Abstract
Definitive chemoradiotherapy is the standard of care for inoperable locoregionally advanced esophageal squamous cell carcinoma (ESCC). Immune checkpoint inhibitors such as anti-PD-1/PD-L1 antibodies have led to a paradigm shift in advanced, metastatic ESCC treatment; however, the effect of incorporating checkpoint inhibitors in the definitive management of ESCC is unclear. Tislelizumab is an anti-PD-1 antibody specifically engineered to minimize FcɣR binding on macrophages to abrogate antibody-dependent phagocytosis, a mechanism of T-cell clearance and potential resistance to anti-PD-1 therapy. The RATIONALE 311 study described here (BGB-A317-311; NCT03957590) is a registrational multicenter, double-blind, placebo-controlled, randomized, Phase III clinical trial designed to evaluate the efficacy and safety of tislelizumab combined with concurrent chemoradiotherapy in patients with inoperable localized ESCC.
Lay abstract
Esophageal cancer is a challenging disease that seriously threatens patients’ health and life. Esophageal squamous cell carcinoma (ESCC) is the most common type of esophageal cancer. Most patients who have inoperable stage II–IV ESCC are currently treated with a sequential combination of chemotherapy and radiation therapy, with the hopes of increasing the positive effects seen from either therapy alone. Immune checkpoint inhibitors such as anti-PD-1/PD-L1 antibodies have shown encouraging results in patients with ESCC, but it is not known if combining checkpoint inhibitors with simultaneous chemotherapy and radiation therapy will provide additional benefits. The safety and efficacy of tislelizumab, an anti-PD-1 antibody specifically engineered to limit potential resistance to anti-PD-1 therapy, is being investigated in combination with simultaneous chemotherapy and radiation therapy in patients with inoperable stage II–IV ESCC in an actively enrolling clinical trial, RATIONALE 311 (NCT03957590). Our trial in progress article explains the reason RATIONALE 311 was started and provides important enrollment information for doctors.
Clinical trial registration: NCT03957590 (ClinicalTrials.gov)
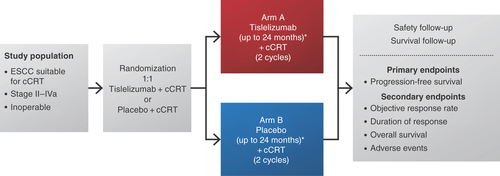
We describe RATIONALE 311, an ongoing multicenter Phase III, randomized, double-blind, placebo-controlled study examining tislelizumab combined with concurrent chemoradiotherapy (cCRT) compared with placebo plus cCRT in patients with inoperable localized esophageal squamous cell carcinoma (ESCC; BGB-A317-311; NCT03957590). The study is sponsored by BeiGene and evaluates progression-free survival (PFS), overall response rate and duration of response (DoR) assessed per RECIST v1.1, as well as overall survival (OS) and the safety and tolerability profile of tislelizumab.
Background & rationale
Current treatment for ESCC
With over 604,000 new cases and 544,000 deaths each year, esophageal cancer is the eighth most common cancer worldwide and the sixth most common cause of cancer-related deaths [Citation1]. Patient survival is correlated with clinical stage [Citation2]. With a few exceptions for rare subtypes, esophageal cancers are classified histologically into esophageal adenocarcinoma or ESCC, with ESCC globally predominant [Citation3]. ESCC is more prevalent (79%) in Southeast and Central Asian countries; more than half of all new global cases of ESCC each year occur in China [Citation4].
Definitive CRT with platinum-based chemotherapy in combination with fluoropyrimidine or taxanes is the standard of care for patients with inoperable ESCC and is recommended by treatment guidelines [Citation5]. Paclitaxel plus cisplatin is one of the recommended chemotherapy regimens for definitive CRT based on a Phase II study which investigated the efficacy of concurrent cisplatin, paclitaxel and radiation as a preoperative regimen for patients with locoregional esophageal carcinoma [Citation6]. In a recent Phase III study (ESO-Shanghai 2), treatment with paclitaxel plus cisplatin also showed a comparable benefit in PFS and OS outcomes and a similar safety profile with paclitaxel plus carboplatin as definitive treatment in patients with locally advanced ESCC [Citation7]. Despite treatment with current standard of care of definitive CRT, many patients experience treatment failure and about 30–40% of patients develop distant metastases; the 5-year survival rate is approximately 20% in patients with ESCC [Citation8–12]. Unfortunately, efforts to optimize treatment outcomes through variations of CRT have been unsuccessful in the past. The addition of the epidermal growth factor receptor inhibitor cetuximab to CRT in the Phase II/III SCOPE1 trial resulted in increased toxicity and significantly worse OS compared with CRT in patients with ESCC [Citation13]. Together, these data highlight an unmet medical need in patients with ESCC.
