Abstract
Aim: To evaluate real-world clinical outcomes of radium-223 or alternative novel hormonal therapy (NHT) following first-line NHT for metastatic castration-resistant prostate cancer (mCRPC). Patients & methods: Retrospective analysis of the US Flatiron database (ClinicalTrials.gov identifier: NCT03896984). Results: In the radium-223 cohort (n = 120) versus the alternative NHT cohort (n = 226), proportionally more patients had prior symptomatic skeletal events and bone-only metastases, and first-line NHT duration was shorter. Following second-line therapy, 49 versus 39% of patients received subsequent life-prolonging therapy; of these, 47 versus 76% received taxane. Median overall survival was 10.8 versus 11.2 months. Conclusion: Real-world patients with mCRPC had similar median overall survival following second-line radium-223 or alternative NHT after first-line NHT. Many patients received subsequent therapy, with less taxane use after radium-223.
Lay abstract
Patients with metastatic castration-resistant prostate cancer are often first treated with novel hormonal therapy (NHT) using abiraterone or enzalutamide. To aid decisions about what treatment to use next, we reviewed information about patients who were treated with an alternative NHT (226 patients) or the nuclear medicine radium-223 (120 patients) after the first NHT. Most patients given radium-223 had cancer that had spread to their bones only, whereas many patients given an alternative NHT had cancer in their bones and other parts of their body. Around one in four patients given radium-223 and one in five given an alternative NHT had symptoms related to their bone metastases after starting treatment. Five in every ten patients given radium-223 received further therapy, including chemotherapy in 50% of these patients, while four in every ten patients given an alternative NHT received further therapy, including chemotherapy in 75%. On average, patients lived for almost a year after starting radium-223 or an alternative NHT.
Tweetable abstract
In men with mCRPC in US real-world practice, survival was similar after radium-223 or alternative novel hormonal therapy (NHT) following first-line NHT. Subsequent therapies were common in both cohorts, but with less taxane use after radium-223.
Graphical abstract
In patients with metastatic castration-resistant prostate cancer (mCRPC) in US real-world practice, median overall survival was similar after second-line radium-223 or alternative NHT following first-line NHT. Use of subsequent life-prolonging therapies was frequent in both cohorts, with proportionally less taxane use in the radium-223 cohort.
The available treatment options for patients with metastatic castration-resistant prostate cancer (mCRPC) have expanded considerably in recent years. However, there is limited evidence to support decisions about optimal sequencing, including choice of second-line therapy following disease progression on first-line novel hormonal therapy (NHT) [Citation1,Citation2]. In real-world settings, many patients receive a second NHT, although the clinical benefit of this sequence may be limited by cross-resistance [Citation2–4]. Taxane chemotherapy is also an option, although patients may prefer to avoid taxanes, because of concerns about the toxicity profile [Citation5–7]. The bone-targeted alpha emitter radium-223 has demonstrated favorable safety and efficacy in patients with bone-predominant mCRPC, including those who experienced disease progression on first-line NHT [Citation8]. A randomized trial of radium-223 versus a second NHT following first-line NHT is ongoing in Europe, Australia, and Asia (ClinicalTrials.gov identifier: NCT04597125). While results of that trial are awaited, to aid physicians who are making clinical decisions about treatment sequencing, we used data from the US real-world Flatiron registry database to evaluate clinical outcomes in patients with mCRPC who received a first-line NHT followed by radium-223 or an alternative NHT.
Patients & methods
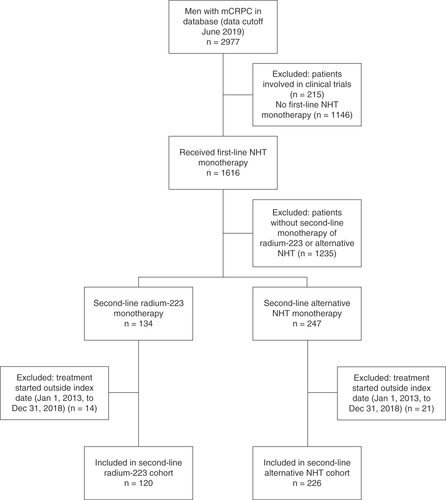
Study design & patients
The Flatiron electronic health record-derived database (https://flatiron.com/real-world-evidence/) is a longitudinal, demographically and geographically varied database comprising electronic health record data from >280 community clinics and seven major academic institutions, with data from almost 3 million patients with cancer in USA available for analysis [Citation7].
