Abstract
Aim: To describe initial treatment patterns and survival of patients diagnosed with non-small-cell lung cancer (NSCLC) in Denmark, before immune checkpoint inhibitor and later-generation tyrosine kinase inhibitor use. Patients & methods: Adults diagnosed with incident NSCLC (2005–2015; follow-up: 2016). Initial treatments and overall survival (OS) are reported. Results: 31,939 NSCLC patients (51.6% stage IV) were included. Increasing use of curative radiotherapy/chemoradiation for stage I, II/IIIA and IIIB NSCLC coincided with improved 2-year OS. Systemic anticancer therapy use increased for patients with stage IV non-squamous NSCLC (53.0–60.6%) but not squamous NSCLC (44.9–47.3%). 1-year OS improved in patients with stage IV non-squamous NSCLC (23–31%) but not squamous NSCLC (22–25%). Conclusion: Trends indicated improved OS as treatments evolved between 2005 and 2015, but the effect was limited to 1-year OS in stage IV disease.
In Denmark, lung cancer ranks first in cancer incidence and cause of cancer death, with almost 5000 new cases and >4000 deaths estimated in 2018 [Citation1]. Historically, lung cancer treatment was limited to surgery for localized disease, radiotherapy (RT) for locally advanced disease and platinum-based doublet chemotherapy for advanced disease. However, expanded options in non-small-cell lung cancer (NSCLC), the most frequent subtype, now include surgery with adjuvant therapy, curative RT, chemoradiation and systemic therapies including tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs). TKIs for EGFR and ALK were first available in Denmark in 2010 and 2012, respectively, and ICIs were approved for NSCLC in Europe for second-line therapy or consolidation therapy beginning in 2015 and from 2017 as first-line therapy [Citation2–8].
SCAN-LEAF, a retrospective longitudinal study, aims to understand the evolution of treatment patterns and overall survival (OS) with the evolution of treatment recommendations between 2005 and 2015 in Scandinavia [Citation8]. This study is part of I-O Optimise, a multinational initiative aimed at developing a research framework to provide insights into evolving real-world management of thoracic malignancies [Citation2].
In this SCAN-LEAF analysis of the Danish NSCLC population, we describe the evolution of initial treatment patterns and OS by stage and histology among adult patients diagnosed with NSCLC in Denmark between 2005 and 2015.
Patients & methods
Study design
The overall SCAN-LEAF study (ClinicalTrials.gov Identifier: NCT02839629) was conducted in accordance with International Society for Pharmacoepidemiology Guidelines for Good Epidemiology Practices and the Declaration of Helsinki. The SCAN-LEAF retrospective observational study adhered to the laws and regulatory requirements of all participating countries; as it used pseudonymized patient data from national registries, informed consent was not applicable.
The Danish SCAN-LEAF cohort was established by linking national registries (the National Patient Register [Citation9] and the Cause of Death Register [Citation10]) and included all inpatient and outpatient adult patients with incident NSCLC from 1 January 2005 to 31 December 2015, based on diagnoses reported in the Danish Cancer Registry.
Patients
Eligible patients aged ≥18 years had a diagnosis of NSCLC, identified by an International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10) code for lung cancer (C34) and an ICD for Oncology third edition (ICD-O-3) code for NSCLC morphology (Supplementary Table 1). The Danish Cancer Registry recorded cancer stage at diagnosis according to the tumor, node and metastasis (TNM) classification (American Joint Committee on Cancer TNM Classification; sixth edition until 2009, seventh edition thereafter) [Citation11,Citation12]. Patients were excluded if they had missing age or sex data, or if they had a concomitant malignant disease within 5 years before NSCLC diagnosis (except non-metastatic non-melanoma skin cancer). Patients were followed from date of first NSCLC diagnosis until death, emigration or end of follow-up (31 December 2016).