Immune checkpoint inhibition as a strategy for treating ESCC
Therapies targeting the immune system, especially single-agent monoclonal antibodies targeting the PD-1/PD-L1 axis, have demonstrated promising results as adjuvant and second-line treatment of esophageal cancer. In the CheckMate 577 trial, adjuvant nivolumab demonstrated a statistically significant improvement in disease-free survival versus placebo (22.4 vs 11.0 months; hazard ratio [HR] 0.69; 96.4% CI: 0.56–0.86; p = 0.0003) in patients with esophageal or gastroesophageal junction cancer who received neoadjuvant CRT plus surgery and did not achieve a pathological complete response at a prespecified interim analysis [Citation14]. In ATTRACTION-3, OS was significantly improved in patients with advanced ESCC refractory or intolerant to previous chemotherapy who received second-line nivolumab compared with those receiving chemotherapy (median OS: 10.9 vs 8.4 months; HR: 0.77; 95% CI: 0.62–0.96; p = 0.019) [Citation15]. An ongoing feasibility study of nivolumab as neoadjuvant chemotherapy for locally advanced esophageal carcinoma (FRONTiER/JCOG1804E) has also been initiated [Citation16]. In the KEYNOTE-181 study, an improvement in OS was seen in patients with locally advanced or metastatic ESCC treated with pembrolizumab as second-line therapy compared with chemotherapy alone (8.2 vs 7.1 months; HR: 0.78; 95% CI: 0.63–0.96; p = 0.0095) [Citation17]. Anti-PD-1 therapies have also shown promising results in combination with chemotherapy as first-line treatment of esophageal cancer. The KEYNOTE-590 trial demonstrated that first-line pembrolizumab in combination with chemotherapy significantly improved OS compared with chemotherapy alone (12.6 vs 9.8 months; HR: 0.72; 95% CI: 0.60–0.88; p < 0.0006) in patients with locally advanced/unresectable or metastatic ESCC [Citation18]. In a randomized Phase III study (CheckMate 648), median OS was significantly better among patients receiving both nivolumab plus chemotherapy (13.2 months; HR vs chemo: 0.74; 99.1% CI: 0.58–0.96; p = 0.0021) and nivolumab plus ipilimumab (12.8 months; HR vs chemo: 0.78; 98.2% CI: 0.62–0.98; p = 0.011) compared with chemotherapy alone (10.7 months) [Citation19].
Tislelizumab as a PD-1 inhibitor for treatment of ESCC
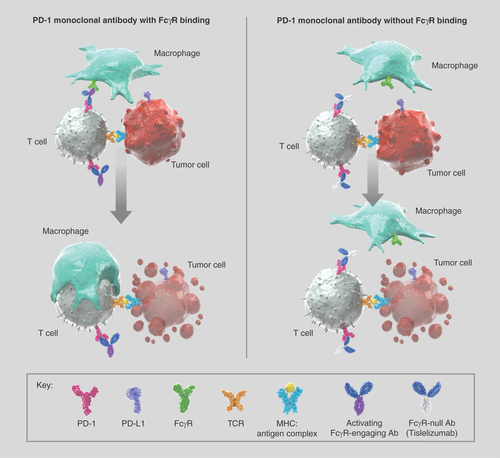
Tislelizumab is a humanized IgG4 monoclonal antibody that binds to and blocks human PD-1 with high affinity and specificity [Citation20]. Unique among monoclonal anti-PD-(L)1 inhibitors, tislelizumab was specifically engineered to minimize FcɣR binding on macrophages in order to abrogate antibody-dependent phagocytosis, a potential mechanism of T-cell clearance and resistance to anti-PD-1 therapy () [Citation21–23]. As an antagonist to PD-L1–mediated cell signaling, tislelizumab leads to increased cytokine production and restoration of T-cell activation, resulting in immune-mediated tumor cell death [Citation21].