This retrospective, observational study (ClinicalTrials.gov identifier: NCT03896984) included all patients in the Flatiron prostate cancer database with a diagnosis of mCRPC, according to International Classification of Diseases 9th and 10th revision diagnostic codes, identified by electronic extraction and processing of patient-level data, and confirmed by an experienced oncology nurse or tumor registrar under the supervision of an oncologist. In addition, patients had to have received a first-line NHT followed by monotherapy with radium-223 or an alternative NHT, together with androgen-deprivation therapy. Second-line therapy had to have started between 1 January 2013 and 31 December 2018. To minimize the impact of other therapies, patients who received radium-223 or NHT in combination with other therapies were excluded. Patients could have received chemotherapy for metastatic hormone-sensitive prostate cancer.
Study outcomes
All patient-level data within the database were subject to extensive review and extraction as described previously [Citation7]. Patient-level data were collated for the following outcomes: duration of therapy; subsequent treatments (including chemotherapy) after second-line therapy and the time to subsequent chemotherapy; frequency of symptomatic skeletal events (SSEs; defined as symptomatic pathologic fracture, spinal cord compression, bone surgery, and external-beam radiation therapy for pain palliation) and time to first SSE after the start of second-line therapy; and overall survival (OS) from the start of second-line therapy.
Statistical analyses
All statistical analyses were conducted using SAS 9.4 software (SAS Institute Inc., NC, USA). On the basis of a feasibility study, which indicated that the sample size was not sufficient for a well-powered comparative study, baseline patient characteristics, treatments and outcomes for each cohort are reported descriptively: frequency and percentage are reported for categorical variables; means (standard deviation) or medians (range) are reported for continuous variables; and Kaplan–Meier estimates, with medians and 95% CIs, are reported for time-to-event variables. Correlative analyses were performed for OS, time to first SSE, and time to first use of subsequent taxane chemotherapy using Cox proportional hazard regression modeling based on non-missing values, with hazard ratios (HRs), 95% CIs, and p-values reported.
Study ethics
No Institutional Review Board review or patient consent was required for this retrospective study from the US Flatiron database (ClinicalTrials.gov identifier: NCT03896984).
Results
Patient disposition & baseline characteristics
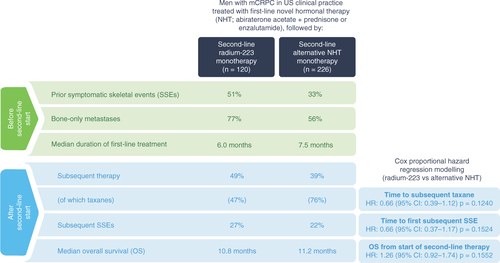
We identified 346 patients who had received first-line NHT for mCRPC and then started second-line monotherapy with radium-223 (n = 120) or alternative NHT (n = 226) during the index period (). The Kaplan–Meier estimated median duration of follow-up for OS was 25.8 months (95% CI: 16.1–38.0; range: 0–61.9) in the radium-223 cohort and 21.7 months (95% CI: 17.2–28.8; range: 0–55.5) in the alternative NHT cohort.
mCRPC: Metastatic castration-resistant prostate cancer; NHT: Novel hormonal therapy.
Compared with the alternative NHT cohort, the radium-223 cohort had proportionally more patients with a history of prior SSEs and with bone-only metastases at baseline and a slightly shorter median duration of first-line NHT (). Other patient and disease characteristics at baseline (i.e., within 6 months before or at the start of second-line therapy) were generally similar between the two cohorts.
Table 1. Patient demographics and disease characteristics.
Second-line treatment exposure
The median duration of second-line therapy was 5.6 months (range: 0.03–31.1) in the radium-223 cohort and 4.7 months (range: 0.03–34.2) in the alternative NHT cohort. In the radium-223 cohort, 50% of patients received five or six of the maximum six scheduled injections.
