Abstract
The present study aimed to explore patient preferences for attributes of advanced hepatocellular carcinoma (HCC) treatments. A stated preference survey was completed by 150 patients with HCC living in Europe. Overall survival (OS) was the most important attribute, closely followed by risk of diarrhea and hypertension, and other adverse event (AE) risks. Patients were willing to trade OS to reduce AE risks. While less important than OS and AEs, patients also preferred shorter waiting times, and one-off administration of selective internal radiation therapy and oral tablets over intravenous infusions. Although patients placed the most value on extending OS, they were willing to forego OS to avoid risk of treatment-related AEs, to maintain their quality of life.
Lay abstract
This study aimed to understand patient preferences for characteristics of advanced hepatocellular carcinoma (HCC) treatments. A total of 150 people with HCC in Europe were presented a series of questions asking them to choose between two hypothetical treatments. Overall, length of life was the most important issue for patients, followed by avoiding diarrhea and hypertension, and then other side effects and treatment risks. Patients were willing to forego some months of life to avoid side effects or risks. Patients preferred to be given their treatment via a single minimally invasive hospital procedure or oral daily tablets compared with intravenous drips. In conclusion, although patients placed the most value on overall length of life, side effects and treatment risks were also important.
Hepatocellular carcinoma (HCC) is the most prevalent primary liver cancer [Citation1], accounting for 70–90% of liver cancer deaths worldwide [Citation2,Citation3]. Treatment is not curative for most patients, who often experience poor clinical outcomes, with approximately three-quarters of patients dying within five years of diagnosis [Citation4]. Patients with HCC have worse health-related quality of life (HRQL) than people with chronic liver disease [Citation5]. Impairments have been reported in physical and psychological health and functional wellbeing. Fan and colleagues suggest that compromised physical wellbeing may often be a consequence of treatment side effects. Pain is the main symptom reported by patients, with an increasing impact on HRQL as patients approach the end of life [Citation4].
Sorafenib has been the standard of care for advanced HCC for over 10 years, following the SHARP trial [Citation6–8]. The trial showed a median overall survival (OS) of 10.7 months for sorafenib versus 7.9 for placebo [Citation6]. Lenvatinib, another oral tyrosine kinase inhibitor (TKI), was subsequently introduced based on the REFLECT trial, showing noninferiority versus sorafenib and a relatively similar tolerance profile [Citation9].
The combination therapy of atezolizumab plus bevacizumab appears set to replace TKIs as the first-line treatment for advanced HCC for most patients in most Western countries [Citation10,Citation11]. The IMbrave150 trial reported a median OS of 19.2 months for atezolizumab plus bevacizumab, versus 13.4 months for sorafenib [Citation12]. The incidence of grade 3 or 4 adverse events (AEs) was similar in both groups (56.5 vs 55.1% for atezolizumab plus bevacizumab vs sorafenib, respectively); however time to deterioration in quality of life and physical and role functioning were longer for patients receiving atezolizumab plus bevacizumab, suggesting better tolerability for patients compared with sorafenib [Citation12]. Despite advances in therapy, options for treating advanced HCC remain limited. An unmet medical need persists for therapy options with minimal impact on patients' HRQL.
Patients with portal vein thrombosis or a tumor-induced degradation of their performance status are considered to have advanced HCC, even if the cancer has not spread outside the liver [Citation13]. Selected patients without extrahepatic spread and with adequate tumor morphology and vascularization can be considered for an alternative locoregional treatment, such as selective internal radiation therapy (SIRT), after assessment by a multidisciplinary team. SIRT is an intra-arterial procedure usually requiring a single administration through a femoral or radial puncture [Citation14], performed in an in- or outpatient hospital setting. The SARAH trial randomized 467 patients to SIRT with SIR-Spheres® Y-90 resin microspheres or to sorafenib, in 25 centers in France [Citation15]. The primary end point (survival difference) was not met, however benefit in terms of HRQL was observed for SIRT versus the sorafenib group. Specifically, patient-reported global health status was significantly better in the SIRT versus the sorafenib group, with between group differences increasing over time. Further, the number of patients with at least one treatment-related grade 3 to 5 AE was 92 (41%) of 226 patients in the SIRT group versus 136 (63%) of 216 patients in the sorafenib group. A similar pattern of results emerged from the SIRveNIB trial whereby OS did not differ between SIRT and sorafenib but SIRT had a better toxicity profile [Citation16].