Ab: Antibody; MHC: Major histocompatibility complex.
In reports from two early phase studies, single-agent tislelizumab was generally well tolerated and reported adverse events were manageable and generally mild-to-moderate in severity across numerous malignancies [Citation24,Citation25]. In the global, Phase IA/IB, first-in-human study (BGB-A317-001; NCT02407990) of tislelizumab in patients with advanced solid tumors, patients received tislelizumab 0.5–10 mg/kg every 2 weeks; 2 or 5 mg/kg administered every 2 or every 3 weeks; or 200 mg every 3 weeks. Among all patients (n = 451), 47% (n = 210) had received two or more prior systemic drug therapies, 53% (n = 241) had received prior radiotherapy, and 72% (n = 323) had undergone prior anticancer surgery. Tislelizumab median treatment duration for all patients (n = 451) was 2.8 months (range: 0.13–43.7) and 65 patients (14.4%) received treatment for ≥12 months [Citation24]. Fatigue (28%), nausea (25%) and decreased appetite (20%) were the most commonly reported adverse events. Most adverse events were grade 1–2 in severity; anemia (4.9%) was the most common grade 3–4 adverse event. Treatment-related adverse events led to discontinuation in 5.3% of patients [Citation24]. In the open-label Phase I/II study (BGB-A317-102; NCT04068519), median treatment duration of patients with advanced solid tumors (n = 300) receiving tislelizumab 200 mg was 18 weeks (range: 0.9–95.7) [Citation25]. Most treatment-related adverse events were grade 1 or 2 (62%), with the most common being anemia (n = 70; 23%) and increased aspartate aminotransferase (n = 67; 22%). Treatment-emergent adverse events led to discontinuation in 8% of patients [Citation25]. Together, the data from these two early phase clinical trials show that treatment of tislelizumab for multiple advanced tumor types was generally well tolerated.
Tislelizumab, alone and in combination with chemotherapy, has also demonstrated preliminary antitumor activity in patients with esophageal carcinoma. Among the 54 heavily pretreated patients with advanced esophageal cancer who were response-evaluable and received tislelizumab in the first-in-human study (BGB-A317-001; NCT02407990), an objective response rate of 11.1% (95% CI: 4.2– 22.6) was observed [Citation24]. Similar results were observed in the open-label Phase I/II study (NCT04068519). Among evaluable patients with heavily treated advanced ESCC (n = 26), the objective response rate was 8% (95% CI: 0.9–25.1) [Citation25]. In a Phase II study of first-line tislelizumab in combination with chemotherapy (BGB-A317-205; NCT03469557), patients with inoperable, locally advanced or metastatic ESCC (n = 15) had a confirmed objective response rate of 46.7% (95% CI: 21.3–73.4), with a median DoR of 12.8 months (95% CI: 3.5–12.8) [Citation26]. No new safety signals were observed when tislelizumab was combined with chemotherapy and most of the reported adverse events were mild-to-moderate in severity. A recent global Phase III study (RATIONALE 302; NCT03430843) evaluating tislelizumab versus investigator choice of chemotherapy as second-line treatment in patients with advanced or metastatic ESCC met its primary end point by demonstrating that tislelizumab significantly improved median OS over chemotherapy (8.6 vs 6.3 months; HR: 0.70; 95% CI: 0.57–0.85; p = 0.0001) [Citation27].
Single-agent tislelizumab is currently approved in China for the treatment of relapsed or refractory classical Hodgkin lymphoma and as third-line treatment and for locally advanced or metastatic urothelial carcinoma in patients with PD-L1–high expression who had received prior platinum-containing chemotherapy [Citation28]. In January 2021, tislelizumab was also approved for use in combination with chemotherapy as first-line treatment for locally advanced or metastatic squamous non-small-cell lung cancer (NSCLC).
