Abstract
The prognosis of stage III gastric cancer (GC) is not satisfying and the specific chemotherapy regimens for GC of stage IIIC based on the 8th edition of the UICC/AJCC TNM staging system are still inconclusive. Peritoneal recurrence is the common and severe relapse pattern. Nanoparticle albumin-bound paclitaxel (Nab-PTX) is safer and more effective than PTX in the peritoneal metastasis. Clinical trial has demonstrated the safety and efficacy of sintilimab in GC. A combination of Nab-PTX, S-1 and sintilimab could be a promising triplet regimen as adjuvant therapy for GC. The aim of this article is to describe the design of this prospective Dragon-VII trial, conducted to evaluate the safety and efficacy of the combination of Nab-PTX, S-1 and sintilimab.
Clinical trial registration: NCT04781413
Lay abstract
The prognosis of stage IIIC gastric cancer is poor and the treatment for it is not satisfying. This is a clinical trial that aims to explore a more effective therapy in gastric cancer patients of stage IIIC. Patients with stage IIIC gastric cancer must meet all of the inclusion criteria and none of the exclusion criteria to be eligible for this trial. The eligible patients will be given eight cycles of combinatory therapy of albumin-bound paclitaxel, a chemotherapy (day 1 and day 8), and S-1, another chemotherapy (days 1 to 14), plus sintilimab, a type of immunotherapy called an immune checkpoint inhibitor (day 1) every 3 weeks and then sintilimab maintenance for up to 12 months.
Gastric cancer (GC) is the fifth most commonly diagnosed cancer and fourth leading cause of cancer-related death in the world [Citation1]. Benefiting from the advances in early screening strategy, the incidence rate of GC has decreased over the years; however, GC-caused mortality has not changed. GC is derived from the mucosal epithelial cells at the most superficial layer of the gastric wall and invades the gastric wall of different depths and widths. Surgical resection is the only potential chance of cure for GC, and adjuvant therapy has been demonstrated to be beneficial in numerous clinical trials worldwide. According to the ACTS-GC [Citation2] and CLASSIC [Citation3] trials, international consensus recommends fluorouracil-based chemotherapy regimens for adjuvant chemotherapy of GC, but these two trials both adopted the 6th edition of the UICC/AJCC TNM staging system for GC; therefore, the specific chemotherapy regimens for GC of stage IIIC according to the 8th edition of the UICC/AJCC TNM staging system for GC are still inconclusive and remain to be verified in large clinical trials. In spite of curative resection and perioperative chemotherapy, because of the heterogeneity of gastric carcinoma or the homogenous insensitivity of tumors to chemotherapy, recurrence is common, especially in stage III GC. Peritoneal metastasis was the most common recurrence site [Citation2–5]. A retrospective study showed that the 5-year disease-specific survival rate of patients with stage IIIC GC was only 30% based on the 7th TNM staging system [Citation6]. In a retrospective study comparing the 7th and 8th TNM staging systems, the 3-year relapse-free survival (RFS) of stage IIIC was 49.6% and 42.3% respectively [Citation7]. In 2019, the JACCRO GC-07 trial [Citation4] demonstrated that docetaxel plus S-1 significantly improved overall survival (OS) and RFS compared with S-1 monotherapy for patients with stage III GC. Subgroup analysis of RFS showed that the addition of docetaxel provided modest efficacy in patients with stage IIIC GC (hazard ratio [HR]: 0.693; 95% CI: 0.466–1.03); furthermore, docetaxel plus S-1 failed to reduce the incidence of peritoneal surface recurrence (12.9% vs 9.3%; p = 0.092).
Dragon-VII trial
This study aims to assess the feasibility of Nab-PTX, S-1 and sintilimab as an adjuvant regimen for patients with stage IIIC GC. The study was designed as a Phase I/II trial, with Phase I part evaluate the safety feasibility and recommended dose of this combination and Phase II to explore potential anti-tumor activities at the recommended dose and safety in extended population.
Background & rationale
Paclitaxel (PTX) was known to exhibit high intraperitoneal concentration when administered intravenously [Citation8]. In advanced GC, including cases with malignant ascites, PTX had shown good response rates [Citation9]. However, premedication with steroids is essential before the administration of PTX to reduce allergic and hypersensitivity reactions in the clinical setting.
