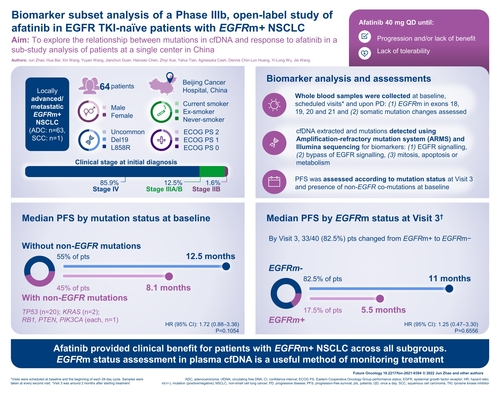
Abstract
Aim: To explore the relationship between mutations in cfDNA and response to afatinib. Patients & methods: In total, 64 patients from one Chinese site with locally advanced/metastatic EGFRm+ non-small-cell lung cancer, who received afatinib 40 mg once daily, were included. Results: Overall, 33 (82.5%) patients became EGFRm- by visit 3; median progression-free survival was longer in these patients vs those who did not (11.0 vs 5.5 months). Progression-free survival was shorter in 42 (45.2%) patients with non-EGFR co-mutations at baseline vs those without (8.1 vs 12.5 months). Neither difference was significant. Conclusion: Afatinib provided clinical benefit for patients with EGFRm+ non-small-cell lung cancer across all subgroups. EGFRm status assessment in plasma cfDNA is a useful method of monitoring treatment.
Plain language summary
We conducted a study in 64 Chinese patients with non-small-cell lung cancer to investigate the relationship between cancer mutations detected in the blood and the response to treatment with afatinib, which is known to be effective against EGFR mutations. Technology is now available to detect these mutations in the blood, as an alternative to obtaining and testing lung tissue samples. All 64 patients had EGFR mutations (and some patients had additional types of mutations) when afatinib was started (visit 1 in the study). By visit 3, most patients (82.5%) no longer had EGFR mutations detected in their blood, and these patients responded better to afatinib than those who still had EGFR mutations in their blood. Patients with additional types of mutations generally did not respond as well as those who had only EGFR mutations. Although results showed clinical benefit with afatinib using assessment of mutation status in the blood, statistical significance could not be shown due to the small size of the study.
Clinical Trial Registration: NCT01953913 (ClinicalTrials.gov)
Background
The prevalence of EGFRm+ non-small-cell lung cancer (NSCLC) is relatively high in eastern Asian populations [Citation1–5]. Estimates from a systematic review and meta-analysis found an overall pooled prevalence of EGFR mutations of 32.3%, ranging from 14.1% in Europe to 38.4% in China [Citation5]; however, one study found a prevalence of 63% among Chinese patients with lung adenocarcinoma [Citation4].
EGFR tyrosine kinase inhibitors (TKIs) are recommended as first-line treatment options in EGFRm+ NSCLC [Citation6–8]. Afatinib is a second-generation TKI that irreversibly blocks members of the ErbB family [Citation9]. This broader inhibition of receptors relative to first-generation EGFR TKIs may delay the development of acquired resistance [Citation10]. Acquisition of the EGFR T790M mutation is an important mechanism of resistance to afatinib [Citation11–13]. Development of the EGFR C797S mutation is also among other potential mechanisms of resistance to afatinib, although its influence on resistance is less than that of T790M [Citation11]. Afatinib is approved in China, the USA and the EU, as well as many other countries, for the treatment of EGFRm+ TKI-naive patients with locally advanced or metastatic NSCLC, and for the treatment of locally advanced or metastatic NSCLC of squamous histology progressing after platinum-based chemotherapy [Citation9,Citation14–16]. Afatinib is recommended as first-line treatment for patients with EGFRm+ NSCLC in Chinese and Pan-Asian guidelines [Citation6,Citation15]. In addition, afatinib has demonstrated efficacy in NSCLC patients harboring certain uncommon (as well as common) EGFR mutations [Citation17].
Tumor biomarker analysis is a crucial method of guiding treatment decisions and has become routine clinical practice. Current guidelines recommend that all patients with advanced probable or definitive adenocarcinoma should be tested for oncogenic drivers, and genetic testing for EGFR mutations and rearrangements of ALK and ROS1 are considered mandatory [Citation6,Citation8,Citation15,Citation16,Citation18]. However, more information is needed on how activating mutations change over the course of treatment in patients with EGFRm+ NSCLC. In addition, the role of co-mutations (i.e., non-EGFR mutations occurring in the same patient) in influencing treatment efficacy and outcomes is unclear. Liquid biopsy and analysis of cfDNA is an emerging and attractive technique. It overcomes problems associated with invasive tumor biopsies (e.g., tumor heterogeneity) and allows monitoring of the response to treatment and the onset of resistance [Citation19]. Highly sensitive methodologies are needed to identify cfDNA above the background germline DNA, and techniques such as next-generation sequencing and Amplification Refractory Mutation System have successfully been used to analyze cfDNA in patients with EGFRm+ NSCLC [Citation19].