Potential synergy between radiation therapy & immune checkpoint inhibition
While both radiotherapy and immune checkpoint inhibitors (CPIs) have demonstrated efficacy in the treatment of locally advanced ESCC, preclinical evidence suggests that synergism may result when they are administered together. Early studies of in vitro stimulation of fibroblasts and in vivo immunosuppression of mice showed that modulation of the immune system altered the dose of radiation needed to control tumor growth [Citation29]. Because immune checkpoint inhibition stimulates the immune system, it was hypothesized that combining radiation therapy and immune checkpoint inhibition would result in a similar enhanced effect of radiation therapy. Indeed, combining an anti-PD-1 antibody (RMP1-14) with carboplatin and paclitaxel plus radiotherapy (10 daily fractions of 2 Gy at 290 cGy/min with 220 kV x-rays) resulted in less tumor growth than that seen in mice treated with either RMP1-14 or chemotherapy alone [Citation29]. Additionally, anti-PD-1 treatment combined with radiation increased the number of tumor-infiltrating CD28+ T lymphocytes, while simultaneously decreasing the number of anergic tumor-infiltrating lymphocytes [Citation29]. Together, these data suggested that immune CPIs could potentially synergize with radiotherapy and CRT and warranted further investigation in clinical trials.
The Phase III PACIFIC trial examined and proved a sequential approach of using a PD-L1 inhibitor after prior CRT in NSCLC cancer. In patients with stage III, unresectable NSCLC who did not have disease progression after two or more cycles of platinum-based CRT (n = 709, randomized 2:1 to receive durvalumab or placebo), durvalumab consolidation therapy had significantly improved median PFS (16.8 months) compared with placebo (5.6 months) and a significantly decreased risk of disease progression (stratified HR: 0.52; 95% CI: 0.42–0.65; p < 0.001) [Citation30].
Relevance of the current trial
While previous trials have investigated the potential benefit of sequential PD-L1 inhibition and CRT, concurrent anti-PD-1/PD-L1 therapy combined with CRT had not been examined in patients with ESCC in a Phase III trial prior to the initiation of the RATIONALE 311 study. Other randomized trials evaluating the activity of CPIs combined with CRT in ESCC with or without esophageal adenocarcinoma have since begun recruiting patients [Citation16,Citation31,Citation32]. Standard of care for patients with inoperable ESCC is definitive CRT consisting of fluoropyrimidine in combination with platinum-based chemotherapy, but the 5-year survival rate still remains at approximately 20% [Citation8,Citation11,Citation12]. The preclinical data outlined above suggest that CPIs can synergize with radiotherapy, and the Phase III PACIFIC trial demonstrated that a sequential addition of a PD-L1 inhibitor after prior CRT resulted in clinical benefits in patients with NSCLC. RATIONALE 311 (BGB-A317-311; NCT03957590) was designed to evaluate the efficacy and safety of tislelizumab, an anti-PD-1 antibody, in combination with cCRT compared with placebo in patients with inoperable localized ESCC. RATIONALE 311 started enrolling patients in June 2019, and the estimated study completion date is April 2022.
Clinical trial design
Study design
This Phase III, randomized, double-blind, placebo-controlled study (RATIONALE 311; BGB-A317-311; NCT03957590) evaluates the efficacy and safety of tislelizumab in combination with cCRT in patients with inoperable localized ESCC (). The primary objective of the study is to compare PFS, as assessed by a Blinded Independent Review Committee (BIRC) per RECIST v1.1. Secondary objectives include a comparison of tislelizumab plus cCRT with the placebo control in terms of efficacy assessments (overall response rate, DoR and OS), measures of health-related quality of life, as well as safety/tolerability. Eligible patients will be randomized in a 1:1 ratio to receive either tislelizumab or placebo in combination with cCRT up to approximately 6 weeks followed by tislelizumab or placebo for a total of up to 24 months. Randomization will be stratified according to Eastern Cooperative Oncology Group (ECOG) performance status (0 vs 1) and clinical staging (II/III vs IVa). Archival tumor tissues are required for PD-L1 assessment and exploratory biomarker analysis at a central laboratory or sponsor designed laboratory. In both arms, treatment beyond the initial BIRC-assessed, RECIST v1.1-defined disease progression is permitted if the patient is clinically stable and likely to benefit from continued treatment, provided that the patient agrees to continue treatment beyond progression and provides written informed consent.