Subsequent therapy
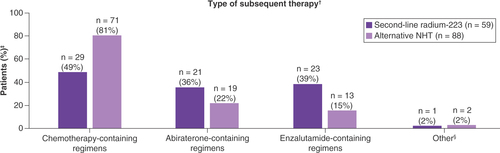
Subsequent life-prolonging therapy was received by 59/120 patients (49%) and 88/226 patients (39%), respectively (; ). Among patients who received subsequent therapy, 28/59 (47%) in the radium-223 cohort and 67/88 (76%) in the alternative NHT cohort received subsequent taxane therapy (docetaxel and/or cabazitaxel); 1/59 (2%) and 4/88 (5%), respectively, received other chemotherapies.
Table 2. Subsequent life-prolonging therapy after second-line therapy.
†Patients were counted once for each treatment type, regardless of the number of lines received; patients who received more than one treatment type were counted in each category.
‡The denominator is the number of patients in each cohort who received any subsequent therapy (radium-223 n = 59; alternative NHT n = 88).
§In the radium-223 cohort, one patient received subsequent radium-223 monotherapy; in the alternative NHT cohort, one patient received subsequent pembrolizumab monotherapy, and one patient received subsequent sipuleucel-T monotherapy.
NHT: Novel hormonal therapy.
According to multivariable Cox regression modeling (n = 264 without missing values), the time to first use of subsequent taxanes was numerically longer in the radium-223 cohort than in the alternative NHT cohort, although the difference was not statistically significant (HR: 0.66; 95% CI: 0.39–1.12; p = 0.1240). Factors significantly associated with longer time to first use of subsequent taxanes after second-line therapy were age (HR: 0.96; 95% CI: 0.93–0.98; p = 0.0010) and hemoglobin >12.8 g/dl versus ≤12.8 g/dl at the start of second-line therapy (HR: 0.56; 95% CI: 0.33–0.95; p = 0.0323; ).
Table 3. Multivariable Cox proportional hazards regression model for time to first use of subsequent taxane therapyTable Footnote†, Table Footnote‡.
Symptomatic skeletal events
From the start of second-line therapy until the end of the observation period, 32/120 patients (27%) in the radium-223 cohort and 49/226 patients (22%) in the alternative NHT cohort experienced SSEs. Before the start of second-line therapy, 61/120 patients (51%) in the radium-223 cohort and 75/226 patients (33%) in the alternative NHT cohort had experienced SSEs. Among these patients, 18/61 (30%) in the radium-223 cohort and 23/75 (31%) in the alternative NHT cohort had at least one SSE after the start of second-line therapy, including five and 12 patients, respectively, who had multiple SSEs.
The median time from the start of second-line therapy to first SSE was 33.9 months (95% CI: 16.4–43.6) in the radium-223 cohort and could not be estimated (95% CI: 24.7–not estimable) in the alternative NHT cohort; however, censoring resulted in the number of patients at risk being very low at the median timepoints in both cohorts (Supplementary Figure 1). After controlling for patient characteristics and baseline factors (n = 264 without missing values), the time to first subsequent SSEs after the start of second-line therapy was numerically longer in the radium-223 cohort versus the alternative NHT cohort (HR: 0.66; 95% CI: 0.37–1.17; p = 0.1524). Age (HR: 0.95; 95% CI: 0.92–0.98; p = 0.0014) and prior use of bone health agents (BHAs) (HR: 0.49; 95% CI: 0.29–0.82; p = 0.0071) were associated with a longer time to first subsequent SSEs, whereas a medical history of SSEs (HR: 2.51; 95% CI: 1.48–4.26; p = 0.0006) was strongly predictive of earlier occurrence of subsequent SSEs ().
Table 4. Multivariable cox proportional hazards regression model for symptomatic skeletal event occurrenceTable Footnote†, Table Footnote‡.