A recent phase IIIb, multicenter, single-arm study (NCT01953913) evaluating afatinib 40 mg/day in 541 patients in five Asian countries with EGFR TKI-naive, locally advanced or metastatic EGFRm+ NSCLC revealed no unexpected safety findings, and only 3.8% of patients discontinued treatment due to afatinib-related adverse events. Median time to symptomatic progression (TTSP) was 14.0 months and median progression-free survival (PFS) was 12.1 months [Citation20].
This biomarker sub-study analysis of the phase IIIb ‘near real-world’ study aimed to explore the relationship between tumor mutation type and patients’ response to afatinib in terms of efficacy and the correlation between change in EGFR mutation status to patients’ response and tolerability.
Patients & methods
Study design & patients
The study was a phase IIIb, multicenter, open-label, single-arm trial with afatinib in a broad population of TKI-naive EGFRm+ Asian patients with locally advanced or metastatic NSCLC [Citation21]. The overall study was conducted at 34 sites in China, Hong Kong, India, Singapore and Taiwan. This biomarker analysis was only conducted in patients entering the study at the Beijing Cancer Hospital, China.
Inclusion criteria included: age ≥18 years, locally advanced or metastatic EGFRm+ NSCLC (including uncommon mutations) and Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–2. Exclusion criteria included: previous use of an EGFR TKI; use of any anti-cancer medication within 2 weeks; radiotherapy within 4 weeks (except for palliative treatment) and major surgery within 4 weeks of the first dose of afatinib; history or presence of cardiovascular abnormalities; and symptomatic brain metastases.
The initial regimen of afatinib was 40 mg once daily orally, which was continued until investigator-assessed tumor progression and/or lack of clinical benefit, or lack of tolerability. Afatinib could be continued beyond radiologic progression if the clinical investigator deemed that the patient would continue to benefit from treatment. The protocol allowed treatment interruptions and dose reductions with afatinib when necessary for management of treatment-related adverse events, as detailed in the Supplementary Information.
Ethics approval was obtained at the Beijing Cancer Hospital, China, as was the case at each of the other participating centers in the overall study. The study was approved by the Institutional Review Board or Independent Ethics Committee and conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, Good Clinical Practice and local laws. All patients provided written informed consent. Participants at the Beijing Cancer Hospital also provided written informed consent for blood sample collection for biomarker testing.
Biomarker analysis
Each cycle was 28 (-7/+2) days and patients visited the clinic at the start of each cycle. Whole blood samples (10 ml) were collected at scheduled visits (visits 1 [screening visit, during which baseline parameters were measured and patients received the first treatment dose], 3, 5, 7, 9, 11, 13 and every subsequent second visit during the treatment period); and at upon progressive disease (PD). Samples collected at visits 3, 7, 11, 13 and every subsequent second treatment were assessed for EGFR mutations in exons 18, 19, 20 and 21. DNA was extracted using a Circulating Nucleic Acid Kit (Qiagen, Shanghai, China) and mutations were detected using an Amplification Refractory Mutation System kit (Qiagen). Samples collected at visits 1, 5, 9 and at the PD visit (i.e., the visit where PD was first reported) were analyzed for somatic mutational changes associated with the study treatment. Using Illumina sequencing technology, up to 485 genes were analyzed for (but not limited to): biomarkers downstream of or associated with EGFR signaling, biomarkers indicating a bypass of EGFR signaling pathways and biomarkers related to mitosis, apoptosis or metabolism. These allowed the identification of potential biomarkers associated with efficacy of afatinib treatment and patient prognosis.
Assessments
Efficacy end points were assessed in an exploratory manner: TTSP as determined by investigator, PFS, objective response (OR) rate, best overall response; duration of disease control, assessment of cancer-related symptoms, overall assessment of clinical benefit and the Lung Cancer Symptom Scale (LCSS).
