?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim: To investigate the efficacy, safety and optimal dosage of bevacizumab in non-squamous, non-small-cell lung cancer (NSCLC) patients with malignant pleural effusion (MPE). Methods: 20 patients were enrolled and received intrapleural injection of bevacizumab (group A: 2.5 mg/kg d1, d8; group B: 5 mg/kg d1, d8; group C: 7.5 mg/kg d1, d8). Results: The objective response rate (ORR) of MPE was 50%. The median progression-free survival (PFS) of MPE was 7.0 months (95% CI 4.9–9.2). The ORR and PFS of MPE from group B were better than those of group A and group C. The most common adverse events (AEs) were hypertension (15%) and anemia (15%). Conclusion: Bevacizumab has certain efficacy in non-squamous NSCLC patients with MPE.
Clinical Trial Registration: NCT02942043 (ClinicalTrials.gov).
Malignant pleural effusion (MPE) is a common complication in patients with non-small-cell lung cancer (NSCLC). It implies that the disease is at advanced stage. Lung cancer patients with MPE usually have poor prognosis, and the median overall survival (OS) of them is only about 6 months [Citation1,Citation2]. A large amount of MPE can seriously affect the quality of life of the patients. It may usually cause dyspnea, chest tightness, affect heart function and even threaten the life of the patient. Treatment for MPE is crucial to improve the patients’ quality of life.
Once the diagnosis of MPE is confirmed, treatment should be considered as soon as possible. Treatment methods include diuretic drugs, pleural aspiration, intercostal tube drainage and intrapleural injection with sclerosing agents, chemotherapy or biological agents. Administration of diuretic drugs, pleural aspiration or intercostal tube drainage is prone to relapse, and it may cause electrolyte disturbance, significant loss of protein, chest infection, etc [Citation3,Citation4]. Although the effective rate of pleurodesis with sclerosing agents can reach at 80%, the pleurisy reactions such as chest pain and fever are usually obvious [Citation5]. Intrapleural injection of chemotherapy like cisplatin is widely used, and the effective rate is 50–80% [Citation6,Citation7]. But adverse reactions such as bone marrow suppression and gastrointestinal reactions are common. Intrapleural injection of biological agents has limited curative effect, and it might have adverse reactions such as fever, allergies and chest pain. In short, these treatment methods have different effects, and they all have certain limitations. How to improve the treatment effect and quality of life for patients with MPE are the future research directions.
VEGF are key regulators for the formation of MPE by increasing vascular permeability [Citation8]. It promotes vascular leakage by opening endothelial intercellular junctions and creating fenestrations in the endothelial lining [Citation9]. Bevacizumab is a recombinant humanized monoclonal antibody that selectively binds to VEGF. It interferes with its receptor interactions and control the formation of pleural effusion [Citation10,Citation11].
In recent years, many literatures have reported the efficacy of bevacizumab intrapleural injection for the treatment of MPE. In a retrospective study, bevacizumab was infused at a dose of 100 or 200 mg, and this treatment was repeated every week until a response was observed. The results showed that objective response rate (ORR) of MPE for bevacizumab was better than traditional chemotherapy, biological agents and intercostal tube drainage. The ORR of MPE for bevacizumab intrapleural injection was 82% [Citation12]. In a prospective study, ten patients were administered intrapleural infusion of paclitaxel (175 mg/m2) alone, and 14 patients took a combination intrapleural infusion of paclitaxel and bevacizumab (5 mg/kg). The ORR of MPE for paclitaxel was 50%, and the ORR of the combination therapy for paclitaxel and bevacizumab was 78.6% [Citation13]. In other study, a total of 72 NSCLC patients were given intrapleural administration of either a combination of 30 mg of cisplatin plus 300 mg of bevacizumab or 30 mg of cisplatin monotherapy. This treatment was given every 2 weeks for three cycles. Besides, these patients were given conventional chemotherapy comprising paclitaxel (175 mg/m2) and carboplatin (area under the curve = 6). The ORR of MPE for cisplatin was 50%, and the ORR of MPE for cisplatin plus bevacizumab was 83.3% [Citation7].
These results suggest that intrapleural injection of bevacizumab has a certain efficacy and safety. However, most of these prospective clinical studies were about the treatment of intrapleural injection of bevacizumab combined with other anti-tumor drugs. Moreover, the intrapleural injection dose of bevacizumab reported in literatures is not uniform. There is no established standard for the pleural administration dose of bevacizumab. This study explores the favorable dose by comparing the effectiveness of different intrapleural injection doses of monotherapy bevacizumab in the treatment of MPE.