Overall survival
Median OS from the start of second-line therapy was 10.8 months (95% CI: 9.3–12.9) in the radium-223 cohort and 11.2 months (95% CI: 10.0–13.2) in the alternative NHT cohort (). After controlling for baseline characteristics and other factors (n = 264 without missing values), no statistically significant difference in OS from the start of second-line therapy was observed between the two cohorts (radium-223 vs alternative NHT: HR: 1.26; 95% CI: 0.92–1.74; p = 0.1552). Factors significantly associated with shorter OS from the start of second-line therapy were site of metastasis (visceral ± lymph node/bone vs bone only; HR: 1.69; 95% CI: 1.17–2.42; p = 0.0048) and baseline hemoglobin >12.8 g/dl versus ≤12.8 g/dl (HR: 0.58; 95% CI: 0.40–0.84; p = 0.0038). Patients with bone + lymph node metastases had a similar prognosis to that of patients with bone-only metastases ().
(A) Radium-223 cohort. (B) Alternative NHT cohort.
NHT: Novel hormonal therapy; OS: Overall survival.
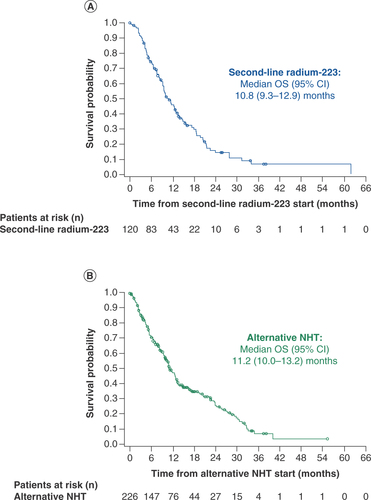
Table 5. Multivariable Cox proportional hazards regression model for overall survivalTable Footnote†, Table Footnote‡.
Discussion
This was a retrospective study of second-line monotherapy with radium-223 or an alternative NHT, following first-line NHT, in patients with mCRPC in US real-world clinical practice. The study provided information about patient and disease characteristics that may influence disease management decisions, sequence of therapies, and potential outcomes in a real-world setting.
Baseline characteristics and medical history likely had an impact on treatment selection, starting with the choice of radium-223 or alternative NHT as second-line treatment and continuing through the later treatment lines. Following first-line NHT, patients with bone-only metastases or prior SSEs are likely to receive bone-targeting therapy with radium-223, in line with its licensed indication and treatment guidelines [Citation5,Citation9–11]. This approach is reflected in our study: at the start of second-line therapy, a markedly higher proportion of patients in the radium-223 cohort than in the alternative NHT cohort had bone-only metastases (77 vs 57%) and prior SSEs (51 vs 33%).
The duration of first-line NHT in both cohorts (<8 months) was substantially shorter than has been reported in clinical trials [Citation12–15], potentially reflecting the broader range of patients, including those with more advanced disease or poorer general health and performance status, in the real world compared with clinical trial settings. Alternatively, the decision to proceed to second-line therapy may have been based on an increase in prostate-specific antigen rather than radiographic progression, as required in clinical trials. However, data are unavailable regarding the reasons for stopping first-line therapy, and we are unable to ascertain the exact reasons why physicians selected one therapy over another.
Thirteen percent of patients in the radium-223 cohort and 6% in the alternative NHT cohort received chemotherapy before first-line NHT for mCRPC. Although the reasons for prior chemotherapy were not specified in the database, a key factor may have been the presence of symptomatic or high-volume disease before the mCRPC stage [Citation16,Citation17]. A similar proportion of patients in each cohort received subsequent life-prolonging therapy after second-line therapy. Among patients who received subsequent therapy following second-line radium-223, almost 50% received chemotherapy (27% as third line, 22% as fourth line) and 75% received further NHT (63% as third line, 12% as fourth line). Use of radium-223 after first-line NHT might reflect physician or patient preference to delay chemotherapy and instead use a treatment with a distinct and relatively tolerable adverse event profile [Citation8,Citation18]. The high proportion of patients who received subsequent NHT in this cohort may indicate that radium-223 postpones rather than replaces further NHT in patients who have received first-line NHT, but it also highlights the lack of sufficient options to treat patients with mCRPC.