In the context of a median OS of 10 to 19 months with existing treatment options, preserving HRQL is an important consideration in clinical decision-making. Choices between treatment options for patients should therefore reflect their views regarding OS, treatment convenience and the risk of adverse events, particularly in the palliative setting. The present study was designed to elicit patient treatment preferences in advanced HCC using standardized stated preference methods. Discrete choice experiments (DCEs) are an accepted and widely-used method for eliciting treatment preferences of patients [Citation17,Citation18]. Previous research using similar methods has explored patient views of transarterial chemoembolization (TACE) for HCC [Citation19].
Methods
The current study was designed to systematically elicit patient preferences for OS, avoidance of side effects and different modes of treatment administration and schedules in the context of advanced HCC. The DCE survey described HCC treatments in terms of different attributes (i.e., characteristics or features) of HCC treatments, which were selected to reflect current treatment options. Survey participants were asked to choose between pairs of hypothetical treatments that varied in terms of these attributes. By analyzing which treatments participants choose, the relative importance of the different attributes can be estimated [Citation20].
Identification of treatment attributes
Treatment attributes were initially selected based on data from clinical trials for treatments for advanced HCC, including sorafenib, lenvatinib, atezolizumab plus bevacizumab and SIR-Spheres® [Citation9,Citation12,Citation15,Citation16,Citation21]. Information from patient support group websites (Cancer.net, Cancer Research UK website, SIRT UK Network) was also used. Attributes were considered relevant if they reflected differences between the main treatment options; treatment differences also informed how attributes were presented. To ensure the hypothetical profiles presented in the choice questions would be plausible, the inclusion of two or more related attributes was avoided, either through a selection process or by combining them. For example, both OS and progression-free survival (PFS) were not included simultaneously, and nausea, vomiting and loss of appetite were combined into a single attribute. The initial selection of attributes reflecting AEs was based on consideration of the severity and frequency of these events between treatment options. Seventeen possible attributes were considered (OS, PFS, tumor response, treatment type, treatment waiting time, discontinuation rates, dose reduction, fatigue, diarrhea, hand-foot syndrome, hypertension, weight loss, alopecia, skin conditions, anorexia, nausea/vomiting and proteinuria). Of the seventeen possible attributes, nine were included in the initial selection based on the criteria described above (OS, treatment schedule/mode of administration, treatment waiting time, fatigue, nausea/vomiting/loss of appetite, diarrhea, skin irritation, hypertension and alopecia). The initial selection was also designed to include attributes that represented both key benefits and limitations of all relevant treatments in order to avoid favoring one treatment over another. Draft lay descriptions of the initial selection of attributes and attribute levels were developed based on the literature.
Survey development: clinician feedback & patient cognitive debriefing
Semistructured interviews with a radiologist, two medical oncologists and a hepatologist, all with clinical expertise in HCC, were undertaken to review the attributes. Clinicians were asked to provide feedback on the attributes in terms of clinical relevance, levels described in the survey, accuracy of the lay descriptions and any potential omissions. A revised set of attributes was incorporated into a survey draft. Interviews with patients with HCC (n = 5) elicited feedback on the relevance of the attributes and on the acceptability and understanding of the survey.
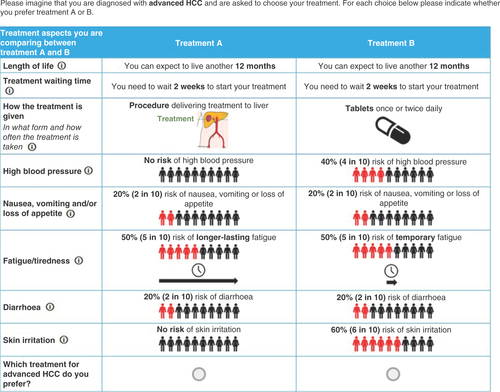
The final survey included the following attributes: length of life (OS); treatment waiting time; how the treatment is given (combining mode of administration and treatment schedule); high blood pressure; nausea, vomiting and/or loss of appetite; fatigue/tiredness; diarrhea; and skin irritation. All attributes were described according to three levels (). The attribute levels were designed to reflect the realistic range of each attribute based on existing HCC treatment options, so it was possible to understand their overall importance. The effectiveness of the treatments was described in terms of remaining life expectancy. How the treatment is administered was described as daily tablets, intravenous infusion in hospital every three weeks or a one-off hospital procedure. Treatment waiting time was included to reflect variation in typical waiting times between systemic therapies (no waiting time) and SIRT (up to 4 weeks). AEs included risk of high blood pressure (described as carrying a risk of developing severe high blood pressure which, if not managed, could become life-threatening) and nausea, vomiting and/or loss of appetite. Diarrhea and skin irritation were described as mostly grade 1–2 with some experiencing grade 3 (see for full descriptions). The final attribute descriptions and levels are presented in Appendix A.