Patients & methods
Study design
Our study was a phase II, open label, prospective randomized controlled clinical study (ClinicalTrials.gov identifier: NCT02942043). The objectives of this study were to investigate the efficacy and safety of bevacizumab in the treatment of MPE for advanced non-squamous NSCLC and compare the efficacy of different doses of bevacizumab. The primary end point for our study was ORR of MPE. The secondary end points were progression-free survival (PFS) of MPE, OS, ORR, disease control rate (DCR), efficacy comparison between three groups, correlation between ultrasonographic estimated volume (UEV) and actual drained volume (ADV) of MPE, and toxicity.
Patients’ eligibility
Inclusion criteria included the following: 1) age between 18 and 75 years old; 2) histologically or cytologically confirmed NSCLC; 3) a chest radiograph, ultrasound or computed tomography (CT) scans estimation was a large amount of pleural effusion, and cytologically confirmed MPE; 4) an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; 5) expected survival time ≥8 weeks; 6) volunteered to participate in the study and signed the informed consent.
Exclusion criteria included the following: 1) squamous cell lung cancer (including adeno-squamous cell lung cancer) and small-cell lung cancer (SCLC; including compound SCLC); 2) previous intrapleural injection of bevacizumab; 3) received antitumor treatment in the prior 2 weeks, including chemotherapy, thoracic radiotherapy, targeted therapy, immunotherapy and biotherapy; 4) received VEGF monoclonal antibody therapy or VEGF small molecule tyrosine kinase inhibitor (TKI) in the prior 4 weeks; 5) underwent surgery in the prior 4 weeks or had a nonhealing wound; 6) occurrence of an embolism within 12 months; 7) tendency for bleeding, risk of massive hemoptysis, obvious coagulation disorders; 8) chest radiograph or CT showed obvious pneumothorax or hydropneumothorax; 9) encapsulated pleural effusion or bilateral moderate to large amount of pleural effusion; 10) coronary heart disease with obvious clinical symptoms or heart failure, uncontrolled arrhythmia and myocardial infarction within the prior 6 months; 11) uncontrolled hypertension (≥160/100 mmHg); 12) active or uncontrolled serious infections; 13) inadequate bone marrow reserve function; 14) other laboratory results abnormal, including: total bilirubin ≥1.5 times of the upper limit of normal; AST and ALT ≥2.5 times of the upper limit of normal (if liver metastasis, ≥5 times of the upper limit of normal); serum creatinine ≥1.5 times of the upper limit of normal or creatinine clearance rate <40 ml/min; serum ALB <30 g/l; urine protein ≥++, or 24-h urine protein ≥1.0 g; 15) pregnant or lactating women.
Randomization
All patients recruited in our study were randomly assigned to group A for low-dose bevacizumab treatment, group B for medium-dose bevacizumab treatment, or group C for high-dose bevacizumab treatment. The central computer system has provided random number and the treatment group. This was an open-label study. Treatment started within 7 days after randomization.
Treatment
After the pleural effusion was fully drained, the patients received intrapleural injection of bevacizumab (group A: 2.5 mg/kg d1, d8; group B: 5 mg/kg d1, d8; group C: 7.5 mg/kg d1, d8) dissolved in 250 ml of 0.9% saline solution once every 3 weeks for one–two cycles. After injection, the patients were required to lie in bed for 1 h and turn over every 10 min.
Study assessments
The data cut-off date was 18 December 2020. The pleural effusion therapeutic effect was assessed by WHO criteria [Citation14] using ultrasonic examination at baseline, before each intrapleural injection, at the end of treatment. Complete response (CR) was defined as pleural effusion completely disappeared and lasted over 4 weeks; partial response (PR) was defined as pleural effusion reduced more than 50% and lasted over 4 weeks; stable disease (SD) was defined as pleural effusion reduced less than 50%; progressive disease (PD) was defined as pleural effusion increased after treatment. ORR of MPE was calculated using the rate of CR plus PR of MPE. PFS of MPE was defined as the duration from the beginning of bevacizumab treatment to MPE progression or death (according to which occurred first). ADV was defined as the actual drained volume for MPE.
The calculation formula of UEV for MPE:
Where EV = estimated effusion volume (ml); X = craniocaudal extent of the effusion at the dorsolateral chest wall measured in the erect/sitting position with the probe oriented longitudinally; LDD = lung base to mid-diaphragm distance/sub-pulmonary height of the effusion (cm); 70 = empirical factor/constant [Citation15].