TTSP and PFS analyses were conducted in the following patient subgroups: age (<65 years vs ≥65 years), ECOG PS (0, 1, 2), EGFRm type at study entry (common [Del19/L858R] vs uncommon mutations). PFS was assessed according to mutational status for: change in mutation status at visit 3 and presence of non-EGFR mutations at baseline. In addition, the appearance/detection of the EGFR T790M mutation was monitored at each visit throughout the study.
The safety of afatinib was also monitored throughout the study; details can be found in Supplementary Table 1.
Statistical analyses
Efficacy end points were analyzed using Kaplan–Meier estimates with 95% CI for the 25th, median and 75th percentiles of survival distribution. Cumulative frequencies were calculated for time to OR by 4-week periods. Two-sided 95% CIs were calculated for the best response rate using the exact 95% Clopper–Pearson CI. The change from baseline of LCSS physical and functional quality of life dimensions in patients was analyzed descriptively.
Results
Patients & treatment exposure
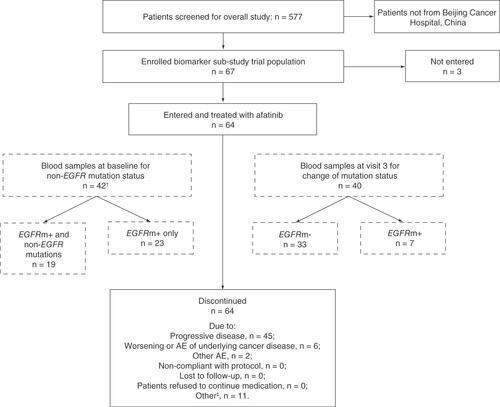
A total of 541 patients included in the overall treated set were treated with afatinib. Of these, 64 (11.8%) patients were enrolled at Beijing Cancer Hospital and provided consent for blood sample collection and biomarker analysis. Among the 64 patients included in the biomarker analysis group, 42 (65.6%) were assessed for non-EGFR mutations at baseline and 40 (62.5%) were assessed for EGFR mutation status at visit 3 to determine if there had been a change from baseline EGFRm+ status ().
†Of the 48 patients who had both blood and tissue samples analyzed, only two had EGFR mutations detectable per tissue samples but not per blood samples.
‡Includes patients who switched to commercial drug.
AE: Adverse event.
Demographic characteristics of the biomarker analysis group and the overall study population are shown in . All patients in the biomarker analysis group were of Chinese origin compared with 76.2% of the overall study population. In general, most other baseline demographic and clinical characteristics were similar, except that the biomarker analysis group had proportionally more females (70.3 vs 52.9%) and patients with ECOG PS 0 (75.0 vs 18.3%), more patients received afatinib as first-line treatment (47 vs 26%), more patients had L858R (50.0 vs 40.5%) than Del19 mutations (42.2 vs 48.2%) and fewer had uncommon mutations (9.4 vs 11.8%).
Table 1. Baseline demographic and clinical characteristics of the biomarker analysis group and overall study population.
In the biomarker analysis group, afatinib was administered for a median duration of 12.7 months and the maximum duration of treatment was 41.4 months. Afatinib was the first systemic treatment in 53 patients, second in seven patients, third in two patients and fourth and fifth in one patient each. All 64 patients started on afatinib 40 mg/day, 18 (28.1%) had their dose reduced to 30 mg/day and five (7.8%) had their treatment reduced to 20 mg/day.
Efficacy findings in the overall biomarker analysis group
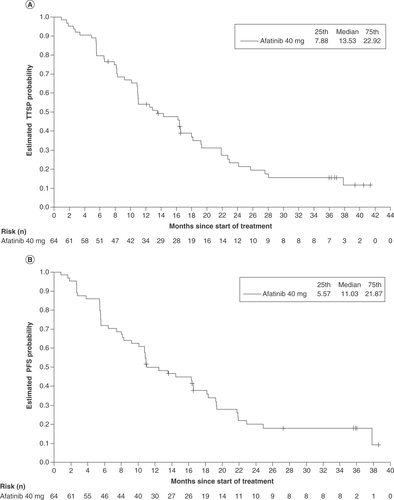
In the biomarker analysis group, median TTSP was 13.5 months (95% CI: 10.9–18.0) and median PFS was 11.0 months (95% CI: 10.1–16.6; ); in the overall trial, median TTSP was 14.0 months and median PFS was 12.1 months. OR was reported in 39 of 64 patients (60.9%; 95% CI: 47.9–72.9), including five patients (7.8%) with complete response and 34 patients (53.1%) with partial response. Median duration of OR was 13.8 months (95% CI: 10.8–19.0). Disease control was reported in 58 of 64 patients (90.6%; 95% CI: 80.7–96.5) and the median duration of disease control was 14.5 months (95% CI: 10.8–19.2). Subgroup analyses of TTSP and PFS for the biomarker analysis group were generally consistent with the overall study population (shown in Supplementary Table 2).