Table 1. Final attributes of the survey with the description of each attribute and associated attribute levels.
Survey development: experimental design & descriptive survey items
The attributes and levels were combined into 18 choice sets using a D-efficient design generated with NGene software version 1.2.1 [Citation22]. A sample choice question is shown in .
The survey captured brief sociodemographic and clinical data. Patient-reported tumor numbers and location, and overall health were used as a proxy for cancer stage, informed by the Barcelona Clinic Liver Cancer (BCLC) staging system and described in Appendix B. Comprehension questions were also included to check understanding of the hypothetical treatment scenario and attribute descriptions. The survey was developed in English and translated into German, French and Spanish for data collection in those countries.
Participant recruitment
This study was reviewed and received exempt status determination by the Western Institutional Review Board (WIRB). All participants gave informed consent prior to taking part in the study. Patients with HCC were recruited through a healthcare research recruitment agency between July and November 2020. Potential participants were invited to take part in the study via email with a link to the screening questionnaire. Participants were eligible if they had a self-reported diagnosis of HCC (any stage of disease), were resident of a study country (France, Germany, Spain or UK) and were willing and able to provide informed consent. If eligible, participants were directed to the main online survey. Patients with any HCC stage were eligible for inclusion in the study, but they were asked to imagine they had advanced stage HCC. A description shown in Appendix C was developed to provide background information on symptoms, prognosis and how treatment options differ at this stage of the disease, which was also assessed in the patient interviews.
Statistical analysis
Choice data were analyzed in R using Apollo, an R package for choice model estimation [Citation23]. Descriptive data were analyzed using Stata version 16.0. Discrete choice data were analyzed using logit models; based on goodness-of-fit properties (log likelihood, Akaike information criterion and Bayesian information criterion), the results from the random parameter logit (RPL) model are presented. The model was estimated using the maximum simulated likelihood approach. The logit model estimates beta coefficients for each attribute which indicate their importance compared with a reference category. Beta coefficients were converted to odds ratios (ORs) to aid interpretation. For categorical variables, the strength of preference associated with each attribute level was measured with respect to a reference level. For continuous variables, the strength of preference relates to a 1-unit change in the attribute (i.e., a 1-month increase in the length of life attribute, a 1-week increase in the treatment waiting time attribute, or a 10% increase in risk of high blood pressure, nausea/vomiting/loss of appetite, diarrhea or skin irritation attributes). Any left bias in people's choices (e.g., selecting the choice shown on the left) was accommodated using an alternative-specific constant (i.e., a constant was added in the model for choosing treatment B over treatment A, independent of the treatment attributes). The extent to which participants were willing to trade between treatment attributes was explored using marginal rates of substitution, defined as the number of months of OS a patient is willing to give up to improve another treatment attribute (for example accepting a shorter OS if there was lower risk of an AE).
Results
Demographic and clinical characteristics of the sample are shown for the total sample and by country in . BCLC staging based on patient-reported tumor numbers and location, and overall health was (very) early/intermediate HCC in 21%, advanced HCC in 43%, and end stage HCC in 36% of the cases.
Table 2. Sample characteristics.
All treatment attributes were independent predictors of treatment choice (). This means that, for the sample as a whole, all features of the treatments were considered by the participants when making their choices, and that participants were willing to make trade-offs between those. The weight given to each attribute (patients' strength of preference for each attribute) is presented with confidence intervals in and .
Graph shows the importance of changes in the levels of each attribute in the survey (based on beta coefficients from the logit model results presented in with 95% CI).
*p < 0.05; **p < 0.001. For continuous variables, p-values for trend are shown; for categorical variables, p-values for attribute levels are compared with reference level (how the treatment is given: SIRT is the reference level; fatigue/tiredness: no risk of fatigue is the reference level).