In Japanese clinical practice, nanoparticle albumin-bound paclitaxel (Nab-PTX) has also been developed for chemotherapy of gastric cancer [Citation10]. Nab-PTX is made of albumin-bound PTX particles. Compared with PTX and docetaxel, Nab-PTX is more metastatic to tumor tissues and less likely to cause hypersensitivity reactions due to its different chemical composition. The ABSOLUTE trial [Citation11] showed that weekly Nab-PTX was non-inferior to weekly solvent-based PTX in terms of OS; the advantages of the Nab-PTX formulation make it a potential regimen for the treatment of GC. Subgroup analysis of the ABSOLUTE trial in patients with peritoneal metastasis showed better OS and progression-free survival in patients receiving weekly Nab-PTX than in those receiving solvent-based PTX. In patients with peritoneal metastasis, the HR for OS in the weekly Nab-PTX arm compared with the weekly PTX arm was 0.78 (95% CI: 0.59–1.03; p = 0.011). These findings suggest that Nab-PTX might be more effective than PTX for patients with peritoneal metastasis. In an exploratory analysis of the ABSOLUTE trial, the median survival time in the Nab-PTX arm was longer than that of the solvent-based PTX arm (9.9 months vs 8.7 months; p = 0.006) when patients had apparent peritoneal metastasis [Citation12], indicating that Nab-PTX might be more effective in peritoneal metastasis. A preclinical study demonstrated that intravenous Nab-PTX was more effective than solvent-based PTX at equitoxic doses involving a subcutaneous xenograft model, using a peritoneal metastatic model of GC [Citation13].
S-1 is an orally active combination of tegafur, gimeracil and oteracil in a molar ratio of 1:0.4:1 [Citation14]. The pharmacokinetics of the fluorouracil that is derived from S-1 is not influenced by gastrectomy [Citation15], and S-1 has become a standard postoperative adjuvant regimen for locally advanced GC in Asia.
The combination of S-1 and Nab-PTX has been demonstrated to be effective and safe in several solid malignancies in Phase I/II trials [Citation16–22]. A Phase I, dose escalation study aiming at unresectable or recurrent GC revealed that the recommended dose was determined to be S-1 80 mg/m2 twice daily plus Nab-PTX 260 mg/m2 on day 1 [Citation19]. In a Phase II trial in China [Citation22], it was found that S-1 80 mg/m2 plus Nab-PTX given at a dose of 120 mg/m2 on days 1 and 8 every 3 weeks as first-line treatment for patients with metastatic GC resulted in an overall response rate of 58.9% and a median OS of 14.6 months. In the ABSOLUTE trial [Citation11], median OS was 10.3 months in the group that received Nab-PTX every 3 weeks and 11.1 months in the weekly Nab-PTX group with a lower incidence rate of grade 3 or worse adverse drug reactions.
Immunotherapy targeting the checkpoint molecule programmed cell death 1 (PD-1) or programmed death ligand 1 (PD-L1) has been shown to be safe and effective in the management of several malignant tumors [Citation23–27]. The first Phase III randomized, controlled trial ATTRACTION-2 [Citation27] demonstrated the superiority of the survival and safety benefits of nivolumab to placebo in third-line treatment of gastric and gastroesophageal junction cancer. Later on, two globally and regionally Phase III randomized, controlled trials, Checkmate-649 [Citation28] and ATTRACTION-04 [Citation29], further approved the survival benefits and tolerability of nivolumab in combination with chemotherapy at first-line treatment. In 2021 May, the US FDA approved the use of nivolumab plus FOLFOX/XELOX in patients with esophageal, gastric and gastroesophageal junction adenocarcinoma with HER2 negative status. A global Phase III clinical trial (ATTRACTION-05, NCT03006705) is designed to verify the efficacy and safety of adjuvant therapy of nivolumab and chemotherapy compared with chemotherapy in stage III GC.