Eligibility criteria
Adult patients (aged 18–75 years) with histologically confirmed localized ESCC, stage II–IVa inoperable ESCC (medically unsuitable for surgery or patient refusal of surgical intervention) who received less than three cycles of prior chemotherapy without radiotherapy are eligible. Patients will be excluded if they have a history of surgery for esophageal cancer or of fistula due to primary tumor invasion, a high risk of fistula or signs of perforation, show evidence of distant metastases or are intolerable/resistant to protocol-specified chemotherapy. Patients must have an ECOG performance status ≤1 and could have received prior chemotherapy (no more than three cycles), but not prior radiotherapy, targeted therapies or therapies targeting PD-1, PD-L1, PD-L2 or other immune-oncology therapies.
Planned sample size
Roughly 366 study patients will be recruited from approximately 32 centers in China. Sample size was determined based on the assumed number of events required to demonstrate PFS superiority of tislelizumab plus cCRT (Arm A) to placebo plus cCRT (Arm B) in the intention-to-treat (ITT) analysis set.
Planned study period
This study opened to accrual in June 2019 and is currently recruiting patients. The estimated primary completion date of this study is April 2022 and the estimated study completion date is October 2023.
Study procedures
Patients will be randomized 1:1 to receive either IV tislelizumab 200 mg every 3 weeks (Day 1 of each 21-day cycle) plus cCRT (Arm A) or placebo (IV every 3 weeks) plus cCRT (Arm B). Concurrent chemotherapy will consist of cisplatin (25 mg/m2 IV; days 1–3 of each 3-week cycle) in combination with paclitaxel (135 mg/m2 IV; day 1 of each 3-week cycle) and will be administered for two cycles; radiotherapy will be delivered in 28 fractions (total dose, 50.4 Gy). Duration of treatment will be up to 24 months (~35 cycles) for tislelizumab (Arm A) or placebo (Arm B) or until progressive disease, unacceptable toxicity or death, or until another discontinuation criterion is met, including roughly 6 weeks (two cycles) for concurrent administration of chemotherapy and radiotherapy. Cisplatin in combination with paclitaxel was chosen as chemotherapy for this trial because it is recommended by treatment guidelines [Citation5] and has shown a comparable benefit in PFS and OS outcomes with a similar safety profile as other regimens [Citation7]. An Independent Data Monitoring Committee will assess the adverse events in the first 20 enrolled patients (~10 patients per treatment arm) after they have had at least 6 weeks of follow-up after the last dose of radiotherapy. The Independent Data Monitoring Committee will also perform regular safety and efficacy monitoring throughout the conduct of the trial (at least every 6 months).
Outcome measures
The primary end point of this study is PFS, defined as the time from randomization to the first documented disease progression, as determined by BIRC per RECIST v1.1, or death from any cause, whichever occurs first, in the ITT analysis set for patients receiving tislelizumab in combination with cCRT (Arm A) and placebo in combination with cCRT (Arm B). While the most convincing measure of clinical benefit in oncology drug trials is generally considered to be an improvement in OS, these data require large patient numbers, long follow-up, and can be confounded by in-trial crossover, effective subsequent-line therapies and mortality unrelated to disease [Citation33]. For these reasons, OS is defined as one of the secondary efficacy end points. Other secondary efficacy end points for this study include objective response rate and DoR by BIRC per RECIST v1.1.