Data collection
The Danish Patient Registry captured procedure codes and dates of lung surgery (KGD and KGWW96–98), RT (BWG and ZPZ, including RT on metastases) and systemic anticancer therapy (SACT; BWHA). Initial treatment was defined as the first treatment received within 6 months of diagnosis, associated with any other treatment received within a certain time period following first treatment (Supplementary Table 2). Concurrent chemoradiation was defined by RT starting within 6 weeks of the start of SACT in patients with stage I–IIIA NSCLC. It was not possible to differentiate sequential chemoradiation SACT from SACT associated with palliative RT. In this study, all RT received among stage IIIB and IV patients was considered palliative. Untreated patients were defined as those who had not received SACT, RT or surgery. Mortality data (date of death) were retrieved from the Cause of Death Register.
Data analysis & statistical methodology
Data were categorized based on time of diagnosis; the diagnostic time period was divided into two 5-year periods with the most recent year on its own: 2005–2009, 2010–2014 and 2015. Population characteristics and initial treatments were summarized using descriptive statistics.
OS was defined as the time from initial NSCLC diagnosis until death from any cause during the observation period. Kaplan–Meier methodology was used to estimate OS probability (95% CI) at 1 and 2 years by histology (non-squamous [NSQ] or squamous [SQ] cell carcinoma), TNM stage and year of diagnosis. No specific trend tests were used. The changes in 1- and 2-year OS were evaluated using 95% CIs. A change in OS probability was considered statistically significant when there were non-overlapping 95% CIs between the earliest and latest periods.
The variables obtained from the National Patient Register and the Cause of Death Register had near-complete coverage for all patients. Some variables had missing values (e.g., disease stage at diagnosis), but for others the absence of a diagnostic or procedure code was assumed to indicate that an event had not occurred (e.g., comorbidities). Missing values were reported in the descriptive analyses.
Results
Population characteristics
Between 2005 and 2015, 35,383 patients were diagnosed with NSCLC (); if these, 3026 had a concomitant malignant disease (except for non-metastatic skin cancer or benign tumors) within 5 years prior to NSCLC diagnosis, 367 had missing data on age or sex and 51 had prior lung cancer; the remaining 31,939 patients were included in this analysis.
†Except for non-metastatic skin cancer or benign tumors (prespecified).
NSCLC: Non-small-cell lung cancer.
As previously reported [Citation8], median age at diagnosis was 69 years and 52% of the patients were male. TNM stage distribution at diagnosis was: stage I, 13.0% (n = 4138); stage II, 7.3% (n = 2322); stage IIIA, 11.3% (n = 3594); stage IIIB, 11.7% (n = 3735); and stage IV, 51.6% (n = 16,486; missing TNM stage, 5.2% [n = 1664]). Overall, 54.4% of patients (n = 17,386) had NSQ NSCLC, 26.5% (n = 8457) had SQ NSCLC and 14.6% (n = 4675) had a diagnosis of NSCLC not otherwise specified.
Patients’ characteristics by histology, stage and time periods are summarized in . The proportion of patients older than 75 years at diagnosis, as well as the proportion of males, was higher in the SQ group than in the NSQ group. Patients with stage IV NSCLC had higher Charlson comorbidity index scores than patients with stage I–IIIB disease, regardless of histology.
Table 1. Patient and clinical characteristics by stage and histology.
Initial treatment
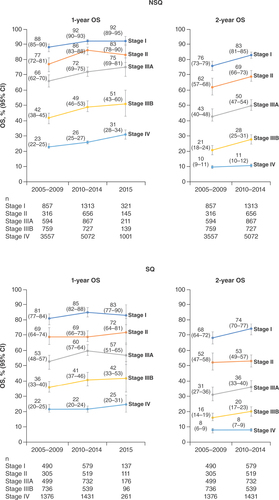
Most patients with stage I disease received surgery alone in all time periods (without RT or SACT; ). Among patients with NSQ and SQ stage I, respectively, the proportions receiving RT alone increased from 8.1 (67/828; 95% CI: 6.2–9.9) and 11.9% (56/471; 95% CI: 9.0–14.8) in 2005–2009 to 25.1 (78/311; 95% CI: 20.3–29.9) and 31.6% (43/136; 95% CI: 23.8–39.4) in 2015.