The tumor therapeutic effect was assessed at baseline and at the end of treatment using RECIST 1.1 criteria. Overall survival (OS) was defined as the duration from the beginning of bevacizumab treatment to the time of death. ORR was calculated using the rate of CR plus PR. DCR was calculated using the rate of CR plus PR and SD.
The safety of bevacizumab treatment was assessed using the Common Terminology Criteria for Adverse Events version 4.03 (CTCAE 4.03).
Statistical analysis
All efficacy and safety analyses were conducted on all patients who received at least one-dose intrapleural injection of bevacizumab. Statistical analyses were performed using SPSS version 26.0. Survival curves were generated using the Kaplan–Meier method. A log-rank test was used for the univariate analysis of PFS of MPE and OS between groups. Fisher’s exact test was used to compare the rates between groups. One-way ANOVA test was used to compare the mean values between groups. Pearson’s correlation analysis was performed to determine the extent of correlation between UEV and ADV. The degrees of correlation were classified as follows: r = 0–0.20, very low and probably meaningless correlation; r = 0.21–0.40, low correlation that might warrant further investigation; r = 0.41–0.60, reasonable correlation; r = 0.61–0.80, high correlation; and r = 0.81–1.0, excellent/very high correlation [Citation16]. Statistical significance was defined as p < 0.05.
Results
Patients’ characteristics
There were 20 patients from Peking University Cancer Hospital recruited in this study between January 2017 and August 2019. Their baseline demographic and clinical characteristics were collected, including: gender, age, EGFR status, ALK rearrangement status, number of distant metastases, brain metastases, smoking history (defined as a smoking index >10 pack-years), ECOG performance status and previous intrapleural infusion history. There were no significant differences for these demographic and clinical characteristics between three groups ().
Table 1. Baseline demographic and clinical characteristics.
Treatment efficacy & patient survival
The median PFS of MPE was 7.0 months (95% CI 4.9–9.2; ). The median OS was 19.2 months (95% CI 14.0–24.3; ). The MPE of one patients was assessed as CR, nine patients were assessed as PR, nine patients were assessed as SD and one patients were assessed as PD. The ORR of MPE was 50%. For tumor efficacy, one patient was assessed as PR, 11 patients were assessed as SD and six patients were assessed as PD. There were two patients did not completed tumor efficacy evaluation. The ORR was 6% and DCR was 67% (). The ORR of MPE and PFS of MPE from group B were better than group A and group C, but there were no significant differences for ORR of MPE, ORR of tumor, DCR of tumor, PFS of MPE and OS between group A, B and C ( and ). The PFS of MPE in EGFR sensitizing mutation and EGFR wild type patients were 4.5 months (95% CI: 0.4–8.6) and 7.5 months (95% CI: 4.9–10.1) respectively (p = 0.623). The ORR of MPE in EGFR sensitizing mutation and EGFR wild type patients were 45 and 56%, respectively (p = 1.000). The PFS of MPE in ALK rearrangement and ALK non-rearrangement patients were 11.5 months (95% CI 2.7–20.3) and 6.6 months (95% CI: 2.1–11.1), respectively (p = 0.444). The ORR of MPE in ALK rearrangement and ALK non-rearrangement patients were 100 and 41%, respectively (p = 0.211). There were no significant differences between EGFR sensitizing mutation and EGFR wild type patients for PFS of MPE and ORR of MPE. And there were also no significant differences between ALK rearrangement and ALK non-rearrangement patients for PFS of MPE and ORR of MPE.
(A) Progression-free survival of malignant pleural effusion for all 20 patients. (B) Overall survival for all 20 patients. (C) Progression-free survival of malignant pleural effusion stratified by groups. (D) Overall survival stratified by groups.
mOS: Median overall survival; mPFS: Median progression-free survival; MPE: Malignant pleural effusion.
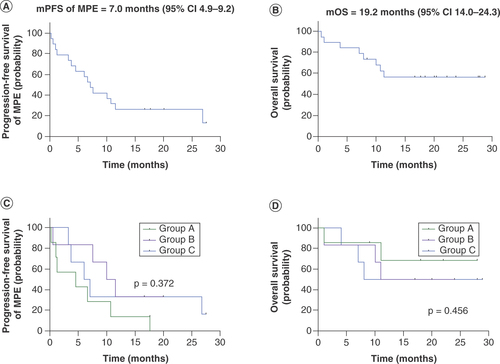
Table 2. Overall treatment efficacy and patient survival.