Sintilimab is a highly selective, fully human, monoclonal IgG4 antibody that inhibits interactions between PD-1 and its ligands, with strong anti-tumor response [Citation30]. In the Phase Ib study (NCT02937116), the results from the gastric/gastroesophageal junction adenocarcinoma cohort demonstrated manageable safety and favorable anti-tumor activity of sintilimab combined with a CapeOx regimen as first-line treatment for unresectable advanced metastatic gastric/gastroesophageal junction adenocarcinoma [Citation31]. The Phase III randomized, controlled trial (ORIENT-16 trial, NCT03745170) of sintilimab plus CapeOx in first-line treatment of GC has completed enrollment and the primary outcomes will be announced soon. A Phase II trial (NCT04065282) designed to assess the feasibility of sintilimab in combination with CapeOx as neoadjuvant therapy for patients with resectable locally advanced GC demonstrated a pathological complete response of 23.1% in ASCO 2021 Poster presentation (#211P).
It is safe to hypothesize that anti-PD-1 antibodies in combination with chemotherapy may further improve the clinical outcomes of patients with GC.
Design
The study has been approved by the Ruijin Hospital Ethics Committee, Shanghai Jiao Tong University School of Medicine, China (No. 2021–19) and has been registered in the ClinicalTrial.gov as NCT04781413.
Target population
The target population consists of patients with stage IIIC GC who underwent radical resection with D2 lymphadenectomy at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Inclusion criteria
Age 18 years to 80 years | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Primary GC or gastroesophageal junction cancer that is pathologically diagnosed as adenocarcinoma | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Patients who have undergone radical resection with D2 lymphadenectomy and histologically proven to be stage IIIC GC according to the 8th edition of the UICC/AJCC TNM staging system for gastric cancer [Citation32] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Patients who have received no prior chemotherapy, radiotherapy or immunotherapy for GC or gastroesophageal junction cancer | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
No peritoneal metastasis by laparoscopy and no tumor cells in peritoneal fluid on cytologic analysis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Adequate organ function for chemotherapy as follows:
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8. | Written (signed) informed consent | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9. | Good compliance with the study procedures, including examination and treatment | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10. | Surgeons should have experience doing this type of surgery (>50 procedures per year). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11. | Patients have recovered from the operation and have no unresolved postoperative complications (such as postoperative infection, anastomotic leakage, gastrointestinal bleeding, pancreatic leakage) during baseline evaluation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12. | Start first treatment within 6 weeks after surgery and there is no potential disease recurrence at the baseline evaluation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13. | The serum or urine human chorionic gonadotropin (HCG) test of the female patients of childbearing age must be negative within 72 hours before registration . | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14. | During the study treatment period and within 3 months after the end of the study treatment period, a medically recognized contraceptive measure (such as IUD, contraceptive pill or condom) should be used by the enrolled patients. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Exclusion criteria
Distant metastatic disease evaluated by chest–abdomen–pelvis computed tomography, bone scan and head magnetic resonance imaging (MRI) with central nervous system symptoms or positron emission tomography-computed tomography (PET-CT).
R1 or R2 surgical margins
Hospital stays exceeding 60 days
Patients with a history of prior or concurrent malignant tumors; however, subjects who have been disease-free for 5 years, or subjects with a history of completely resected non-melanoma skin cancer or successfully treated in situ carcinoma, are eligible
Patients who received study drug treatment within 4 weeks before enrollment (participate in other clinical trials)
Patients with serious complications such as
- uncontrolled cardiovascular disease, angina and arrhythmia
- myocardial infarction in the past 6 months
- uncontrolled diabetes mellitus
Patients with a history of receiving anti-PD-1, anti-PD-L1, anti-PD-L2 or any other T cell co-simulation or checkpoint inhibitor therapy (e.g., CTLA-4, OX-40, CD137)
Patients who have received any anti-cancer for this disease, including chemotherapy, radiotherapy, immunotherapy or Chinese traditional herb therapy
Patients who refuse to provide blood/tissue samples
Female patients who are pregnant or lactating or are planning to become pregnant or lactating
Patients who have active autoimmune disease or a history of refractory autoimmune disease; subjects with hypothyroidism requiring only hormone replacement therapy and skin diseases without systemic treatment (e.g., vitiligo, psoriasis or alopecia) can be selected
Patients for whom steroid or other systemic immunosuppressive therapy was used 14 days before admission, excluding local or physiological doses of systemic glucocorticoids (e.g., no more than 10 mg/day of prednisone or other glucocorticoids of equivalent dose) by nasal spray, inhalation or other routes, or hormones used to prevent allergy of contrast agents
Patients with uncontrollable pleural effusion, pericardial effusion or ascites
Patients with a history of allogeneic organ transplantation and allogeneic hematopoietic stem cell transplantation
Patients with a history of hypersensitivity to any drugs in this study
It may affect the absorption of S-1 in patients with upper gastrointestinal obstruction/bleeding, abnormal digestive function or malabsorption syndrome.