†No SACT, RT or surgery reported.
‡Combinations of SACT and RT (other than chemoradiation) in patients with stage I–IIIA NSCLC.
§Surgery alone or surgery associated with SACT or RT (categories merged due to limited sample size).
¶Combinations of SACT and RT (other than chemoradiation) in patients with stage IIIB–IV NSCLC.
#SACT alone or SACT + RT (categories merged due to limited sample size).
Percentage labels are only shown for values ≥3.0%.
NSCLC: Non-small-cell lung cancer; NSQ: Non-squamous; RT: Radiotherapy; SACT: Systemic anticancer therapy; SQ: Squamous.
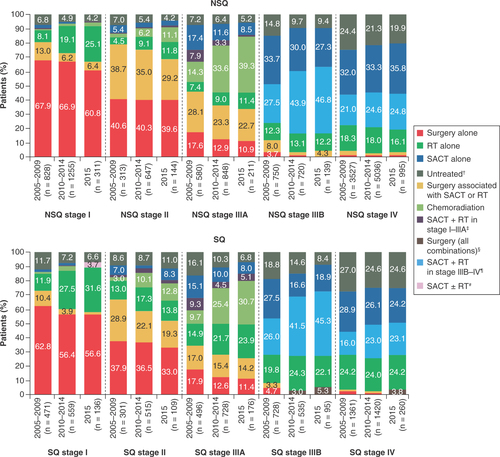
For patients with stage II NSQ and SQ histology, 38.6% (783/2029) received surgery alone; this was consistent across the study period. The proportion of patients with stage II disease treated with surgery associated with SACT and/or RT decreased from 38.7% (121/313; 95% CI: 33.3–44.1) in 2005–2009 to 29.2% (42/144; 95% CI: 21.7–36.6) in 2015 in patients with NSQ and from 28.9% (87/301; 95% CI: 23.8–34.0) to 19.3% (21/109; 95% CI: 11.9–26.7) in patients with SQ during the same time periods. Among stage II patients treated with surgery associated with SACT and/or RT, 91.3% of NSQ patients (355/389) and 81.5% of SQ patients (181/222) received adjuvant SACT only. The decreased use of surgery with adjuvant SACT in stage II between 2005–2009 and 2015 was associated with an increased proportion of patients receiving RT (either alone or with SACT), from 8.3% (26/313; 95% CI: 5.2–11.4) to 24.3% (35/144; 95% CI: 17.3–31.3) in patients with NSQ disease and from 17.6% (53/301; 95% CI: 13.3–21.9) to 28.4% (31/109; 95% CI: 20.0–36.9) in patients with SQ histology. Compared with patients with stage I and II NSCLC (NSQ and SQ) receiving surgery, those treated with RT alone were older (median age: RT alone, 75 and 77 years; surgery alone, 69 and 70 years; surgery with neoadjuvant SACT, 63 and 65 years, for stage I and II, respectively) and had more chronic pulmonary disease (RT alone, 34.8% and 26.2%; surgery alone, 12.1% and 9.3%; surgery with neoadjuvant SACT, 9.6% and 5.6% for stage I and II, respectively).
Among patients with stage IIIA disease, while surgery (alone or with SACT or RT) was the main initial treatment in 2005–2009 (NSQ, 45.7% [265/580]; SQ, 34.9% [173/496]), by 2015 a higher proportion of patients received concurrent chemoradiation (NSQ, 39.3% [83/211]; SQ, 30.7% [54/176]) or RT alone (NSQ, 11.4% [24/211]; SQ, 23.9% [42/176]). This was associated with a decrease in the proportion of stage IIIA patients receiving only SACT, from 17.4% (101/580; 95% CI: 14.3–20.5) to 8.5% (18/211; 95% CI: 4.8–12.3) in NSQ patients and from 15.1% (75/496; 95% CI: 12.0–18.3) to 8.0% (14/176; 95% CI: 4.0–12.0) in SQ patients.