Table 3. Treatment efficacy and patient survival between three groups.
Correlation between UEV & ADV
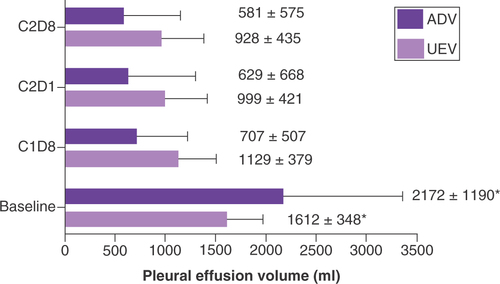
We collected all the UEV and ADV, which including baseline, eighth day of the first cycle (C1D8), the first day of the second cycle (C2D1), the eighth day of the second cycle (C2D8) for these 20 patients, and calculated the average value for UEV and ADV. The average value of UEV was 1218 ± 474 ml, and the average value of ADV was 1138 ± 1086 ml. It showed high correlation between UEV and ADV (r = 0.713, p < 0.001).
Volume changes of MPE after treatment
For both UEV and ADV, there were significant differences between baseline and after treatment, which included C1D8, C2D1 and C2D8 (p < 0.05). The MPE volume was significantly reduced after bevacizumab treatment. The more times of bevacizumab perfusion, the less MPE volume. But there was no significant difference for UEV and ADV between different treatment cycles ().
Analysis of toxicity
The adverse events (AEs) with an incidence of ≥5% were hypertension (15%), anemia (15%), thoracic infection (10%), hypoproteinemia (10%), pain (10%), elevated transaminase (10%), proteinuria (5%), venous thrombosis (5%), prolonged APTT (5%), urine occult blood (5%), elevated γ-glutamyl transpeptidase (5%) and elevated creatine kinase (5%). Hypertension (10%) and thoracic infection (10%), which can be well controlled, were assessed as a grade 3–4 AE with an incidence of ≥5% ().
Table 4. Adverse events.
Discussion
This was a phase II, open-label, prospective, randomized controlled clinical study. In this study, different doses of bevacizumab were perfused into the pleural cavity to treat MPE of advanced non-squamous NSCLC. As far as we know, there were only few clinical studies of similar design before. According to the results of this study, the ORR of MPE was 50%, the median PFS of MPE was 7.0 months (95% CI 4.9–9.2), the median OS was 19.2 months (95% CI: 14.0–24.3) and the MPE volume was significantly reduced after bevacizumab treatment. These results suggest that intrapleural injection of single-agent bevacizumab has a certain effect in the treatment of MPE for advanced non-squamous NSCLC. However, according to the results of several prospective clinical studies, the ORR of MPE for intrapleural injection of bevacizumab combined with other anti-tumor drugs was about 80% [Citation7,Citation13]. In this study, only bevacizumab intrapleural injection was given to patients, which might be the cause for lower ORR of MPE in this study. In a clinical study for intrapleural injection of bevacizumab and cisplatin versus cisplatin with a systemic chemotherapy treatment, the bevacizumab and cisplatin group showed that the median OS was 10.3 months [Citation7]. Although bevacizumab was the only treatment in this study, OS was longer than studies for bevacizumab combined with other anti-tumor treatments. A total of 55% of the enrolled patients had EGFR sensitive mutations, and 15% of the patients were ALK-positive in this study. Therefore, some patients received target therapy as subsequent treatments. That might be the underlying reason of longer OS in this study. In this study, we did not compare the efficacy of intrapleural infusion and intravenous infusion of bevacizumab. But in a prospective randomized controlled study which enrolled 43 patients, bevacizumab intrapleural infusion showed higher efficiency and safety than intravenous infusion in the management of MPE caused by NSCLC. The ORR of MPE was 80 and 66.7%, and the median duration of response for MPE was 4.50 months and 3.70 months, respectively [Citation17].
It showed high correlation between ADV and UEV (r = 0.713; p < 0.001) in our study, which was consistent with the previous literature reports [Citation15]. The results suggested that this calculation formula of ultrasonographic estimated pleural effusion volume can be used to measure pleural fluid volume. It also suggested that ultrasound is reliable in the measurement of pleural effusion. For both UEV and ADV, the MPE volume was significantly reduced after bevacizumab treatment, and the MPE volume of C2D8 was the least (). In this study, the MPE volume of C2D8 was reduced by 42% than baseline for the UEV, and the MPE volume of C2D8 was reduced by 73% than baseline for the ADV. The differences between these two values might be attributed to the large MPE volume of baseline for some patients, they needed to drain pleural effusion continuously for several days. Pleural effusion still increased during these time, so the UEV was lower than ADV at the baseline. The ORR of MPE in this study might also be underestimated because the ORR of MPE was calculated according by UEV.