Tumor assessments will occur at baseline, every 9 weeks for the first 54 weeks and every 12 weeks thereafter – regardless of treatment delays – until disease progression. Screening assessments will include CT scans or MRI of the neck, chest and abdomen, and esophagography. If feasible, esophagoscopy, including esophagoendoscopic ultrasonography will also be included. 18F-fluorodeoxyglucose positron emission tomography may be used if clinically indicated. Tumor assessments during the study will include CT scans (with oral/intravenous contrast, unless contraindicated) or MRI (with preference for CT) of the neck, chest and abdomen, as well as esophagography. Esophagoscopy and biopsy (if disease progression is suspected) may also be included. Survival follow-up information will be collected every 3 months after treatment discontinuation until death, loss to follow-up, withdrawal of consent or study termination by the sponsor. The safety and tolerability of tislelizumab in combination with cCRT will be evaluated by examining the incidence and severity of treatment-emergent adverse events graded according to the National Cancer Institute Common terminology for adverse events (NCI-CTCAE) v5.0 and will be monitored throughout the study. Patient-reported health-related quality of life outcomes will be assessed using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-C30 and EORTC QLQ-Oesophageal Cancer Module (EORTC QLQ-OES18) and will be collected at screening or baseline, prior to dosing of every treatment cycle for the first six cycles, then after every other cycle until the end of treatment.
Statistics
All efficacy analyses will be assessed in the ITT analysis set, defined as all randomized patients. Progression-free survival per BIRC will be compared between Arm A and Arm B using a stratified log-rank test by using the stratification factors at randomization (ECOG performance status: 0 vs 1 and clinical stage: II/III vs IVa per American Joint Committee on Cancer eighth edition staging primer for cancer of the esophagus and esophagogastric junction [Citation34]). Median PFS with 95% CI, if estimable, will be constructed using a generalized Brookmeyer and Crowley method. The cumulative probability of PFS at 3, 6, 9, 12 and 24 months (if estimable) will be calculated and presented with two-sided 95% CI using Greenwood’s formula. The HR of PFS will be estimated using a Cox proportional hazards model, and the 95% CI for the HR will be provided. For objective response rate, the proportion of patients with either a complete or partial response by BIRC per RECIST v1.1 in the ITT analysis set will be presented and Clopper–Pearson 95% CIs will be calculated for each treatment arm. Patients without any postbaseline assessment will be considered nonresponders. Methodology similar to that used to evaluate PFS will be applied to OS and DoR; the population used for DoR will only include patients with responses. Safety/tolerability data will be summarized from the safety analysis set, which includes patients receiving at least one dose of study drug. Health-related quality of life data will also be summarized.
Ethical conduct
This study will be performed in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice guidelines and the principles of informed consent. Written informed consent will be obtained from each patient prior to screening. The protocol will be approved by regulatory authorities and independent ethics committees prior to initiation.
Conclusion
Data from Phase III studies have shown that immune CPIs have improved outcomes for patients with ESCC with a tolerable safety profile. The Phase III, randomized, double-blind, placebo-controlled RATIONALE 311 study is among the first registrational Phase III, multicenter, controlled clinical trials designed to examine the efficacy and safety of a CPI in combination with cCRT. This study may help to address the unmet need for new therapeutic options for patients with localized ESCC.
Executive summary
Current treatment for esophageal squamous cell carcinoma
Definitive chemoradiotherapy (CRT) is the standard of care for inoperable locoregionally advanced esophageal squamous cell carcinoma (ESCC).
Outcomes of patients with inoperable locally advanced ESCC are suboptimal, as many patients experience treatment failure after definitive CRT and the 5-year survival rate is around 20%.
Immune checkpoint inhibitors (CPIs), such as anti-PD-(L)1 antibodies, have led to a paradigm shift in ESCC treatment and preclinical evidence suggests that synergism may result when radiotherapy and immune CPIs are administered together for the treatment of locally advanced ESCC.
Tislelizumab as a PD-1 inhibitor for treatment of ESCC
Tislelizumab is a humanized IgG4 monoclonal antibody with high affinity and binding specificity for PD-1.
Tislelizumab was specifically engineered to minimize FcɣR binding on macrophages to abrogate antibody-dependent phagocytosis, a mechanism of T-cell clearance and potential resistance to anti-PD-1 therapy.
Tislelizumab monotherapy has demonstrated a survival benefit as second-line treatment of ESCC and tislelizumab in combination with chemotherapy and has also shown preliminary antitumor activity as first-line treatment of ESCC.
RATIONALE 311 study
This Phase III, randomized, double-blind, placebo-controlled study evaluates the efficacy and safety of tislelizumab in combination with concurrent CRT (cCRT) in patients with inoperable localized ESCC.