Patients who have not fully recovered from toxicity or complications caused by any intervention before starting treatment
Patients who are HIV antibody positive or have active hepatitis B or C (hepatitis B: HBsAg positive and HBV DNA ≥10 copies/ml; hepatitis C: HCV antibody and HCV-RNA positive, requiring antiviral treatment at the same time)
Patients who receive live attenuated vaccine within 4 weeks before the first dose of the study treatment or during the study period
Patients with severe or uncontrolled systemic disease:
severe cardiovascular diseases such as symptomatic coronary heart disease, congestive heart failure ≥level II, uncontrolled arrhythmia and myocardial infarction within 12 months before admission
- active infection that requires systemic treatment
- active tuberculosis
- central nervous system disorder, peripheral nervous system disorder or psychiatric disease
- a history of primary immunodeficiency
- complicated with severe, uncontrolled concurrent infection or other serious uncontrolled concomitant diseases or moderate or severe renal injury
Other factors that may affect the safety or test compliance of the subjects according to the judgment of the researchers
Registration
Subjects must meet all of the inclusion criteria and none of the exclusion criteria to be eligible for this trial. Treatment should be planned to start within 6 weeks after surgery. Once the registration process has been completed, the subject will be assigned a subject study number.
Treatment methods
The eligible patients will be given eight cycles of combinatory therapy of Nab-PTX (day 1 and day 8) and S-1 (days 1 to 14) plus sintilimab (day 1) every 3 weeks and sintilimab maintenance up to 12 months.
S-1
Patients will orally receive two doses of S-1 according to body-surface area per day in a 2-weeks-on followed by 1-week-off manner ().
Table 1. S-1 dose.
Sintilimab
Patients receive 200 mg sintilimab on day 1 of each cycle. After the eighth course, treatment with sintilimab continues on day 1 of 3-week cycles for up to 1 year.
Nab-PTX
During the dose escalation, patients will be treated with Nab-PTX at a dose of 80 mg with escalations to 120 mg ().
Table 2. Nanoparticle albumin-bound paclitaxel dose levels.
Phase I part
The Phase I trial is a dose escalation via a standard 3 + 3 model followed by expansion cohorts (). Initial treatment starts from level 1 ().
DLT: Dose-limiting toxicities; MTD: Maximum tolerated dose.
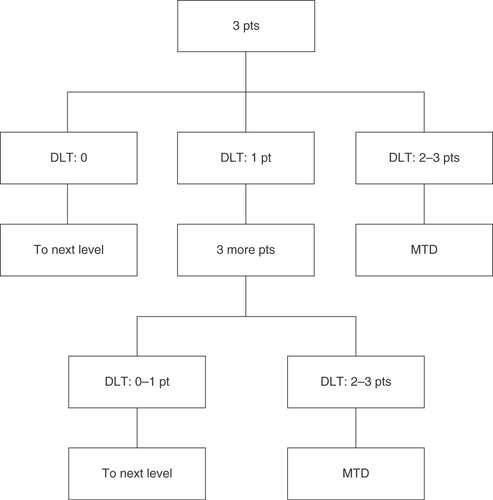
Table 3. Dose escalation in Phase I part.
The recommended dose (RD) is defined as a dose equal to the maximum tolerated dose (MTD). If 1 of 3 patients experiences dose-limiting toxicities (DLTs), 3 more patients will be enrolled at the same dose level. The MTD is defined as the dose level at which 2 or more of 3 patients, or at least 3 of 6 patients, have DLTs during one cycle.
Adverse events are monitored and graded based on the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 [Citation33]. The following adverse drug reactions are defined as DLTs:
≥Grade 3 febrile neutropenia
Grade 4 thrombocytopenia
Grade 4 neutropenia over 7 days
≥Grade 3 non-hematological toxicities (excludes nausea and vomiting)
Delay of starting cycle 2 longer than 15 days due to adverse event
If MTD is not reached in level 3, the RD will be determined as level 3. There will be no further dose escalation. The primary objectives are treatment tolerability and RD determination for Phase II.