To our knowledge, there was only a few prospective studies for the effective dose of bevacizumab intrapleural injection treatment. A retrospective study in which 71 patients were recruited showed that there was no significant difference in PFS and OS between low-dose (median dose was 100 mg/week) and high-dose (median dose was 200 mg/week) intrapleural bevacizumab treatment for MPE in NSCLC patients [Citation18]. In this study, for the comparison of treatment effects and survival between three groups, the ORR of MPE and PFS of MPE from group B were better than group A and group C. The ORR of MPE for group B was 67%, group A was 29% and group C was 50%. The PFS of MPE for group B was 10.0 months (95% CI: 5.2–14.8), group A was 4.5 months (95% CI: 0–13.0) and group C was 6.0 months (95% CI: 4.9–9.2). But there were no significant differences for ORR of MPE, PFS of MPE between group A, B and C. There were no significant differences for ORR of tumor, DCR of tumor and OS between group A, B and C.
Regarding the safety of bevacizumab treatment, the types of adverse reactions for bevacizumab intrapleural injection in this study were generally consistent with systemic treatment. The incidence of adverse reactions for bevacizumab pleural perfusion in this study was equal or lower than systemic treatment, and the severity of adverse reactions was equal to or lighter than systemic treatment. There were one patients (5%) founded grade 1 urinary occult blood, and there were no tumor related bleeding and serious bleeding events. As for adverse reactions of thromboembolism, there was one patient (5%) founded grade 2 venous thrombosis in lower extremities. The incidence was lower and severity was lighter for adverse reactions of thromboembolism in this study than reported in the literature [Citation19]. In this study, the incidence of any grades hypertension was 15%, and the incidence of grade 3–4 hypertension was 10%. Previous studies have reported that the overall incidence of hypertension with bevacizumab intravenous application was 20–30%, and the incidence of grade 3–4 hypertension was 6–9%. In this study, the incidence was lower and severity was lighter for adverse reactions of hypertension than bevacizumab intravenous administration. In this study, one patient (5%) had grade 1 proteinuria, and the incidence and severity of proteinuria were consistent with intravenous administration [Citation20]. In addition, no adverse reactions such as gastrointestinal perforation, fistula and heart failure were found in this study. In conclusion, the adverse reactions for pleural effusion of bevacizumab were controllable and predictable.
As patients with advanced NSCLC have increased systemic treatment options, such as targeted therapy, chemotherapy and immunotherapy, etc., only small number of patients can accept single agent bevacizumab treatment. This was the poor recruitment reason for our study. To further confirm the efficacy, safety and optimal dosage of bevacizumab in the treatment of MPE for advanced non-squamous NSCLC patients, future studies are warranted by combining bevacizumab with EGFR TKI or immunotherapy in future.
Conclusion
In this study, the single agent of bevacizumab has certain efficacy in non-squamous NSCLC patients with MPE. Among the three groups, the MPE was controlled better in group B. The adverse reactions for pleural effusion of bevacizumab were controllable and predictable. Considering the limitations of this study, studies by combining bevacizumab with EGFR-TKI or immunotherapy can be conducted to further confirm the efficacy, safety and optimal dosage of bevacizumab in the treatment of MPE for advanced non-squamous NSCLC.
Some studies suggested that intrapleural injection of bevacizumab has a certain efficacy and safety. However, most of these clinical studies were about the combination of bevacizumab with other anti-tumor drugs. Moreover, the intrapleural injection dose of bevacizumab reported in literatures is not uniform.
This was a Phase II, open label, prospective randomized controlled clinical study of intrapleural injection of single agent bevacizumab in non-squamous non-small-cell lung cancer (NSCLC) patients with malignant pleural effusion (MPE). The objective response rate (ORR) of MPE was 50%. The median PFS of MPE was 7.0 months (95% CI: 4.9–9.2). The ORR of MPE and progression-free survival (PFS) of MPE from group B were better than those of group A and group C, but there were no significant differences for ORR of MPE, PFS of MPE between three groups.