Adult patients (aged 18–75 years) with histologically confirmed localized ESCC with an Eastern Cooperative Oncology Group performance status ≤1 for whom cCRT is suitable and surgery is unsuitable/declined are eligible.
The primary objective of RATIONALE 311 is to compare progression-free survival, as assessed by a Blinded Independent Review Committee per RECIST v1.1.
Secondary objectives will include a comparison of tislelizumab plus cCRT with the placebo control in terms of efficacy assessments (overall response rate, duration of response rate and overall survival), measures of health-related quality of life, as well as safety/tolerability.
Conclusion
Among the first registrational Phase III, multicenter, controlled clinical trials designed to examine the efficacy and safety of a CPI in combination with cCRT, the current study may help to address the unmet need for new therapeutic options for patients with localized ESCC.
Author contributions
All authors were involved in the design of this study. Additionally, all authors agreed to the submission of this manuscript and vouch for the completeness and accuracy of the information contained within. The corresponding author was responsible for the decision to submit the manuscript for publication.
Ethical conduct of research
The authors state that the study will be performed in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice guidelines and the principles of informed consent. Written informed consent will be obtained from each patient prior to screening. The protocol will be approved by regulatory authorities and independent ethics committees prior to initiation.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions, BeiGene will consider requests for the protocol, data dictionary and statistical analysis plan. Data requests may be submitted to [email protected].
Acknowledgments
The authors acknowledge the investigative center study staff, the study patients and their families.
Financial & competing interests disclosure
The study protocol was developed by BeiGene, Ltd. in collaboration with the study steering committee. BeiGene, Ltd. was also involved in data collection, analysis and interpretation of results. Statistical analyses were performed by statisticians at BeiGene, Ltd. Professional medical writers, funded by BeiGene, Ltd., assisted with the development and submission of this manuscript under the authors’ guidance. W Huang, L Li, W Yu, C Wei, Y Wang and W Shen are employees of Beigene. R Yu, W Wang, T Li, J Li, K Zhao, W Wang, L Liang, H Wu, D Ai and Z Xiao declare no conflicts of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
BeiGene, Ltd. provided financial support for this manuscript, including writing and editorial assistance by S Lindsey and E Hermans, (Peloton Advantage, LLC, an OPEN Health company, NJ, USA).
Additional information
Funding
References
- International Agency for Research on Cancer . Oesophagial fact sheet, 2020. Global Cancer Observatory (2020). https://gco.iarc.fr/today/data/factsheets/cancers/6-Oesophagus-fact-sheet.pdf
- Qiu MJ , YangSL, WangMMet al. Prognostic evaluation of esophageal cancer patients with stages I–III. Aging (Albany NY)12(14), 14736–14753 (2020).
- Abbas G , KrasnaM. Overview of esophageal cancer. Ann. Cardiothorac. Surg.6(2), 131–136 (2017).
- Arnold M , SoerjomataramI, FerlayJ, FormanD. Global incidence of oesophageal cancer by histological subtype in 2012. Gut64(3), 381–387 (2015).
- National Health Commission of the People's Republic of China . Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version). Chin. J. Cancer Res.31(2), 223–258 (2019).
- Urba SG , OrringerMB, IanettonniM, HaymanJA, SatoruH. Concurrent cisplatin, paclitaxel, and radiotherapy as preoperative treatment for patients with locoregional esophageal carcinoma. Cancer98(10), 2177–2183 (2003).
- Ai D , YeJ, ChenYet al. Final results of a Phase III randomized trial of comparison of three paclitaxel-based regimens concurrent with radiotherapy for patients with local advanced esophageal squamous cell carcinoma (ESO-Shanghai2). Rad. Oncol.103(3), ( abstract 91) 4564 (2020).
- Stahl M , BudachW. Definitive chemoradiotherapy. J. Thorac. Dis.9(Suppl. 8), S792–S798 (2017).
- Barbetta A , HsuM, TanKSet al. Definitive chemoradiotherapy versus neoadjuvant chemoradiotherapy followed by surgery for stage II to III esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg.155(6), 2710–2721e2713 (2018).