Phase II part
Primary end point
The primary end point is 3-year RFS (based on Response Evaluation Criteria in Solid Tumors [RECIST] V.1.1 criteria [Citation34]).
Secondary end points
The secondary end points are 5-year OS, 5-year RFS, 3-year OS; adverse events (based on the CTCAE, version 4.0) and treatment compliance; peritoneal metastasis rate; and time to peritoneal metastasis and quality of life.
Exploratory objectives
The changes of ct-DNA will be explored.
Number of enrollments
In Phase I, 3 to 6 subjects are enrolled at each dose level (a maximum of 18 patients). In Phase II trial, the RD level is expanded to further estimate the efficacy and safety of this triplet regimen. The 3-year RFS rate under the null hypothesis was defined as 42.3% based on the retrospective study [Citation7]. The expected study subjects will be enrolled for over 1 year and followed up for 5 years. With an expectation of the 3-year RFS of 65%, the simulation results indicated a sample size of 38 with α = 0.1 (single side), a power of 80% and a dropout rate of 10%. Finally, there will be 20 subjects enrolled in the Phase II part.
Treatment discontinuation
Study treatment will be discontinued for any of the following reasons:
Progressive disease is documented by the investigator.
Unacceptable toxicity is determined by the patient or investigator.
The investigator determines that the continuation of treatment is not in the patient's best interest.
Exclusion criteria occur.
The patient fails to comply with the protocol.
The patient declines further study treatment or withdraws his or her consent to participate in the study.
The reasons for discontinuing treatment will be documented in the medical record. Tumor assessment follow-up will be continued for 5 years after surgery or until death.
Follow-up
Physical examinations and blood tests are performed every week during the 8 triplet-cycles and every 3 weeks during sintilimab monotherapy. Imaging evaluation and endoscopy are conducted every 3 months during the treatment.
The blood tests include a complete blood cell count, measurement of liver and renal function, coagulation parameters, myocardial enzymogram, thyroid function and tumor marker evaluations (CEA, CA199, CA125, CA724, AFP). Imaging evaluation includes chest computed tomography (CT), enhanced abdomen-pelvis CT scan, bone scan and head MRI when having central nervous system symptoms.
After the treatment, regular outpatient follow-up is scheduled every 3 months during the first 1 year and every 6 months for the next 3 years with physical examination and blood tests. Chest–abdomen–pelvis CT and endoscopy are performed every 6 months. Liver MRI, bone scan, head MRI and PET-CT are optional. If signs or symptoms indicate a possible recurrence, investigations are done to verify whether the patient is disease free. All patients are followed up for 5 years after surgery or until death.
Discussion
There is no clinical trial covering the adjuvant treatment of stage IIIC GC based on the 8th edition TNM system. The JACCRO GC-07 trial demonstrated the superiority of S-1 plus docetaxel (66%) to S-1 (50%) for 3-year RFS (HR: 0.632; 99.99% CI: 0.400–0.998; p < 0.001) in stage III GC patients, but subgroup analyses of RFS showed that the addition of docetaxel provided no survival benefit in patients with stage IIIC GC (HR: 0.693; 95% CI: 0.466–1.03). Notably, staging of this trial was based on the 7th edition TNM system.
In the JACCRO GC-07 trial, the common recurrence patterns were peritoneal, hematogenous and lymph node relapse. Compared with the S-1 group, the S-1 plus docetaxel group had a significantly lower recurrence rate in hematogenous sites and lymph nodes, whereas no difference was observed in the incidence of local recurrence and peritoneal relapse (9.3% vs 12.9%; p = 0.092).
Although the S-1 plus docetaxel showed no survival benefit in stage IIIC GC, there was still a longer 3-year RFS in comparison with historical data. Since Nab-PTX is more effective in peritoneal metastasis and the use of docetaxel requires hormone pretreatment, the authors choose Nab-PTX to combine with PD-1 and S-1 as adjuvant therapy for patients with stage IIIC GC. It is the first use of Nab-PTX combined with PD-1 as adjuvant therapy in patients with GC; thus, the authors designed this dose-escalation study.