(A) Median TTSP and (B) median PFS in the overall biomarker cohort (n = 64).
PFS: Progression-free survival; TTSP: Time to symptomatic progression.
Improvement in cancer-related symptoms, as assessed by the clinical investigator, occurred in 25.0% of patients at week 4, 25.4% at week 8, 45.0% at week 12 and 9.8% at week 28. Investigator-assessed clinical benefit rates were 98.4% at week 4, 96.8% at week 8, 96.7% at week 12 and 96.1% at Week 28. On the LCSS scale completed by 50 patients, 17 patients (34%) improved from baseline by ≥10 points on the average symptom burden index at any time during the study; 14 (28.0%) and 19 (38.0%) of the remaining patients were stable or worsened, respectively.
Biomarker analyses
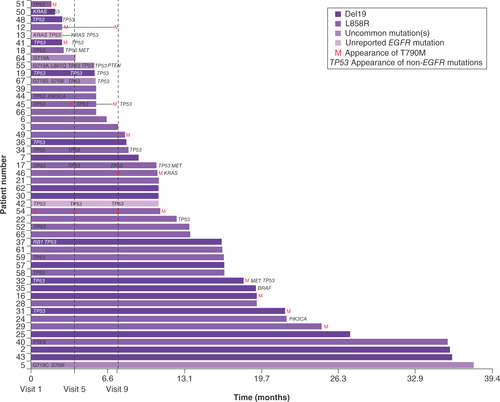
At baseline, 42 patients were tested for the presence of non-EGFR mutations; these were detected in 19 patients (45.2%; ). Non-EGFR mutations detected at baseline included TP53 (n = 19), KRAS (n = 2), RB1 (n = 1), PTEN (n = 1) and PIK3CA (n = 1). Other non-EGFR mutations that emerged during treatment included TP53 (n = 3), MET (n = 3), PTEN (n = 1), BRAF (n = 1), KRAS (n = 1) and PIK3CA (n = 1). Median PFS was shorter for patients who harbored non-EGFR mutations at baseline than for those who did not (8.1 vs 12.5 months), although the difference was not statistically significant (hazard ratio [HR]: 1.72; 95% CI: 0.88–3.36; p = 0.1054; A).
(A) Median PFS in patients with non-EGFR mutations at baseline and with only EGFR mutations. (B) Median PFS in patients whose EGFR mutation status changed from EGFRm+ to EGFRm- at visit 3 and those whose EGFR mutation status did not change.
HR: Hazard ratio; NE: Not evaluable; PFS: Progression-free survival.
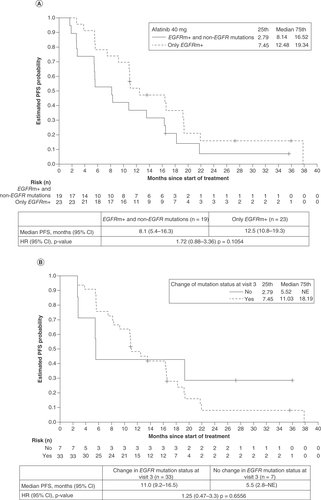
Among the 40 patients who were evaluated for a change in mutation status at visit 3, the status of 33 patients (82.5%) changed from EGFRm+ to EGFRm-. Median PFS was longer among patients in whom EGFRm was not detected at visit 3 than among those without a change in EGFR mutation status (11.0 vs 5.5 months), although the difference was not statistically significant (HR: 1.25; 95% CI: 0.47–3.30; p = 0.6556; ).
Among the 64 patients included in the biomarker analysis group, 11 patients (17.2%) who did not have T790M at baseline acquired it during study treatment (). The mean and median time to appearance of T790M (i.e., detection in blood samples) was 13.8 (standard deviation 7.1 months) and 12.9 months (range 3.7–23.9 months), respectively. There was no significant difference in PFS (10.8 vs 13.6 months; HR: 1.62; 95% CI: 0.83–3.18; p = 0.1591) or TTSP (16.2 vs 13.5 months; HR: 1.22; 95% CI: 0.62–2.39; p = 0.5585) between patients who acquired T790M compared with those who did not.