In stage IIIB, the proportion of SACT-treated patients increased between 2005–2009 and 2015, driven by increases in those receiving SACT and RT (NSQ, 27.5 [206/750; 95% CI: 24.3–30.7] to 46.8% [65/139; 95% CI: 38.5–55.1]; SQ, 26.0 [189/728; 95% CI: 22.8–29.1] to 45.3% [43/95; 95% CI: 35.3–55.3]). In 2015, 21.6% (30/139; 95% CI: 14.7–28.4) of stage IIIB NSQ patients and 30.5% (29/95; 95% CI: 21.3–39.8) of stage IIIB SQ patients were still not receiving any SACT treatment.
For stage IV disease, the proportion of SACT-treated patients (SACT alone or with palliative RT) increased from 53.0% (1869/3527; 95% CI: 51.3–54.6) in 2005–2009 to 60.6% (603/995; 95% CI: 57.6–63.6) in 2015 for NSQ; no substantial changes were observed in SQ (44.9% [611/1361; 95% CI: 42.3–47.5] to 47.3% [123/260; 95% CI: 41.2–53.4]). In 2015, 36.0% (358/995; 95% CI: 33.0–39.0) of stage IV NSQ patients and 48.8% (127/260; 95% CI: 42.8–54.9) of stage IV SQ patients were still not receiving any SACT treatment.
Survival outcomes
Among stage I patients, 2-year OS (95% CI) improved between 2005–2009 and 2010–2014 (NSQ, 76% [95% CI: 73–79] to 83% [95% CI: 81–85]; SQ, 68% [95% CI: 64–72] to 74% [95% CI: 70–77]; & Supplementary Figures 1 & 2). Among stage II NSQ patients, 1- and 2-year OS improved between 2005–2009 and 2010–2014, from 77% (95% CI: 72–81) to 86% (95% CI: 83–88) and from 62% (95% CI: 57–68) to 69% (95% CI: 66–73), respectively. No changes were observed in OS for stage II SQ patients over this period. In stage IIIA patients, 1-year OS improved between 2005–2009 and 2010–2014, from 66% (95% CI: 62–70) to 72% (95% CI: 69–75) in NSQ patients and from 53% (95% CI: 48–57) to 60% (95% CI: 57–64) in SQ patients; similar trends were observed for 2-year OS. Among stage IIIB patients, 1-year OS improved from 42% (95% CI: 38–45) to 49% (95% CI: 46–53) in NSQ patients and from 36% (95% CI: 33–40) to 41% (95% CI: 37–46) in SQ patients between 2005–2009 and 2010–2014; similar trends were observed for 2-year OS. Patients with stage IV NSQ showed improvements in 1-year OS between 2005 and 2015 (23% [95% CI: 22–25] in 2005–2009, 26% [95% CI: 25–27] in 2010–2014 and 31% [95% CI: 28–34] in 2015); no change was observed in the SQ group. Among patients with stage IV disease, 2-year OS remained low (NSQ, 10% [95% CI: 9–11] and 11% [95% CI: 10–12]; SQ, 8% [95% CI: 6–9] and 8% [95% CI: 7–9]) in 2005–2009 and 2010–2014, respectively). Across all stages, OS was higher in patients with NSQ versus SQ histology.
Discussion
Building upon the recent findings from the SCAN-LEAF study [Citation8], this population-based analysis of patients in Denmark diagnosed with NSCLC found that patterns of initial treatment changed across disease stages and coincided with improvements in survival from 2005 to 2015 as new treatment approaches became available and were recommended. Greater improvements in survival were seen in patients with NSQ versus SQ histology; however, SQ NSCLC has a poorer prognosis and is generally considered a more difficult-to-treat form of the disease [Citation13]. Consistent with treatment recommendations [Citation3], surgery was the predominant treatment for stage I/II patients. Increasing RT use (including stereotactic body RT) in patients with early-stage disease over the study period coincided with benefits in 1- and 2-year OS among patients with stage I or II NSQ and stage I SQ disease. No improvement was observed in the SQ stage II population, of whom 20% were untreated or received palliative SACT in 2015.