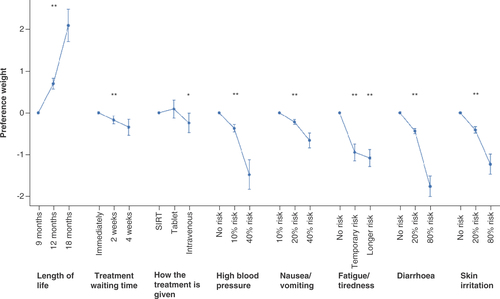
Table 3. Patient preference results from the random parameters model (n = 150).
Patient preferences for overall survival
While OS (described as length of life in the survey) was the most important treatment attribute for the sample, the results show that participants also considered AE risks and other treatment attributes when making their decisions. Regarding OS, patients strongly preferred a treatment with 12 or 18 months OS compared with 9 months (). Patients were 26% more likely to prefer a treatment for each additional month of life (OR = 1.26, 95% CI: 0.88–0.96).
Patient preferences for treatment waiting time & administration
For treatment waiting time, patients preferred to start their treatment as soon as possible. They were 8% less likely to prefer a treatment for each additional week that they had to wait to receive it (OR = 0.92, 95% CI: 0.88–0.97), that is, they were 16% less likely to choose a treatment with a 4-week waiting time compared with a 2-week waiting time. There was no difference in patients' preference for receiving their treatment as a tablet or as a liver-directed procedure (SIRT). Participants preferred to avoid intravenous therapy, as is required for the administration of atezolizumab plus bevacizumab; if all attributes are held constant, participants indicated that they would be 22% less likely to choose an intravenous therapy compared with the SIRT procedure (OR = 0.78, 95% CI: 0.62–0.99).
Patient preferences for avoidance of adverse events
Patients strongly preferred treatments with a lower risk of hypertension, described as potentially life-threatening if not treated, an AE related to all of the systemic therapies included in the survey. If the risk of high blood pressure increased by 10% with a treatment, participants were 31% less likely to choose the treatment (OR = 0.69, 95% CI: 0.63–0.75). Patients also preferred to avoid treatments that caused mild to moderate nausea, vomiting and loss of appetite. They were 20% less likely to choose a treatment that was associated with a 10% increased risk of nausea, vomiting and appetite loss (OR = 0.80, 95% CI: 0.76–0.85). Similarly, patients preferred to avoid diarrhea. Diarrhea was described as mild to moderate for most patients with up to six episodes a day, but some may develop severe diarrhea. If a treatment increased the risk of diarrhea by 10%, patients were 20% less likely to prefer such a treatment (OR = 0.80, 95% CI: 0.78–0.83). Hand-foot skin reaction is a known AE of TKIs and was described in the survey as a skin rash with mild to moderate irritation, some pain, and that could limit usual activities. Treatments which increase the risk of skin rash by 10% were 18% less likely to be chosen by participants, all other attributes being held constant (OR = 0.82, 95% CI: 0.78–0.85). Fatigue was described in terms of a 50% risk of developing either temporary fatigue (lasting up to a week) or longer term fatigue (likely to last months). Compared with no fatigue, patients were 61% less likely to choose a treatment with temporary fatigue (OR = 0.39; 95% CI: 0.32–0.48) and 66% less likely to choose a treatment with long-term fatigue (OR = 0.34; 95% CI: 028–0.42).
Trade-offs between overall survival & other treatment attributes
The importance of each attribute for participants was estimated in terms of marginal rates of substitution with OS. Marginal rates of substitution indicate how many months of life patients may be willing to forego to achieve improvements in other attributes. The results in show the extent to which patients were willing to trade treatment convenience and AEs against OS. also shows information regarding the profile of sorafenib, atezolizumab plus bevacizumab and SIRT in terms of the survey attributes.
Table 4. Marginal rates of substitution for differences in attribute levels with reference to treatment-specific characteristics.
To reduce the risk of high blood pressure by 10%, participants were willing to forego 1.6 months of OS (95% CI: 1.2–2.0); considering the incidence of hypertension reported for systemic therapies in advanced HCC, patients may be willing to forego up to 6 months of life to completely avoid the risk of this adverse event. For a 10% reduction in risk of nausea, vomiting and/or loss of appetite, participants were willing to forego 1.0 month of OS (95% CI: 0.7–1.2). To avoid fatigue completely, participants were willing to trade over 4 months of OS.