The baseline EGFR mutation is shown by the color of the bar. Appearance of the EGFR T790M mutation is denoted by an ‘M’ at either baseline (visit 1), visit 5, visit 9 or end of treatment. Non-EGFR mutations present at baseline are shown on the left against the y-axis; non-EGFR mutations that were detected at other timepoints, or at end of treatment, are shown when they appeared. Uncommon EGFR mutations are detailed on the left of the bar to which they apply.
Discussion
We conducted a biomarker sub-study analysis of patients at a single center in China taking part in a near ‘real-world’, phase IIIb, multicenter study of afatinib in EGFRm+ NSCLC. In general, results of the efficacy analysis with afatinib in this group of Chinese patients were similar to those of randomized controlled trials with afatinib in EGFRm+ NSCLC [Citation22,Citation23], and the safety analysis did not reveal any new safety signals (see Supplementary Information). Analysis of TTSP and PFS in the biomarker subgroups showed that afatinib was beneficial in all subgroups with adequate numbers of patients, as has been shown in other clinical studies (Supplementary Table 2) [Citation23,Citation24].
Just under half (≈45%) of the EGFRm+ NSCLC patients in the biomarker analysis harbored non-EGFR mutations at baseline, illustrating that heterogeneity among EGFRm+ tumors is relatively common. PFS was lower, but not significantly, in these patients compared with patients who harbored only EGFR mutations. This is in keeping with the literature, which shows that patients with EGFRm+ NSCLC and concurrent mutations tend to have worse outcomes than those without other driver mutations [Citation25–29]. Evidence from randomized clinical trials is required to understand if EGFR TKIs combined with radiotherapy, cytotoxic chemotherapies or immunotherapy could be an effective treatment approach for such patients.
The co-mutations observed here have been described in other studies and are well known oncogenes [Citation25–27]. They may provide an escape mechanism for the tumor in the presence of inhibition of EGFR by afatinib. Thorough baseline sequencing of cfDNA in patients with EGFRm+ may therefore guide potential additional treatments. Indeed, a number of patients without non-EGFR mutations at baseline developed these mutations during treatment or by the end of therapy, illustrating the evolution of escape mechanisms under pressure from afatinib. Many of the co-mutations observed in this study, both at progression and at baseline, can be targeted by approved treatment (e.g., MET is inhibited by crizotinib and cabozantinib; BRAF is inhibited by vemurafenib and dabrafenib; and inhibitors are in development for PTEN and PIK3CA) [Citation30].
Approximately 17% of patients in the biomarker analysis group acquired T790M during the study, and the median time to appearance was >1 year. Other reports suggest T790M is the most common mechanism of acquired resistance to first- and second-generation EGFR TKIs, occurring in 32–70% of patients [Citation13,Citation31–36]. Therefore, the rate of T790M acquisition observed in our analysis appears to be relatively low. This could be because the rate of T790M acquisition is lower in L858R than Del19 tumors; more patients had L858R (50.0%) than Del19 mutations (42.2%) in the biomarker analysis group [Citation37]. A couple of patients had T790M at baseline and throughout treatment with afatinib (); one of them (patient 51) had a very short PFS of only 1.6 months, which may have been expected. However, the other (patient 54) had a PFS of 11.0 months, suggesting they derived some benefit from afatinib treatment despite the presence of T790M. The third-generation EGFR TKI osimertinib is a treatment option for patients who have T790M at baseline or acquire it during first-line EGFR TKI therapy [Citation31,Citation38,Citation39], with a median PFS of 10.1 vs 4.4 months with platinum-pemetrexed therapy [Citation39]. Osimertinib is also effective as a first-line treatment in patients with EGFRm+ tumors [Citation40,Citation41], raising the question of whether it should be used first to avoid development of T790M. However, resistance mechanisms to osimertinib are heterogeneous and not currently treatable with targeted therapy [Citation42]. The GioTag observational study was conducted to assess the total treatment duration of sequential afatinib and osimertinib in patients with EGFRm+ NSCLC with T790M-acquired resistance, and found an overall median time on treatment of 27.7 months in all patients, with a particularly long time on treatment in Asian patients (37.1 months) [Citation43]. This suggests that sequential afatinib and osimertinib remains an attractive strategy.
Mutation status of most (83%) patients treated with afatinib changed from EGFRm+ at baseline to EGFRm- by visit 3 (approximately 2 months after treatment initiation). PFS was shorter, although not significantly, for patients who had no change in their EGFR mutation status by visit 3, but this group was small (n = 7). Reduction of EGFRm+ to undetectable levels during treatment with EGFR TKIs has been observed previously [Citation44–47], and loss of EGFRm+ status has also been associated with improved PFS compared with retention of EGFRm+ [Citation45–47]. For example, in an analysis by Lee et al., patients with NSCLC treated with EGFR TKIs (n = 40; all with EGFR mutations at baseline) had a marked (>50%) reduction of mutant copies in plasma during the first 2 weeks of treatment, and median PFS was significantly longer for those with undetectable vs those with detectable EGFR mutations at 2 months (10.1 vs 6.3 months; HR: 3.88; 95% CI: 1.48–10.19; p = .006) [Citation45].