The use of concurrent chemoradiation increased markedly in patients with stage IIIA NSQ and SQ disease over time. This was associated with a reduction in the proportion of stage IIIA patients receiving SACT only. These changes coincided with improvements in 1- and 2-year OS over time in stage IIIA patients. However, a study limitation is the definition of chemoradiation, which was defined as the start of RT within 6 weeks of SACT initiation, as dose and RT type were unavailable; therefore some patients may have been classified as receiving chemoradiation when in fact they received palliative RT after SACT. Conversely, sequential chemoradiation, where RT follows chemotherapy, was not captured in the analysis, and such treatment may have been classified as SACT only.
Among stage IIIB patients, the proportion treated with SACT and RT increased during 2005–2015 and coincided with an increase in 1- and 2-year OS. We suspect that this was partly due to increased chemoradiation use with curative intent in stage IIIB NSCLC; however, it was not possible to differentiate palliative RT from chemoradiation. Despite these apparent improvements, a substantial proportion of stage IIIB patients with NSQ (one-fifth) and SQ (almost one-third) were still not receiving any SACT in 2015.
Among stage IV patients, the only notable survival improvements over time were in 1-year OS in NSQ disease. This may be explained by the fact that even in 2015, a substantial proportion of patients with stage IV NSQ (more than one-third) and SQ disease (approximately one-half) did not receive SACT, and many patients received no treatment (i.e., no SACT, surgery or RT). Although the proportion of patients with advanced disease (combined stage IIIB/IV) who did not receive SACT was relatively high in this study (38% for NSQ: from 40% in 2005–2009 to 34% in 2015; 46% for SQ: from 47% in 2005–2009 to 44% in 2015), it was lower than the proportions reported in publications from the UK (2007–2012), Netherlands (2008–2012) and Canada (2009), where approximately 52–71% of patients with advanced disease did not receive SACT [Citation14–16]. Studies in the UK [Citation16] and Canada [Citation14] showed higher SACT use in patients at advanced disease stages, with better performance status. However, comparisons with other studies are limited by the patient characteristics of the populations, geographical region and treatment time periods. Because of the descriptive nature of the study, it is not possible to determine why so many patients with late-stage disease did not receive SACT or other treatment. The poor survival outcomes are in line with previous reports from Denmark and other countries and reflect the difficulty in treating advanced NSCLC [Citation14,Citation17,Citation18]. Increasing the proportion of patients with advanced NSCLC who are treated with SACT may improve survival outcomes. In the Canadian study, OS was shown to be greater in patients treated with SACT versus best supportive care, even when controlling for age, sex, performance status and histology [Citation14].
A strength of this study is the use of nationwide registry data with near-complete coverage, which increases the generalizability of the findings and reduces the risk of selection bias and loss to follow-up. These findings have been derived from Danish cancer registries previously validated to monitor treatment trends [Citation19]. Limitations include a lack of information on changes in important lifestyle factors (e.g., smoking) and diagnostic practices (e.g., mutation testing), which may have influenced mortality trends, as well as the lack of information on the type of RT received. In addition, the changes in TNM classification in 2010 could also explain in part some of the changes observed in treatment patterns and OS. In this analysis we report 1- and 2-year OS. The 5-year OS from the 2010–2014 period should be interpreted cautiously as only patients diagnosed in 2010–2011 could be followed for 5 years due to the study period ending in December 2016. Longer follow-up may provide further insights into OS trends over time.
This analysis highlights the need for newer, more effective treatment options and earlier diagnosis, as patients with advanced disease were less likely to have received appropriate treatment and derived survival benefits compared with patients with early-stage disease, based on the period studied. Prior studies have described delays in cancer diagnoses and the corresponding poor survival rates in Denmark and have discussed national strategies to achieve earlier diagnosis [Citation20,Citation21].