Reductions in the risk of either skin rash or diarrhea by 10% were considered to be equivalent to 0.9 months of OS. Patients may therefore be willing to forego approximately 4 months of life to completely avoid the risk of skin rash associated with sorafenib and lenvatinib (between 2.1 and 4.9 months of OS based on a 23–55% risk of skin rash) and up to 6 months of life for diarrhea (between 2.7 and 6.9 months of OS based on a 30–77% risk of diarrhea). Patients are also willing to trade approximately 1.7 months of OS to avoid a 18.8% risk of having diarrhea as an AE of atezolizumab plus bevacizumab, and up to 1 month of OS to avoid a 2–13% risk of diarrhea associated with SIRT. Having to undergo an intravenous infusion every 3 weeks was equivalent to 1.1 months of OS (95% CI: 0.0–2.1). To avoid waiting for treatment, participants were willing to trade up to 1.6 months of OS (assuming that treatment waiting times could be up to 4 weeks).
Discussion
The current stated preference survey provides several important insights into patient preferences for advanced HCC treatments. All treatment attributes included in the survey were significant independent predictors of patient choice. While some attributes were more important than others, the findings show that participants considered all of them when making their choices. Length of life was the most important treatment attribute. Patients also had a strong preference to avoid diarrhea, high blood pressure, fatigue, skin irritation and nausea, vomiting and/or loss of appetite. All of these AEs are associated with one or more of the current treatment options for advanced HCC.
Remaining life expectancy for patients with advanced HCC is very low. Therefore, their treatment choices reflect a trade-off between maximizing the length of their remaining life and maintaining their quality of life. Treatment-related AEs are more common for systemic therapies compared with SIRT [Citation9,Citation12,Citation15,Citation16]. While AEs such as diarrhea, fatigue and hand-foot skin reaction are known for their negative impact on patients' quality of life [Citation24], the present study demonstrates that patients are also willing to trade length of life to avoid the risk of other AEs such as hypertension and nausea/vomiting. These results are consistent with previous research that has also reported that patients with cancer are willing to trade survival to avoid severe AEs [Citation25,Citation26].
Although the present study, by design, evaluated patient preferences for individual types of AEs as separate attributes of the treatments, it is worth noting that people receiving treatment for advanced HCC will commonly have more than one AE and that the risk of events persists over the duration of their treatment. In the SARAH trial, patients who had any treatment-related AE reported a mean of 14.0 events of any grade (2837 events for 203 patients), and 3.0 events of grade 3 or 4 (411 events for 136 patients) during the follow-up period of the trial [Citation15]. Atezolizumab, an immune checkpoint inhibitor, can further result in immune-related AEs involving multiple organs [Citation27].
Hypertension is an adverse event commonly associated with systemic therapies for advanced HCC. The present study results suggest that patients may be willing to trade 2.2, 4.8 or 6.7 months of life to avoid the risks of hypertension associated with sorafenib (14%), atezolizumab plus bevacizumab (29.8%) or lenvatinib (42%), respectively, in their pivotal trials. Because the reported incidence of any grade hypertension with SIRT is 0 to 3% [Citation15,Citation16], the study findings indicate that this attribute is a significant driver of patient preference toward SIRT.
Nevertheless, the magnitude of patients' aversion for hypertension may not be clinically plausible. While the description of high blood pressure was developed by the authors for the survey, validated by clinicians and tested in cognitive debriefing interviews with patients to reflect all grades of severity of this AE, it cannot be ruled out that surveyed patients may have focused on the more severe aspects of the description when making their choices. This may have been caused in part by the reference to “life-threatening” consequences of hypertension: indeed, the corresponding attribute described how “[patients] may not have symptoms, but […] may develop severe high blood pressure which, if it is not managed, could increase [their] risk of serious life-threatening health conditions (e.g., heart attack and stroke).”
Although hypertension is described in the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v5.0 classification as having “life-threatening consequences,” this is especially true for grade 4 hypertension. Furthermore, while deaths due to cardiovascular events were reported in clinical trials of systemic therapies, it is uncertain if hypertension contributed to these deaths. Finally, the description may not have sufficiently emphasized the possibility of managing hypertension using additional treatment, described as “If you develop high blood pressure you would need to take additional medication or reduce your cancer treatment.” A conservative sensitivity analysis considering only grade 3–4 hypertension risks suggests that the avoidance of this AE is equivalent to trading up to 0.2 to 3.7 months of OS for sorafenib and lenvatinib (1–23% risk) and 2.4 months for atezolizumab plus bevacizumab (15.2% risk).