Plasma-based EGFR mutation analysis using cfDNA represents a noninvasive alternative to tumor rebiopsy, which can be challenging in clinical practice [Citation44,Citation45]. This biomarker analysis has demonstrated that it is possible to longitudinally monitor the disappearance of EGFR mutations and the appearance of T790M using liquid biopsy and cfDNA in a ‘near real-world’ clinical study, which may have implications for patient outcomes, continuation of ongoing treatment and selection of potential new treatments.
A limitation of this biomarker analysis is that it was a subgroup analysis of a larger trial and included a relatively small patient group in a single center in China. Therefore, comparative analyses were not adequately powered to demonstrate any statistically significant differences, and some of the subgroups had too few patients to allow any meaningful conclusions to be drawn. The generalizability of results may also be limited, as the biomarker analysis included only Chinese patients from a single center. There was also a bias between the study cohort (n = 64) and the cfDNA sub-cohort (n = 42), in that 22 patients had EGFR mutations detected only in tissue but not when using cfDNA, a subgroup that may have an improved prognosis. In addition, the biomarker analysis was conducted at set time points, not continually throughout the study. And finally, clinical application of cfDNA has a relatively low sensitivity (60–70%), therefore the absence of a specific mutation using cfDNA does not exclude the possibility of the mutation being present in tumor tissue [Citation19].
Conclusion
Median PFS in patients included in the biomarker analysis was longer, but not significantly, among Chinese patients with NSCLC whose mutation status changed to EGFRm- during afatinib treatment compared with those whose status remained EGFRm+ at month 2. Median PFS with afatinib was shorter for patients who harbored non-EGFR co-mutations at baseline compared with those who did not, although the difference did not achieve statistical significance. This exploratory analysis suggests that afatinib provides clinical benefit for patients with EGFRm+ NSCLC across all subgroups assessed, and that assessment of EGFRm status in cfDNA from the plasma may be a useful method of monitoring treatment.
Future perspective
This exploratory analysis suggests that afatinib provides clinical benefit for patients with EGFRm+ NSCLC across all subgroups assessed. Assessment of EGFRm status in cfDNA from the plasma may be a useful method of monitoring treatment. Clinical utility of monitoring by liquid biopsy may be investigated in a larger trial.
EGFRm+ non-small-cell lung cancer (NSCLC) tumors inevitably develop resistance upon treatment with a tyrosine kinase inhibitor.
The aim of this study was to explore the relationship between mutations in cfDNA and response to afatinib.
Overall, 64 patients from one site in China with locally advanced or metastatic EGFRm+ NSCLC who received afatinib 40 mg once daily were included.
Median progression-free survival was shorter, but not significantly, in patients who had non-EGFR co-mutations at baseline compared with those who did not have co-mutations in a subanalysis of a phase IIIb ‘near real-world’ study of Chinese patients with EGFRm+ NSCLC treated with afatinib.
Median progression-free survival was longer, but not significantly, in the 83% of patients who became EGFRm- during afatinib treatment compared with those who remained EGFRm+.
Analyzing cfDNA is a useful method for monitoring response to treatment and the onset of resistance.
Author contributions
The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development and have approved the final version.