In patients in the alternative NHT cohort who received subsequent therapy, 76% received a taxane, and 26% received a third line of NHT. This cohort had a higher proportion of patients with visceral metastases at baseline compared with the radium-223 cohort. These patients would therefore not be eligible for radium-223 therapy, making a taxane a reasonable choice for third-line therapy. This approach is supported by findings from the multicenter, randomized, open-label CARD study, in which outcome benefits were reported for third-line therapy with cabazitaxel versus an alternative NHT after prior docetaxel and NHT [Citation19].
Despite the differences in baseline characteristics between the two cohorts, median OS and time to first SSE were similar in patients treated with second-line radium-223 or an alternative NHT. In a separate analysis of the Flatiron database, real-world median OS was longer in patients with metastatic prostate cancer who received second-line NHT following first-line NHT than in those who received second-line docetaxel [Citation20], but the reason for this difference might be that patients with rapid disease progression were more likely to be given docetaxel than a second NHT.
Our analysis adds to the growing real-world evidence base on treatment sequencing, while we await data from randomized controlled trials to provide definitive guidance. Recruitment has started in a Phase IV, randomized, open-label study of second-line radium-223 versus alternative NHT in patients with bone-predominant mCRPC that progressed on or after one line of NHT, and results are anticipated in 2024 (ClinicalTrials.gov identifier: NCT04597125). Meanwhile, our analysis indicates the feasibility of second-line radium-223 in patients with mCRPC who require a switch from first-line NHT. The finding of similar OS, but reduced taxane use, in patients who received second-line radium-223 versus an alternative NHT is reassuring for patients with bone-predominant disease who wish to avoid or delay chemotherapy and its associated toxicities [Citation6]. Nonetheless, almost 50% of patients received chemotherapy after radium-223, indicating that radium-223 will not limit a patient's ability to receive subsequent cytotoxic therapy. Another relevant real-world study (the ongoing global REASSURE study; ClinicalTrials.gov identifier: NCT02141438) is also demonstrating the long-term safety profile of radium-223 in a heterogenous population of patients, including a substantial proportion who received prior NHT (abiraterone plus prednisone [45%] and/or enzalutamide [37%]) [Citation21].
Patients with mCRPC will also benefit from greater use of BHA therapy, as recommended by current guidelines [Citation5,Citation11]. Fewer than 60% of patients in the current study had received BHAs before the start of second-line therapy, even though more than 50% of patients in the radium-223 cohort and 30% in the alternative NHT cohort had prior SSEs.
This study is limited by its retrospective nature, small sample size, and potential for selection bias, because the second-line treatment decision was based on factors not ascertained, and we excluded patients who received radium-223 in combination regimens or after sequential NHT–NHT. Furthermore, adjustment for confounding factors was limited to the available data, as indicated in the multivariable Cox proportional hazard regression modeling analyses, and some variables with missing values or unknown factors were not considered.
Conclusion
In real-world clinical practice in USA, patients with mCRPC had a similar survival duration following second-line radium-223 or alternative NHT after first-line NHT. Many patients received subsequent therapies, although proportionally fewer patients in the radium-223 cohort than in the alternative NHT cohort received subsequent taxane. Our findings indicate the feasibility of using radium-223 in the sequence of life-prolonging therapies, including chemotherapy, in patients with bone-predominant mCRPC in real-world settings.
In patients with metastatic castration-resistant prostate cancer (mCRPC) who experience disease progression during or after first-line novel hormonal therapy (NHT), data on optimal sequencing of subsequent therapies are limited.
We used data from the US Flatiron prostate cancer registry database to assess clinical outcomes in patients with mCRPC who received first-line NHT followed by monotherapy with either radium-223 (n = 120) or an alternative NHT (n = 226).
At the start of second-line therapy, the radium-223 cohort had proportionally more patients with prior symptomatic skeletal events (SSEs) and bone-only metastases and a slightly shorter duration of first-line NHT, compared with the alternative NHT cohort, while the alternative NHT cohort had a higher proportion of patients with visceral metastases.
Similar proportions of patients in the two cohorts received subsequent life-prolonging therapy (radium-223 49%; alternative NHT 39%), although proportionally fewer patients in the radium-223 cohort (47%) than in the alternative NHT cohort (76%) received subsequent taxane chemotherapy.
From the start of second-line therapy, similar proportions of patients in each cohort had SSEs (radium-223 27%; alternative NHT 22%).