Conclusion
In this population-based analysis, trends indicated improved survival with evolving treatments for NSCLC between 2005 and 2015. Greater survival improvements were consistently observed in patients with NSQ versus SQ histology, with SQ disease representing a more difficult-to-treat form of NSCLC [Citation13]. These findings will serve as a baseline for future analyses investigating the impact of the introduction of ICIs and later-generation TKIs into routine clinical practice in Denmark when more recent data become available. The results can also serve as a comparison in countries with similar populations and healthcare systems.
Between 2005 and 2015, treatment options for non-small-cell lung cancer (NSCLC) evolved to include surgery with adjuvant therapy, curative radiotherapy (RT), chemoradiation, tyrosine kinase inhibitors and, latterly, immune checkpoint inhibitors.
This SCAN-LEAF retrospective longitudinal study describes the evolution of initial treatment patterns and survival over time among the incident NSCLC population between 2005 and 2015 in Denmark.
As new treatment options became available and were recommended between 2005 and 2015, the initial treatment patterns in patients diagnosed with NSCLC in Denmark changed across disease stages and coincided with improvements in survival.
Surgery was the predominant treatment for stage I/II patients.
Increasing RT use in patients with stage I or II non-squamous (NSQ) and stage I squamous (SQ) disease over the study period coincided with benefits in 1- and 2-year overall survival.
The use of concurrent chemoradiation increased markedly over time in stage IIIA NSQ and SQ NSCLC, as did the use of systemic anticancer therapy (SACT) and RT in stage IIIB NSQ and SQ NSCLC; both coincided with improvements in 1- and 2-year overall survival.
Survival improvements for stage IV NSQ and SQ NSCLC were limited, and even in 2015 >33% and ∼50% of patients with NSQ and SQ histology, respectively, did not receive any systemic anticancer therapy, highlighting the need for earlier diagnosis and newer, more effective treatment options.
The evolving treatment options for patients with NSCLC between 2005 and 2015 coincided with improved survival, particularly in patients with NSQ versus SQ histology, with the SQ type remaining a more difficult-to-treat form of NSCLC.
Author contributions
The authors are fully responsible for all content. All authors contributed to the interpretation of the results and the writing and revising of the manuscript and provided approval of the final version submitted for publication. All authors also contributed to the conception and design of the analysis. A Mette Kejs and P Horvat conducted the analyses.
Ethical conduct of research
The overall SCAN-LEAF study (NCT02839629) was conducted in accordance with International Society for Pharmacoepidemiology Guidelines for Good Epidemiology Practices and the Declaration of Helsinki. The SCAN-LEAF retrospective observational study adhered to the laws and regulatory requirements of all participating countries; as it used pseudonymized patient data from national registries, informed consent was not applicable.
Supplementary Material
Download MS Word (1.4 MB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2021-0746
Financial & competing interests disclosure
This work was supported by Bristol Myers Squibb. IQVIA received funding from Bristol Myers Squibb to perform the data analyses planned in the study protocol. J B Sørensen has received speaker fees from Bristol Myers Squibb. P Horvat, M Rosenlund, A Mette Kejs and D Patel were employees of IQVIA at the time of this study. A Juarez-Garcia, M J Daumont, J R Penrod and J C O’Donnell are employees of Bristol Myers Squibb. A Juarez-Garcia and J R Penrod report stock ownership in Bristol Myers Squibb. L Lacoin is an employee of Epi-Fit and was contracted (paid) as a consultant by Bristol Myers Squibb to support the I-O Optimise initiative. O T Brustugun received honoraria from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Pfizer, Pierre Fabre, Roche and Takeda, and research funding from Pfizer, AstraZeneca, Roche, GlaxoSmithKline and Boehringer Ingelheim. S Ekman is employed by an institution that was remunerated by Bristol Myers Squibb for this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Professional writing and editorial assistance were provided by B Landry of Parexel, funded by Bristol Myers Squibb.