Ethical conduct of research
Ethics approval was obtained at the Beijing Cancer Hospital, China, as was the case at each of the other participating centers in the overall study. The study was approved by the Institutional Review Board or Independent Ethics Committee and was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, Good Clinical Practice and local laws. All patients provided written informed consent. Participants at the Beijing Cancer Hospital also provided written informed consent for blood sample collection for biomarker testing.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data: NCT01953913. To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to relevant material, including participant-level clinical study data, as needed by them to fulfill their role and obligations as authors under the ICMJE criteria. Clinical study documents and participant clinical study data are available to be shared on request after publication of the primary manuscript in a peer-reviewed journal, and if regulatory activities are complete and other criteria met as per the BI Policy on Transparency and Publication of Clinical Study Data (see https://www.mystudywindow.com/msw/datasharing). Bona fide, qualified scientific and medical researchers are eligible to request access to the clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Legal Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request. Prior to providing access, clinical study documents and data will be examined, and, if necessary, redacted and de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Supplementary Table 1
Download MS Word (25.3 KB)Supplementary Information
Download MS Word (15.9 KB)Infographic
Download PDF (246.2 KB)Acknowledgments
This paper was written prior to A Cseh passing away. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Supplementary data
An infographic accompanies this paper. To view or download this infographic in your browser please click here: www.tandfonline.com/doi/suppl/10.2217/fon-2021-0394
Financial & competing interests disclosure
This work was supported by Boehringer Ingelheim International GmbH. The study sponsor participated in the design of the study, the collection, analysis and interpretation of the data, writing this article and the decision to submit the article for publication. The authors did not receive payment related to the development of the manuscript. Y Tian is employed by Boehringer Ingelheim (China) Investment Co., Ltd. A Cseh was employed by Boehringer Ingelheim International GmbH. DC-L Huang was employed by Boehringer Ingelheim Taiwan Limited and is currently employed with Merck Sharp & Dohme (I.A.) LLC, Taiwan Branch. Y-L Wu has received honoraria from AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly, MSD, Pfizer, Sanofi, Roche and reports grants from AstraZeneca, Boehringer Ingelheim and BMS. The authors report no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by C Perry, of Ashfield MedComms, an Ashfield Health company, and T Allinson contracted on behalf of Ashfield MedComms, and funded by Boehringer Ingelheim.
Additional information
Funding
References
- Han B , TjulandinS, HagiwaraKet al. EGFR mutation prevalence in Asia-Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non-adenocarcinoma histology: the IGNITE study. Lung Cancer113, 37–44 (2017).
- Shi Y , AuJS, ThongprasertSet al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol.9, 154–162 (2014).
- Tu HY , WuYL. Afatinib for the first-line treatment of EGFR mutation-positive NSCLC in China: a review of clinical data. Future Oncol.16, 2569–2586 (2020).
- Wang R , ZhangY, PanYet al. Comprehensive investigation of oncogenic driver mutations in Chinese non-small cell lung cancer patients. Oncotarget6, 34300–34308 (2015).
- Zhang YL , YuanJQ, WangKFet al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget7, 78985–78993 (2016).
- Wu YL , PlanchardD, LuSet al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann. Oncol.30, 171–210 (2019).
- Hanna N , JohnsonD, TeminSet al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol.35, 3484–3515 (2017).
- Planchard D , PopatS, KerrKet al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.29, iv192–iv237 (2018).
- Solca F , DahlG, ZoephelAet al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J. Pharmacol. Exp. Ther.343, 342–350 (2012).
- Hirsh V . New developments in the treatment of advanced squamous cell lung cancer: focus on afatinib. Onco. Targets Ther.10, 2513–2526 (2017).
- Nakamura T , NakashimaC, KomiyaKet al. Mechanisms of acquired resistance to afatinib clarified with liquid biopsy. PLoS ONE13, e0209384 (2018).
- Tanaka K , NosakiK, OtsuboKet al. Acquisition of the T790M resistance mutation during afatinib treatment in EGFR tyrosine kinase inhibitor-naive patients with non-small cell lung cancer harboring EGFR mutations. Oncotarget8, 68123–68130 (2017).
- Wu SG , LiuYN, TsaiMFet al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget7, 12404–12413 (2016).
- European Medicines Agency . Giotrif. Afatinib. (2021). www.ema.europa.eu/en/medicines/human/EPAR/giotrif
- Chinese guidelines for diagnosis and treatment of primary lung cancer 2018 (English version). Chin. J. Cancer Res.31, 1–28 (2019).
- Lindeman NI , CaglePT, AisnerDLet al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med.142, 321–346 (2018).
- Passaro A , MokT, PetersSet al. Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations. J. Thorac. Oncol.16, 764–773 (2021).
- Kalemkerian GP , NarulaN, KennedyEBet al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J. Clin. Oncol.36, 911–919 (2018).
- Pasini L , UliviP. Liquid biopsy for the detection of resistance mechanisms in NSCLC: comparison of different blood biomarkers. J. Clin. Med.8, 998 (2019).
- Wu YL , TuH, FengJet al. A phase IIIb open-label, single-arm study of afatinib in EGFR TKI-naive patients with EGFRm+ NSCLC: an interim analysis. J. Thorac. Oncol.12, S2214 (2017).
- Wu Y , TuH, FengJet al. P2.01-99 a phase IIIb open-label study of afatinib in EGFR TKI-naive patients with EGFR mutation-positive NSCLC: final analysis. J. Thorac. Oncol.14, S679–S680 (2019).
