with T790M-mutated advanced non-small cell lung cancer. Target. Oncol.14(3), 307–314 (2019).
- Schmid S , KlingbielD, AeppliSet al. Patterns of progression on osimertinib in EGFR T790M positive NSCLC: a Swiss cohort study. Lung Cancer130, 149–155 (2019).
- Cortellini A , LeonettiA, CatinoAet al. Osimertinib beyond disease progression in T790M EGFR-positive NSCLC patients: a multicenter study of clinicians’ attitudes. Clin. Transl. Oncol.22(6), 844–851 (2020).
- Eide IJ , HellandÅ, BorrisovaSet al. P2.03-037 osimertinib in relapsed EGFR-mutated, T790M-negative non-small cell lung cancer (NSCLC) patients: results from the TREM study. J. Thorac. Oncol.12(11), S2141–S2142 (2017).
- De Marinis F , WuYL, DeCastro Get al. ASTRIS: a global real-world study of osimertinib in >3000 patients with EGFR T790M positive non-small-cell lung cancer. Future Oncol.15(26), 3003–3014 (2019).
- Le X , PuriS, NegraoMVet al. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin. Cancer Res.24(24), 6195–6203 (2018).
- So YJ , FraserA, RivallandG, McKeageM, SullivanR, CameronL. Osimertinib in NSCLC: real-world data from New Zealand. JTO Clin. Res. Reports1(2), 100022 (2020).
- Ahn M , TsaiC, ShepherdFAet al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: long-term follow-up from a pooled analysis of 2 Phase 2 studies. Cancer125(6), 892–901 (2019).
- Yoshimura A , YamadaT, OkuraNet al. Clinical characteristics of osimertinib responder in non-small cell lung cancer patients with egfr-t790m mutation. Cancers (Basel)11(3), 365 (2019).
- Papadimitrakopoulou VA , WuY-L, HanJ-Yet al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann. Oncol.29(Suppl. 8), Abstract LBA51 (2018).
- Ramalingam SS , ChengY, ZhouCet al. LBA50 Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the Phase III FLAURA study. Ann. Oncol.29(Suppl. 8), Abstract LBA50 (2018).
- Leonetti A , SharmaS, MinariR, PeregoP, GiovannettiE, TiseoM. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer121(9), 725–737 (2019).
- Piper-Vallillo AJ , SequistLV, PiotrowskaZ. Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: a review. J. Clin. Oncol.38(25), 2926–2936 (2020).
- Oxnard GR , YangJCH, YuHet al. TATTON: a multi-arm, Phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann. Oncol.31(4), 507–516 (2020).
- Oxnard GR , CantariniM, FrewerPet al. SAVANNAH: a Phase II trial of osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-driven (MET +), locally advanced or metastatic non-small cell lung cancer (NSCLC), following disease progression on osimertinib. J. Clin. Oncol. 37(Suppl. 15), TPS9119–TPS9119 (2019).