Overall, treatment administration/schedule and treatment waiting time were less important to patients than length of life or adverse events, but remained relevant. SIRT and the daily oral administration of tablets applicable for TKIs were both preferred to the intravenous infusions required for atezolizumab plus bevacizumab.
Patients also preferred not having to wait for their treatment. The administration of atezolizumab plus bevacizumab requires a prior endoscopy to rule out the presence of esophageal varices. This requirement may result in a delay to treatment that patients may wish to avoid—the present study found that patients would be willing to trade up to 1.6 months of life to avoid a 4-week delay in receiving treatment. The timing of this study may have impacted patient preferences for treatments requiring hospital visits or stays, as these treatments could be associated with more delays due to the COVID-19 pandemic.
SIRT was described in the present study as being performed via femoral or radial access, and during either one or two sequential hospital visits that were one to two weeks apart. While the description of treatment administration was based on current practice, it should be noted that this is evolving continuously. Patients have a preference for a radial as opposed to a femoral access for the administration of SIRT due to a shorter recovery time and lower levels of pain during the procedure [Citation28], and SIRT is increasingly performed with a radial access. In addition, same-day dosimetry and treatment options for Y-90 resin microspheres support an increasing use of single-session procedures for patients with HCC in real-word clinical practice. The estimated strength of preference for the mode of administration attribute of SIRT in the present study could therefore be conservative.
To our knowledge, this is the first study to explore patient preferences for advanced HCC therapies including the most recent developments in the treatment landscape. This study considered data from the latest clinical trials for recently approved treatment options and expert opinion from clinicians to develop the survey. Overall, although patients are likely to favor treatments that extend length of life, the impacts of treatment on HRQL are also significant drivers of patient preference. In the context of a shared decision-making process and with additional treatment sequences and combinations becoming available in advanced HCC, including novel ways of delivering personalized therapies, treatment decisions should be responsive to patients' views regarding treatment convenience and risk of adverse events as well as life expectancy. Patient preferences for advanced HCC treatment options may differ from those of clinicians.
The interpretation of study findings should consider a number of study limitations. First, the average age of the study sample was lower than the average patient age reported in studies in the literature [Citation15,Citation16,Citation29,Citation30]. This may reflect the method of recruitment and online data collection. It is possible, therefore, that the views of older patients, which may differ from younger patients, may be underrepresented. The study also included patients from four European countries in order to increase the generalizability of the results and achieve a robust sample size. The data were aggregated, but the sample was not sufficient to explore differences between countries; not all treatment options may be available in all four countries. The sample also included participants at different points in their treatment pathway; not all participants had advanced HCC. The survey asked participants to imagine that they had advanced disease (with the associated prognosis) when answering the choice questions; it is possible that survey responses were influenced by the participant's current stage of disease (e.g., milder disease than advanced HCC) despite the instruction to imagine they had advanced HCC. Considering the relatively small numbers of patients with advanced HCC, however, the researchers felt the study would benefit from including a larger sample of patients with any stage of HCC to ensure a sufficient sample size for a relatively robust analysis.
Although DCEs are an established method, it is worth noting that the survey and the choice tasks included relatively complex information on which patients were asked to make their choices. The treatment administration attribute, which required a lengthy description, and the fatigue attribute, describing both the duration and risk of fatigue, were both complex. The survey design included graphics and text to aid comprehension, and survey content was piloted with patients; however, it is possible that at least some participants did not fully understand the complexity of the choice tasks, which may have led to some measurement error. Although the survey was validated by clinicians from a range of backgrounds with experience treating HCC patients, their views may not be representative of the views of the wider community of clinical experts.
A limited number of attributes were selected to avoid presenting patients with overly complex treatment choices and to avoid increasing the required sample size to complete the study. The prevalence of specific AEs in clinical trials and the variations in their association with the treatments were key considerations when selecting potential attributes—not all AEs commonly observed in patients with advanced HCC were included in the survey. This is particularly true for liver failure or decompensation and other related symptoms (e.g., ascites, gastrointestinal bleeding). Considering the lack of variability in the rate of these AEs across the evidence base, they were not considered relevant for the present analysis. Lastly, the study findings reflect preferences in the context of current treatment options for advanced HCC. Further research may be needed as new treatments emerge.