This study is designed to evaluate the safety and efficacy of the combination of Nab-PTX, S-1 and sintilimab as adjuvant therapy for patients with GC. The roles of immunotherapy and adjuvant chemotherapy have individually been investigated, but the combination as postoperative therapy has not been investigated before and is an option worth exploring. This triplet therapy is promising to be a more aggressive prophylaxis against relapse, especially peritoneal recurrence in GC patients undergoing gastrectomy of curative intent.
Conclusion
This is indeed the first prospective study that will be investigating comprehensive immunotherapy combined with chemotherapy as adjuvant therapy in the management of stage IIIC GC based on the 8th edition TNM system. The authors hope to find patients who benefit from this triplet regimen and the results of this study will contribute to establish treatment standards for clinical practice in patients with stage IIIC GC.
Background
The prognosis of stage III gastric cancer (GC) is not satisfying and the specific chemotherapy regimens for GC of stage IIIC are still inconclusive. Peritoneal recurrence is the common and severe relapse pattern.
Nanoparticle albumin-bound paclitaxel (Nab-PTX) is safer and more effective than paclitaxel in peritoneal metastasis. Clinical trial has demonstrated the safety and efficacy of sintilimab in GC.
Dragon-VII trial design
This is a prospective, single-arm, open-label, Phase I/II clinical trial.
Pathological stage IIIC gastric/gastroesophageal junction adenocarcinoma without preoperative treatments was involved to receive combination therapy of Nab-PTX, S-1 and sintilimab every 3 weeks for eight cycles, then sintilimab maintenance up to 12 months.
The Phase I part was designed for optimal dose of Nab-PTX via a 3 + 3 dose-escalation model with the initial 80 mg/m2 (i.v., day 1 and day 8) and up to 120 mg/m2. Flat doses of sintilimab (200 mg, i.v., day 1) and S-1 (40–60 mg b.i.d., days 1–14) will be given concurrently. The primary end points are safety and determination of the recommended dose for subsequent Phase II study.
In the Phase II part, the primary objective is 3-year relapse-free survival.
Author contributions
ZL Zhu, J Zhang and ZG Zhu conceived and designed the trial. Y Mei and M Shi drafted the manuscript. H Yuan, C Yan, C Li and M Yan participated in the administration of this study. TN Feng contributed to the statistical analysis. All the authors gave final approval of the final version of this manuscript to be published.
Ethical conduct of research
This study is approved by the Ruijin Hospital Ethics Committee, Shanghai Jiao Tong University School of Medicine, China (no. 2021-19), has been registered in the ClinicalTrial.gov as NCT04781413 and will follow the principles outlined in the Declaration of Helsinki. The patient or his or her legal representative should read, understand and sign the informed consent form for therapy and keep it in the medical record.
Financial & competing interests disclosure
This study was funded in part by the Medical Engineering Cross Research Fund of Shanghai Jiao Tong University (no. YG2021QN14) and the Multicenter Clinical Trial of Shanghai Jiao Tong University of Medicine (no. DLY201602). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Sung H , FerlayJ, SiegelRLet al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249 (2021).
- Sasako M , SakuramotoS, KataiHet al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol.29(33), 4387–4393 (2011).
- Noh SH , ParkSR, YangH-Ket al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol.15(12), 1389–1396 (2014).
- Yoshida K , KoderaY, KochiMet al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J. Clin. Oncol.37(15), 1296–1304 (2019).
- Chang JS , LimJS, NohSHet al. Patterns of regional recurrence after curative D2 resection for stage III (N3) gastric cancer: implications for postoperative radiotherapy. Radiother. Oncol.104(3), 367–373 (2012).
- Li P , HuangCM, ZhengCHet al. Comparison of gastric cancer survival after R0 resection in the US and China. J. Surg. Oncol.118(6), 975–982 (2018).
- Kim SH , LeeHJ, ParkJHet al. Proposal of a new TNM classification for gastric cancer: focusing on pN3b and cytology-positive (CY1) disease. J. Gastric Cancer19(3), 329–343 (2019).
- Kobayashi M , SakamotoJ, NamikawaTet al. Pharmacokinetic study of paclitaxel in malignant ascites from advanced gastric cancer patients. World J. Gastroenterol.12(9), 1412–1415 (2006).
- Sakamoto J , MatsuiT, KoderaY. Paclitaxel chemotherapy for the treatment of gastric cancer. Gastric Cancer12(2), 69–78 (2009).