The median OS from the start of second-line therapy was around 1 year in both cohorts: radium-223 10.8 months, alternative NHT 11.2 months.
When interpreting these findings, it is important to take into account the retrospective, nonrandomized design, small sample size, and inconsistent data availability from a registry database.
Nonetheless, this real-world study adds to the evidence base for treatment sequencing in patients with mCRPC, indicating that subsequent therapy, including taxane chemotherapy, can be sequenced after first-line NHT and second-line radium-223 therapy.
Author contributions
Study conception and design: All authors. Data acquisition: Not applicable. Data analysis: Statistical analyses were done by H Guo; all authors reviewed and interpreted the results. Drafting and revision of the manuscript: all authors, with support from OPEN Health Communications.
Ethical conduct of research
As this study was a retrospective review of electronic health record data, no Institutional Review Board review or patient informed consent was required.
Data sharing statement
The data underlying this publication were provided by Flatiron under contract to Bayer. Requests for access to the data should be sent to the corresponding author.
Supplemental Materials
Download MS Word (116.7 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2021-0886
Financial & competing interests disclosure
This study was sponsored by Bayer Healthcare, Whippany, NJ, USA. O Sartor reports consulting or advisory fees from Amgen, Bayer, Sanofi, AstraZeneca, Dendreon, Constellation Pharmaceuticals, Advanced Accelerator Applications, Endocyte, Pfizer, Bristol Myers Squibb (BMS), Bavarian Nordic, EMD Serono, Astellas Pharma, Progenics Pharmaceuticals, Blue Earth Diagnostics, Myovant Sciences, Myriad Genetics, Novartis, Clarity Pharmaceuticals, Fusion Pharmaceuticals, Isotopen Technologien, Janssen, Noxopharm, Clovis Oncology, Taiho, Noria Therapeutics, Point Biopharma, TeneoBio, Telix Pharmaceuticals, and Theragnostics; and grant support from Sanofi, Endocyte, Merck, Invitae, Constellation Pharmaceuticals, Arvinas, AstraZeneca, Dendreon, SOTIO, Janssen, and Progenics Pharmaceuticals. D George reports grants and/or fees from Acerta Pharma, the American Association for Cancer Research, Astellas, AstraZeneca, Axess Oncology, Bayer, BMS, Calithera Biosciences, Capio Biosciences, EMD Serono, Exelixis, Flatiron, Ipsen, UroGPO, Janssen, Leidos Biomedical Research, Merck Sharp & Dohme (MSD), Michael J Hennessy Associates, Millennium Medical Publishing, Modra Pharmaceuticals, Myovant Sciences, National Cancer Institute, Nektar Therapeutics, Novartis, Pfizer, Physicians' Education Resource, Sanofi, UroToday, and Vizuri Health Sciences. B Tombal reports grants and fees from Amgen, Astellas, Bayer, Ferring Pharmaceuticals, Janssen and Sanofi Genzyme. N Agarwal reports consultancy to Astellas, AstraZeneca, Aveo, Bayer, BMS, Calithera Biosciences, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics. C Higano reports grants and/or fees from Aptevo, Asana, Aragon Pharma, Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Churchill Pharmaceuticals, Clovis, Dendreon, eFFECTOR Therapeutics, Endocyte, Emergent BioSolutions, Ferring Pharmaceuticals, Genentech, Hinova Pharmaceuticals, Hoffmann-La Roche, Janssen, Medivation, Myriad, Orion and Pfizer. CN Sternberg serves as a consultant for Pfizer, MSD, Merck, AstraZeneca, Astellas, Sanofi Genzyme, Roche-Genentech, Incyte, Immunomedics (now Gilead), BMS, Janssen, Medscape, Foundation Medicine, UroToday, and the National Cancer Institute. K Miller reports fees from Astellas, Bayer, BMS, Ferring Pharmaceuticals, Janssen, MSD, Novartis, Pfizer and Roche. XL Jiao, H Guo, P Sandström, A Bruno and F Verholen are employees of Bayer. F Saad reports fees from Astellas, AstraZeneca, Bayer, Janssen, Merck, Pfizer, and Sanofi. N Shore serves as a consultant/adviser for AbbVie, Bayer, Janssen Scientific Affairs, Dendreon, Tolmar, Ferring Pharmaceuticals, Astellas, Amgen, Pfizer, AstraZeneca, BMS, Myovant Sciences, Merck, Sanofi Genzyme, Invitae, Myriad, Phosphorus, Sema4, Foundation Medicine, and Guardant, and serves on speaker bureaux for Janssen, Bayer and Dendreon. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support for the preparation of this article was provided by D Murdoch, and S Black, ISMPP CMPP™, of OPEN Health Communications (London, UK), with financial support from Bayer HealthCare, Whippany, NJ, USA.