- Paz-Ares L , TanEH, O’ByrneKet al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann. Oncol.28, 270–277 (2017).
- Sequist LV , YangJC, YamamotoNet al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol.31, 3327–3334 (2013).
- Wu YL , ZhouC, HuCPet al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase III trial. Lancet Oncol.15, 213–222 (2014).
- Chen M , XuY, ZhaoJet al. Concurrent driver gene mutations as negative predictive factors in epidermal growth factor receptor-positive non-small cell lung cancer. EBioMedicine42, 304–310 (2019).
- Labbe C , CabaneroM, KorpantyGJet al. Prognostic and predictive effects of TP53 co-mutation in patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung Cancer111, 23–29 (2017).
- Li JM , HuJ, BaiCXet al. The concomitant gene alterations impact the therapeutic efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer patients with epidermal growth factor receptor sensitive mutation. Chinese J. Tuberc. Resp. Dis.41, 778–782 (2018).
- Barnet MB , O’TooleS, HorvathLGet al. EGFR-co-mutated advanced NSCLC and response to EGFR tyrosine kinase inhibitors. J. Thorac. Oncol.12, 585–590 (2017).
- Blakely CM , WatkinsTBK, WuWet al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat. Genet.49, 1693–1704 (2017).
- Guo Y , CaoR, ZhangXet al. Recent progress in rare oncogenic drivers and targeted therapy for non-small cell lung cancer. Onco. Targets Ther.12, 10343–10360 (2019).
- Yang JC , AhnMJ, KimDWet al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J. Clin. Oncol.35, 1288–1296 (2017).
- Arcila ME , OxnardGR, NafaKet al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin. Cancer Res.17, 1169–1180 (2011).
- Hochmair MJ , BuderA, SchwabSet al. Liquid-biopsy-based identification of EGFR T790M mutation-mediated resistance to afatinib treatment in patients with advanced EGFR mutation-positive NSCLC, and subsequent response to osimertinib. Target Oncol.14, 75–83 (2019).
- Sequist LV , WaltmanBA, Dias-SantagataDet al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med.3, 75ra26 (2011).
- Liang SK , HsiehMS, LeeMRet al. Real-world experience of afatinib as a first-line therapy for advanced EGFR mutation-positive lung adenocarcinoma. Oncotarget8, 90430–90443 (2017).
- Tanaka K , NosakiK, OtsuboKet al. Acquisition of the T790M resistance mutation during afatinib treatment in EGFR tyrosine kinase inhibitor-naive patients with non-small cell lung cancer harboring EGFR mutations. Oncotarget8, 68123–68130 (2017).
- Liang H , PanZ, WangWet al. The alteration of T790M between 19 del and L858R in NSCLC in the course of EGFR-TKIs therapy: a literature-based pooled analysis. J. Thorac. Dis.10, 2311–2320 (2018).
- Janne PA , YangJC, KimDWet al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med.372, 1689–1699 (2015).
- Mok TS , WuYL, AhnMJet al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med.376, 629–640 (2017).
- Soria JC , RamalingamSS. Osimertinib in EGFR mutation-positive advanced NSCLC. N. Engl. J. Med.378, 1262–1263 (2018).
- Ramalingam SS , VansteenkisteJ, PlanchardDet al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med.382, 41–50 (2020).
- Hochmair MJ , MorabitoA, HaoDet al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol.14, 2861–2874 (2018).
- Hochmair MJ , MorabitoA, HaoDet al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol.16, 2799–2808 (2020).
- Xiong L , CuiS, DingJet al. Dynamics of EGFR mutations in plasma recapitulates the clinical response to EGFR-TKIs in NSCLC patients. Oncotarget8, 63846–63856 (2017).
- Lee JY , QingX, XiuminWet al. Longitudinal monitoring of EGFR mutations in plasma predicts outcomes of NSCLC patients treated with EGFR TKIs: Korean Lung Cancer Consortium (KLCC-12-02). Oncotarget7, 6984–6993 (2016).
- Shepherd FA , PapadimitrakopoulouVA, MokTet al. Early clearance of plasma EGFR mutations as a predictor of response to osimertinib in the AURA3 trial. J. Clin. Oncol.36, Abstr9027 (2018).
- Zhou C , ImamuraF, ChengYet al. Early clearance of plasma EGFR mutations as a predictor of response to osimertinib and comparator EGFR-TKIs in the FLAURA trial. J. Clin. Oncol.37, Abstr9020 (2019).