Conclusion
This study examined what is important to people with HCC across four European countries when choosing a treatment for advanced HCC, given that OS is limited to approximately 1–2 years for these patients. The DCE survey identified that patients placed most value on extending OS. However, the results also demonstrated that they were willing to forego several months of OS in order to maintain their quality of life, by avoiding the risk of treatment-related adverse events. The study results support clinician and patient discussion around shared decision-making for treatment options in advanced HCC.
Summary points
People with unresectable, advanced hepatocellular carcinoma (HCC) have several first-line treatment options including systemic therapies (sorafenib, lenvatinib), the combination of atezolizumab and bevacizumab, and selective internal radiation therapy (SIRT).
This study aimed to understand patient preferences for attributes associated with advanced HCC treatments, including overall survival (OS), treatment waiting time, mode of administration and schedule, and the following adverse events (AEs): hypertension (defined as potentially life-threatening), nausea/vomiting/loss of appetite, fatigue, diarrhea and skin irritation.
A stated preference survey was completed by 150 patients living in Europe, of whom 20% had a single tumor nodule, 55% had multifocal intrahepatic disease and 21% had extrahepatic disease.
All treatment attributes were independent predictors of patient treatment choices. OS was the most important attribute, closely followed by risk of diarrhea and hypertension, and then by other AEs.
Patients were willing to trade OS to reduce risk of AEs: reducing risk of hypertension by 10% was equivalent to trading 1.6 months of OS; reducing risk of diarrhea, skin irritation or nausea/vomiting/loss of appetite by 10% was equivalent to trading 1.0 month of OS. Patients were willing to trade 4.0 months of OS to avoid a 50% risk of fatigue.
While less important than OS and AEs, patients also preferred shorter treatment waiting times, and one-off SIRT or an oral administration over intravenous infusions.
Study results suggest that although patients placed the most value on extending OS, they were willing to forego several months of OS to avoid the risk of treatment-related AEs and maintain quality of life.
Understanding patient treatment preferences may inform clinical decision-making in advanced HCC, and encourage shared decision-making between clinicians and patients.
Author contributions
SHL, DA and AJL designed the study, analyzed and interpreted the data and drafted the manuscript. RS and CC interpreted the data and revised the draft critically for important intellectual content. SS, FC, VB, VS and IA contributed to the design of the study and revised the draft for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical conduct of research statement
This study was reviewed and received exempt status determination by the Western Institutional Review Board (WIRB; July 10, 2020). All participants gave informed consent prior to taking part in the study.
Acknowledgments
The authors would like to thank Ralph Peters for advising on the study design and sharing clinical insights, the staff at Global Perspectives for their assistance recruiting patients, and the patients who participated in this study.
Financial & competing interests disclosure
This study was sponsored by Sirtex Medical United Kingdom Ltd. Suki Shergill, Fabien Colaone, Victoria K Brennan, Vincenzo A Straccia and Ion Agirrezabal are employees of Sirtex Medical, a manufacturer of yttrium-90 microspheres used for the treatment of hepatocellular carcinoma. Siu Hing Lo, Daniel Aggio and Andrew J Lloyd are employees of Acaster Lloyd Consulting and received funding from Sirtex Medical in support of this study. Rohini Sharma reports advisory roles for Advanced Accelerator Applications (AAA), Eisai, Roche and Sirtex Medical, and research grants from, AAA, Astex Pharmaceuticals and Boston Scientific. Charlotte E Costentin reports advisory roles for Intercept, Ipsen and Sirtex Medical, speaker bureau roles for AbbVie, Intercept and Ipsen, research grants from Gilead, and travel and accommodation expenses from AbbVie, Biotest, Gilead, Intercept and Ipsen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Ferlay J , SoerjomataramI, DikshitRet al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer136(5), E359–E386 (2015).
- Torre LA , BrayF, SiegelRL, FerlayJ, Lortet-TieulentJ, JemalA. Global cancer statistics, 2012. CA Cancer J. Clin.65(2), 87–108 (2015).
- Sartorius K , SartoriusB, AldousC, GovenderPS, MadibaTE. Global and country underestimation of hepatocellular carcinoma (HCC) in 2012 and its implications. Cancer Epidemiol.39(3), 284–290 (2015).
- Aly A , RonnebaumS, PatelD, DolehY, BenaventeF. Epidemiologic, humanistic and economic burden of hepatocellular carcinoma in the USA: a systematic literature review. Hepatic Oncol.7(3), HEP27 (2020).