Additional information
Funding
References
- Shore N , HiganoCS, GeorgeDJet al. Concurrent or layered treatment with radium-223 and enzalutamide or abiraterone/prednisone: real-world clinical outcomes in patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis.23(4), 680–688 (2020).
- Nuhn P , DeBono JS, FizaziKet al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur. Urol.75(1), 88–99 (2019).
- Khalaf DJ , AnnalaM, TaavitsainenSet al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, Phase II, crossover trial. Lancet Oncol.20(12), 1730–1739 (2019).
- Mori K , MiuraN, MostafaeiHet al. Sequential therapy of abiraterone and enzalutamide in castration-resistant prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis.23(4), 539–548 (2020).
- Parker C , CastroE, FizaziKet al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.31(9), 1119–1134 (2020).
- Eliasson L , DeFreitas HM, DeardenL, CalimlimB, LloydAJ. Patients' preferences for the treatment of metastatic castrate-resistant prostate cancer: a discrete choice experiment. Clin. Ther.39(4), 723–737 (2017).
- George DJ , SartorO, MillerKet al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin. Genitourin. Cancer18(4), 284–294 (2020).
- Parker C , NilssonS, HeinrichDet al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med.369(3), 213–223 (2013).
- XOFIGO (radium-223 dichloride) Summary of Product Characteristics (2021). www.ema.europa.eu/en/documents/product-information/xofigo-epar-product-information_en.pdf
- XOFIGO (radium Ra 223 dichloride) injection. Prescribing Information (2021). www.accessdata.fda.gov/drugsatfda_docs/label/2013/203971lbl.pdf
- EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer 2020 (2021). https://uroweb.org/wp-content/uploads/EAU-EANM-ESTRO-ESUR-SIOG-Guidelines-on-Prostate-Cancer-2020v4.pdf
- De Bono JS , LogothetisCJ, MolinaAet al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med.364(21), 1995–2005 (2011).
- Scher HI , FizaziK, SaadFet al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med.367(13), 1187–1197 (2012).
- Ryan CJ , SmithMR, DeBono JSet al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med.368(2), 138–148 (2013).
- Beer TM , ArmstrongAJ, RathkopfDEet al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med.371(5), 424–433 (2014).
- Turpin A , PasquierD, MassardC, BerdahJF, CulineS, PenelN. First-line management of metastatic castrate-resistant prostate cancer patients: audit of real-life practices. Bull. Cancer104(6), 552–558 (2017).
- Kyriakopoulos CE , ChenYH, CarducciMAet al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED Trial. J. Clin. Oncol.36(11), 1080–1087 (2018).
- Sartor O , ColemanR, NilssonSet al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a Phase III, double-blind, randomised trial. Lancet Oncol.15(7), 738–746 (2014).
- De Wit R , DeBono J, SternbergCNet al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N. Engl. J. Med.381(26), 2506–2518 (2019).
- Swarmi U , SinnottJA, HaalandBet al. Overall survival (OS) with docetaxel (D) vs novel hormonal therapy (NHT) with abiraterone (A) or enzalutamide (E) after a prior NHT in patients (Pts) with metastatic prostate cancer (mPC): results from a real-world dataset. J. Clin. Oncol.38(Suppl. 15), Abstract 5537 (2020).
- Higano C , SaadF, SartorOet al. Clinical outcomes and patient (pt) profiles in REASSURE: an observational study of radium-223 (Ra-223) in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol.38(Suppl. 6), Abstract 32 (2020).