Additional information
Funding
References
- GLOBOCAN 2018: Denmark Fact Sheet (2018). http://gco.iarc.fr/today/data/factsheets/populations/208-denmark-fact-sheets.pdf
- Ekman S , GriesingerF, BaasPet al. I-O Optimise: a novel multinational real-world research platform in thoracic malignancies. Future Oncol.15(14), 1551–1563 (2019).
- Postmus PE , KerrKM, OudkerkMet al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.28(Suppl. 4), iv1–iv21 (2017).
- European Medicines Agency . Nivolumab BMS European public assessment report (2016). www.ema.europa.eu/en/medicines/human/EPAR/nivolumab-bms
- European Medicines Agency . Keytruda European public assessment report (2021). www.ema.europa.eu/en/medicines/human/EPAR/keytruda
- European Medicines Agency . Tecentriq European public assessment report (2021). www.ema.europa.eu/en/medicines/human/EPAR/tecentriq
- European Medicines Agency . Imfinzi European public assessment report (2021). www.ema.europa.eu/en/medicines/human/EPAR/imfinzi
- Ekman S , HorvatP, RosenlundMet al. Epidemiology and survival outcomes for patients with NSCLC in Scandinavia in the preimmunotherapy era: a SCAN-LEAF retrospective analysis from the I-O Optimise initiative. JTO Clin. Res. Rep.2 (5), 100165 (2021).
- Schmidt M , SchmidtSA, SandegaardJL, EhrensteinV, PedersenL, SorensenHT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin. Epidemiol.7, 449–490 (2015).
- Helweg-Larsen K . The Danish Register of Causes of Death. Scand. J. Public Health39(Suppl. 7), 26–29 (2011).
- American Joint Committee on Cancer . Cancer Staging Manual. (6th Edition). Springer, NY, USA (2002).
- Detterbeck FC , BoffaDJ, TanoueLT. The new lung cancer staging system. Chest136(1), 260–271 (2009).
- Perez-Moreno P , BrambillaE, ThomasR, SoriaJC. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin. Cancer Res.18(9), 2443–2451 (2012).
- Noonan K , TongKM, LaskinJet al. Referral patterns in advanced non-small cell lung cancer: impact on delivery of treatment and survival in a contemporary population based cohort. Lung Cancer86(3), 344–349 (2014).
- Peters BJM , Cramer-VdWelle CM, SmitAAJ, SchramelF, vande Garde EMW, SanteonNSCLC Study Group. Trends in prescribing systemic treatment and overall survival for non-small cell lung cancer stage IIIB/IV in the Netherlands: 2008–2012. Cancer Epidemiol.51, 1–6 (2017).
- Snee M , CheesemanS, ThompsonMet al. Trends in the prescription of systemic anticancer therapy and mortality among patients with advanced non-small cell lung cancer: a real-world retrospective observational cohort study from the I-O optimise initiative. BMJ Open11(5), e043442 (2021).
- Walters S , MaringeC, ColemanMPet al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax68(6), 551–564 (2013).
- Jakobsen E , RasmussenTR, GreenA. Mortality and survival of lung cancer in Denmark: results from the Danish Lung Cancer Group 2000–2012. Acta Oncol.55(Suppl. 2), 2–9 (2016).
- Lund JL , FroslevT, DeleuranTet al. Validity of the Danish National Registry of Patients for chemotherapy reporting among colorectal cancer patients is high. Clin. Epidemiol.5, 327–334 (2013).
- Olesen F , HansenRP, VedstedP. Delay in diagnosis: the experience in Denmark. Br. J. Cancer101(Suppl. 2), S5–S8 (2009).
- Vedsted P , OlesenF. A differentiated approach to referrals from general practice to support early cancer diagnosis – the Danish three-legged strategy. Br. J. Cancer112(Suppl. 1), S65–S69 (2015).