It showed high correlation between ultrasonographic estimated volume and actual drained volume; r = 0.713; p < 0.001) in our study. The results suggest that this calculation formula of ultrasonographic-estimated pleural effusion volume can be used to measure pleural fluid volume. This result also suggests that ultrasound is reliable for the measurement of pleural effusion.
Bevacizumab has certain efficacy in non-squamous NSCLC patients with MPE. Among the three groups, the MPE was seemingly controlled better in group B. The side effects can be well tolerated.
Financial & competing interests disclosure
We received bevacizumab (Avastin®) from Roche for this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted with approval from the Ethics Committee of Peking University Cancer Hospital, the project no. 2016YJZ22. Written informed consent was obtained from all patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Acknowledgments
The authors thank all the patients and the staffs of Peking University Cancer Hospital who participated in our study.
References
- van de Molengraft FJ , VooijsGP. Survival of patients with malignancy-associated effusions. Acta. Cytol.33(6), 911–916 (1989).
- Porcel JM , GasolA, BielsaSet al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology20(4), 654–659 (2015).
- Roberts ME , NevilleE, BerrisfordRGet al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax65(Suppl. 2), ii32–40 (2010).
- Koegelenberg CFN , ShawJA, IrusenEMet al. Contemporary best practice in the management of malignant pleural effusion. Ther. Adv. Respir. Dis.12, doi:10.1177/1753466618785098 (2018).
- Medford AR , MaskellNA. A national survey of oncologist and chest physicians’ attitudes towards empirical anti-oestrogen therapy, early pleurodesis and preference of sclerosing agent in malignant breast and ovarian pleural disease. Palliat. Med.19(5), 430–431 (2005).
- Seto T , UshijimaS, YamamotoHet al. Intrapleural hypotonic cisplatin treatment for malignant pleural effusion in 80 patients with non-small-cell lung cancer: a multi-institutional phase II trial. Br. J. Cancer95(6), 717–721 (2006).
- Du N , LiX, LiFet al. Intrapleural combination therapy with bevacizumab and cisplatin for non-small cell lung cancer-mediated malignant pleural effusion. Oncol. Rep.29(6), 2332–2340 (2013).
- Bates DO . Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res.87(2), 262–271 (2010).
- Grove CS , LeeYC. Vascular endothelial growth factor: the key mediator in pleural effusion formation. Curr. Opin. Pulm Med.8(4), 294–301 (2002).
- Sabang RL , GandhirajD, FanucchiMet al. Role of bevacizumab in the management of the patient with malignant pleural effusion: more questions than answers. Expert Rev. Respir. Med.12(2), 87–94 (2018).
- Chen Y , MathyNW, LuH. The role of VEGF in the diagnosis and treatment of malignant pleural effusion in patients with non-small cell lung cancer (Review). Mol. Med. Rep.17(6), 8019–8030 (2018).
- Chen D , SongX, ShiFet al. Greater efficacy of intracavitary infusion of bevacizumab compared to traditional local treatments for patients with malignant cavity serous effusion. Oncotarget8(21), 35262–35271 (2017).
- Qi N , LiF, LiXet al. Combination use of paclitaxel and avastin enhances treatment effect for the NSCLC patients with malignant pleural effusion. Medicine (Baltimore).95(47), e5392 (2016).
- Miller AB , HoogstratenB, StaquetMet al. Reporting results of cancer treatment. Cancer47(1), 207–214 (1981).
- Ibitoye BO , IdowuBM, OgunrombiABet al. Ultrasonographic quantification of pleural effusion: comparison of four formulae. Ultrasonography37(3), 254–260 (2018).
- Harris M , TaylorG. Medical statistics made easy.Martin Dunitz, London, UK, 48–52 (2003).
- Nie K , ZhangZ, YouYet al. A randomized clinical study to compare intrapleural infusion with intravenous infusion of bevacizumab in the management of malignant pleural effusion in patients with non-small-cell lung cancer. Thorac. Cancer11(1), 8–14 (2020).
- Chen D , SongX, ZhangYet al. Optimizing intrapleural bevacizumab dosing in non-small-cell lung cancer-mediated malignant pleural effusion: less is more. Future Oncol.14(21), 2131–2138 (2018).
- Scappaticci FA , SkillingsJR, HoldenSNet al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J. Natl Cancer Inst.99(16), 1232–1239 (2007).
- Hirsh V . Emerging safety data for bevacizumab in advanced non-small-cell lung cancer. Clin. Lung Cancer9(Suppl. 2), S62–70 (2008).