- Fan SY , EiserC, HoMC. Health-related quality of life in patients With hepatocellular carcinoma: a systematic review. Clin. Gastroenterol. Hepatol.8 (7), 559–564 (2010).
- Llovet JM , RicciS, MazzaferroVet al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. [Internet].359(4), 378–390 (2008). Available from: https://pubmed.ncbi.nlm.nih.gov/18650514/.
- Rizzo A , RicciAD, BrandiG. Immune-based combinations for advanced hepatocellular carcinoma: shaping the direction of first-line therapy [Internet]. Futur. Oncol.17(7), 755–757 (2021).
- Faivre S , RimassaL, FinnRS. Molecular therapies for HCC: looking outside the box [Internet]. J. Hepatol.72(2), 342–352 (2020)
- Kudo M , FinnRS, QinSet al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet391(10126), 1163–1173 (2018).
- Kelley RK . Atezolizumab plus bevacizumab — a landmark in liver cancer. N. Engl. J. Med.382(20), 1953–1955 (2020).
- Rizzo A , RicciAD, BrandiG. Atezolizumab in advanced hepatocellular carcinoma: good things come to those who wait. Immunotherapy [Internet].13(8), 637–644 (2021).
- Finn RS , QinS, IkedaMet al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med.382(20), 1894–1905 (2020).
- Galle PR , FornerA, LlovetJMet al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J. Hepatol. [Internet].69(1), 182–236 (2018).
- Cancer Research UK . Selective internal radiation therapy (SIRT) (2020). https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/radiotherapy/internal/radioactive-implant-treatment/selective-internal-radiation-therapy-sirt.
- Vilgrain V , PereiraH, AssenatEet al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol.18(12), 624–1636 (2017).
- Chow PKH , GandhiM, TanSBet al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J. Clin. Oncol.36(19), 1913–1921 (2018).
- Johnson FR , LancsarE, MarshallDet al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Heal.16(1), 3–13 (2013).
- Hauber AB , GonzálezJM, Groothuis-OudshoornCGMet al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Heal.19(4), 300–315 (2016).
- Chiba T , HiraokaA, MikamiSet al. Japanese patient preferences regarding intermediate to advanced hepatocellular carcinoma treatments. Patient Prefer. Adherence.13, 637–647 (2019).
- De Bekker-Grob EW , RyanM, GerardK. Discrete choice experiments in health economics: a review of the literature. Health Econ.21(2), 145–172 (2012).
- Cheng A-L , QinS, IkedaMet al. Atezolizumab + bevacizumab vs sorafenib in patients with unresectable hepatocellular carcinoma: phase 3 results from IMbrave150. In: ESMO Asia (2019).
- ChoiceMetrics . Ngene 1.2 USER MANUAL & REFERENCE GUIDE The Cutting Edge in Experimental Design [Internet]. http://www.choice-metrics.com.
- Hess S , PalmaD. Apollo: a flexible, powerful and customisable freeware package for choice model estimation and application. J. Choice Model.32, 100170 (2019).
- Gill J , BaiceanuA, ClarkPJet al. Insights into the hepatocellular carcinoma patient journey: Results of the first global quality of life survey. Futur. Oncol.14(17), 1701–1710 (2018).
- Wong XY , LimAQJ, ShenQet al. Patient preferences and predicted relative uptake for targeted therapies in metastatic colorectal cancer: a discrete choice experiment. Curr. Med. Res. Opin.36(10), 1677–1686 (2020).
- MacEwan JP , Gupte-SinghK, ZhaoLM, ReckampKL. Non–small cell lung cancer patient preferences for first-line treatment: a discrete choice experiment. MDM Policy Pract.5(1), (2020).
- Sangro B , ChanSL, MeyerT, ReigM, El-KhoueiryA, GallePR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. (2020).
- Liu LB , CedilloMA, BishayVet al. Patient experience and preference in transradial versus transfemoral access during transarterial radioembolization: a randomized single-center trial. J. Vasc. Interv. Radiol. [Internet].30(3), 414–420 (2019).
- Sangro B , MainiCL, EttorreGMet al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur. J. Nucl. Med. Mol. Imaging.45(10), 1721–1730 (2018).
- Costentin CE , MouradA, LahmekPet al. Hepatocellular carcinoma is diagnosed at a later stage in alcoholic patients: results of a prospective, nationwide study. Cancer124(9), 1964–1972 (2018).