- Sasaki Y , KatoK. Chemoradiotherapy for esophageal squamous cell cancer. Jpn J. Clin. Oncol.46(9), 805–810 (2016).
- Conroy T , GalaisMP, RaoulJLet al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, Phase II/III trial. Lancet Oncol.15(3), 305–314 (2014).
- Cooper JS , GuoMD, HerskovicAet al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA281(17), 1623–1627 (1999).
- Crosby T , HurtCN, FalkSet al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, Phase II/III randomised trial. Lancet Oncol.14(7), 627–637 (2013).
- Kelly RJ , AjaniJA, KuzdzaJet al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer (EC/GEJC) following neoadjuvant chemoradiation therapy (CRT): first results of the CheckMate 577 study. Ann. Oncol.31(Suppl.4), S1193–S1194 (2020).
- Kato K , ChoBC, TakahashiMet al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, Phase III trial. Lancet Oncol.20(11), 1506–1517 (2019).
- Yamamoto S , KatoK, DaikoHet al. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol.16(19), 1351–1357 (2020).
- Kojima T , ShahMA, MuroKet al. Randomized Phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol.38(35), 4138–4148 (2020).
- Kato K , SunJ, ShahMAet al. Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The Phase III KEYNOTE-590 study. Ann. Oncol.31(Suppl. 4), S1192–S1193 (2020).
- Chau I , DokiY, AjaniJAet al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line treatment for advanced esophageal squamous cell carcinoma (ESCC): first results of the CheckMate 648 study. J. Clin. Oncol.39(Suppl. 15), abstract 4000 (2021).
- Lee A , KeamSJ. Tislelizumab: first approval. Drugs80(6), 617–624 (2020).
- Zhang T , SongX, XuLet al. The binding of an anti-PD-1 antibody to FcγRI has a profound impact on its biological functions. Cancer Immunol. Immunother.67(7), 1079–1090 (2018).
- Dahan R , SegaE, EngelhardtJ, SelbyM, KormanAJ, RavetchJV. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell28(3), 285–295 (2015).
- Hong Y , FengY, SunHet al. Tislelizumab uniquely binds to the CC’ loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio11(3), 782–792 (2021).
- Desai J , DevaS, LeeJSet al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J. Immunother. Cancer8(1), e000453 (2020).
- Shen L , GuoJ, ZhangQet al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, Phase I/II study. J. Immunother. Cancer8(1), e000437 (2020).
- Xu J , BaiY, XuNet al. Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Clin. Cancer Res.26(17), 4542–4550 (2020).
- Shen L , KatoK, KimS-Bet al. RATIONALE 302: randomized, Phase III study of tislelizumab versus chemotherapy as second-line treatment for advanced unresectable/metastatic esophageal squamous cell carcinoma. J. Clin. Oncol.39(Suppl.15), 4012 (2021).
- Liu SY , WuYL. Tislelizumab: an investigational anti-PD-1 antibody for the treatment of advanced non-small cell lung cancer (NSCLC). Expert Opin. Investig. Drugs29(12), 1355–1364 (2020).
- Oh P , DuKL, LeichmanL, AifantisI. PD-1 blockade enhances the efficacy of chemoradiation in a mouse model of esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys.96(2), S127–S128 (2016).
- Antonia SJ , VillegasA, DanielDet al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med.377(20), 1919–1929 (2017).
- Liao XY , LiuCY, HeJF, WangLS, ZhangT. Combination of checkpoint inhibitors with radiotherapy in esophageal squamous cell carcinoma treatment: a novel strategy. Oncol. Lett.18(5), 5011–5021 (2019).
- Kelly RJ , SmithKN, AnagnostouVet al. Neoadjuvant nivolumab plus concurrent chemoradiation in stage II/III esophageal/gastroesophageal junction cancer. J. Clin. Oncol.37(Suppl.4), 142 ((2019).
- Gill S , BerryS, BiagiJet al. Progression-free survival as a primary endpoint in clinical trials of metastatic colorectal cancer. Curr. Oncol.18(Suppl. 2), S5–S10 (2011).
- Rice TW , IshwaranH, FergusonMK, BlackstoneEH, GoldstrawP. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J. Thorac. Oncol.12(1), 36–42 (2017).