- Sasaki Y , NishinaT, YasuiHet al. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci.105(7), 812–817 (2014).
- Shitara K , TakashimaA, FujitaniKet al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol.2(4), 277–287 (2017).
- Takashima A , ShitaraK, FujitaniKet al. Peritoneal metastasis as a predictive factor for nab-paclitaxel in patients with pretreated advanced gastric cancer: an exploratory analysis of the phase III ABSOLUTE trial. Gastric Cancer22(1), 155–163 (2019).
- Kinoshita J , FushidaS, TsukadaTet al. Comparative study of the antitumor activity of nab-paclitaxel and intraperitoneal solvent-based paclitaxel regarding peritoneal metastasis in gastric cancer. Oncol. Rep.32(1), 89–96 (2014).
- Shirasaka T , ShimamatoY, OhshimoHet al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs7(5), 548–557 (1996).
- Kochi M , FujiiM, KanamoriNet al. Effect of gastrectomy on the pharmacokinetics of S-1, an oral fluoropyrimidine, in resectable gastric cancer patients. Cancer Chemother. Pharmacol.60(5), 693–701 (2007).
- Zhang W , DuC, SunYet al. Nab-paclitaxel plus S-1 as first-line followed by S-1 maintenance for advanced pancreatic adenocarcinoma: a single-arm phase II trial. Cancer Chemother. Pharmacol.82(4), 655–660 (2018).
- Shi Y , ZhangS, HanQet al. Nab-paclitaxel plus S-1 in advanced pancreatic adenocarcinoma (NPSPAC): a single arm, single center, phase II trial. Oncotarget8(54), 92401–92410 (2017).
- Morimoto M , TobaH, AoyamaMet al. Phase 1 dose-escalation study of triweekly nab-paclitaxel combined with S-1 for HER2-negative metastatic breast cancer. Clin. Breast Cancer20(6), 448–453 (2020).
- Nakayama N , IshidoK, ChinKet al. A phase I study of S-1 in combination with nab-paclitaxel in patients with unresectable or recurrent gastric cancer. Gastric Cancer20(2), 350–357 (2017).
- Sakaguchi K , NakatsukasaK, TaguchiT. Phase I study of triweekly nab-paclitaxel combined with S-1 in patients with HER2-negative metastatic breast cancer. Anticancer Res.36(12), 6515–6519 (2016).
- Tsurutani J , KuroiK, IwasaTet al. Phase I study of weekly nab-paclitaxel combined with S-1 in patients with human epidermal growth factor receptor type 2-negative metastatic breast cancer. Cancer Sci.106(6), 734–739 (2015).
- He MM , WangF, JinYet al. Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer Sci.109(11), 3575–3582 (2018).
- Robert C , LongGV, BradyBet al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med.372(4), 320–330 (2015).
- Le DT , UramJN, WangHet al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med.372(26), 2509–2520 (2015).
- Reck M , Rodriguez-AbreuD, RobinsonAGet al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med.375(19), 1823–1833 (2016).
- Fuchs CS , DoiT, JangRWet al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol.4(5), e180013 (2018).
- Kang YK , BokuN, SatohTet al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet390(10111), 2461–2471 (2017).
- Moehler M , ShitaraK, GarridoM. LBA6_PR nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): first results of the CheckMate 649 study. Ann. Oncol.31(Suppl. 4), S1191 (2020).
- Boku N , RyuMH, OhDY. LBA7_PR nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann. Oncol.31(Suppl. 4), S1192 (2020).
- Wang J , FeiK, JingHet al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs11(8), 1443–1451 (2019).
- Jiang H , ZhengY, QianJet al. Safety and efficacy of sintilimab combined with oxaliplatin/capecitabine as first-line treatment in patients with locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma in a phase Ib clinical trial. BMC Cancer20(1), 760 (2020).
- Brierley JD , GospodarwiczMK, WittekindC, AminMB. TNM Classifcation of Maligant Tumours (8th Edition).Wiley Blackwell, NJ, USA (2016).
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. NIH National Cancer Institute, Division of Cancer Treatment & Diagnosis (2020). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40
- Eisenhauer EA , TherasseP, BogaertsJet al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer45(2), 228–247 (2009).
