Abstract
Aim: To reveal the treatment patterns of palbociclib and complete blood count (CBC) monitoring in a Japanese real-world setting. Materials & methods: Deidentified data of patients with advanced breast cancer who received palbociclib from 2017 to 2020 were examined from a Japanese claims database. Results & conclusion: We identified 1074 patients. Palbociclib was commonly prescribed as second- or later-line treatment in 2017/2018; thereafter its first-line treatment increased. Regardless of treatment lines, fulvestrant was most commonly prescribed in combination with palbociclib (57–66% in the first–third-line), and this finding differed from that in the USA. Most patients initiated palbociclib at 125 mg/day; however, over a half of patients reduced doses within the first 8 weeks. Although CBC was regularly monitored, some patients did not undergo blood tests. Early dose reduction and CBC monitoring should be performed cautiously to minimize safety concern and prevent early treatment discontinuation.
Breast cancer is the most common type of cancer among women in Japan, with nearly 92,000 new cases diagnosed in 2017 and approximately 15,000 deaths reported in 2018 [Citation1]. Approximately two-thirds of breast cancers are hormone receptor-positive (HR+) [Citation2], and endocrine therapy is the optimal targeted treatment. For HR+/HERR2-negative advanced breast cancer (ABC), endocrine therapy is recommended as the preferred first-line therapy than chemotherapy in the absence of immediate life-threatening situations, visceral crises and/or refractory endocrine therapy [Citation3]. Although endocrine therapy is the foundation treatment for HR+/HER2- ABC, a large number of patients eventually develop resistance to the therapy. The development of the resistance has led to the search for new agents with alternate signaling pathways [Citation4].
Palbociclib (IBRANCE®) is an oral CDK 4/6 inhibitor used in combination with endocrine therapy for HR+/HER2- ABC [Citation5,Citation6]. The CDK4/6 inhibitors prevent progression from G1 to S phases during cell division; and thereby, prevent tumor cell proliferation [Citation4]. In a double-blind phase III study (PALOMA-2), a significantly longer median progression-free survival (PFS) was observed in postmenopausal patients treated with palbociclib plus letrozole than in those treated with placebo plus letrozole in the first-line setting (27.6 vs 14.5 months; hazard ratio [HR] = 0.563; p < 0.0001) [Citation7,Citation8]. In another double-blind phase III study (PALOMA-3) of patients with HR+/HER2- ABC who showed disease progression after previous endocrine therapy, the median PFS was significantly longer in patients treated with palbociclib plus fulvestrant than in those treated with placebo plus fulvestrant (11.2 vs 4.6 months; HR = 0.497; p < 0.0001) [Citation9–12]. Subgroup analyses of patients enrolled in the PALOMA-2 and PALOMA-3 studies further indicated that palbociclib was effective in Japanese patients [Citation13,Citation14]. Although hematologic toxicities were more common among Japanese patients than in the overall population, they were successfully managed with dose modifications.
In Japan, palbociclib was approved in September 2017 and launched in December 2017 [Citation15] for the treatment of inoperable or recurrent breast cancer in combination with endocrine therapy; however, due to its recent launch, data on real-world palbociclib treatment patterns are still limited. First, treatment line and combination partner of palbociclib in this real-world setting is unknown. In the package insert in the USA, palbociclib is indicated to prescribe in combination with an aromatase inhibitor (AI) as the initial endocrine-based therapy in postmenopausal women and in men, or with fulvestrant in patients with disease progression following endocrine therapy [Citation5]. However, the Japanese package insert does not recommend specific treatment line or combination partner of palbociclib [Citation16], leading to the possibility of various treatment patterns in clinical practice. It is important to clarify the treatment line for palbociclib prescribed because despite the similar HRs of PFS (~0.5) regardless of treatment lines observed in clinical trials, the absolute PFS benefit obtained from palbociclib combination was different among treatment lines [Citation7–10]. In addition, it is also important to clarify whether the combination partner of palbociclib is selected in clinical practice in accordance with evidence [Citation7–11,Citation17]. Second, it remains unknown whether treatment-related risk management is appropriately conducted in a real-world setting. Compared with the overall population, myelosuppression including neutropenia and leukopenia was more frequently reported in Japanese patients during clinical trials, and subsequently, palbociclib dose reduction was more common in Japanese patients [Citation13,Citation14]. Furthermore, the timing and number of complete blood count (CBC) tests are not indicated in the Japanese package insert. Thus, there is a need to clarify whether adverse event monitoring and dose adjustments are appropriately implemented in clinical practice.
This study aimed to reveal treatment patterns of palbociclib including treatment lines and its combination partners and CBC monitoring in patients with HR+/HER2- ABC initiating this therapy in the Japanese real-world setting. We conducted this study by using a large-scale Japanese medical claims database.
Materials & methods
Study design & data source
This retrospective, observational database study examined individual-level data on patients with HR+/HER2- ABC who were prescribed palbociclib in combination with endocrine therapy in a Japanese clinical setting. Data were extracted from a hospital-based medical claims database managed by Medical Data Vision ([MDV]; Tokyo, Japan) for a period of April 2008 through December 2020 (study period). The MDV database contains data on approximately 27.95 million patients (as of July 2019) [Citation18] from 383 nationwide medical institutions that use a diagnosis procedure combination per-diem payment system. These institutions comprise 22% of all acute care hospitals across Japan [Citation19], including 185 cancer therapeutic facilities. The data included patient demographics (e.g., age and sex), claims (e.g., diagnosis, medical procedures and prescriptions) and laboratory tests (e.g., blood tests). The database contains anonymously processed information as classified under the amended Act on the Protection of Personal Information 2003 [Citation20]. According to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects, institutional review board approval and patient informed consent were not required for this observational study as it used secondary data without any identifiable patient information.
Patient selection
Eligible patients were required to have: a diagnosis record of breast cancer (coded using the International Statistical Classification of Diseases and Related Health Problems, 10th revision [ICD-10]: C50.xx); at least one prescription record of endocrine therapy (anastrozole, letrozole, exemestane, tamoxifen citrate, toremifene citrate, fulvestrant or medroxyprogesterone acetate; Supplementary Table 1); and at least one record of palbociclib treatment along with a breast cancer surgery or metastasis record (codes listed in Supplementary Table 2) from September 2017 (the month of palbociclib approval) through December 2020. Patients were selected if they had ≥3 months of follow-up from palbociclib treatment initiation, irrespective of whether the palbociclib treatment was continued. Patients with prescription records of HER2-targeted therapy (trastuzumab, trastuzumab emtansine, trastuzumab deruxtecan, pertuzumab or lapatinib tosilate hydrate) were excluded.
ABC treatment lines
To identify breast cancer treatment lines in each patient, baseline was defined as the date of the earliest metastasis or the latest breast cancer surgery record identified in the study period (Supplementary Table 2). The first-line treatment for ABC was identified for each patient who had either a metastasis or surgery record, as defined in . For those who had a surgery record, the first-line treatment was categorized into a gap period of either a <12 months or ≥12 months following adjuvant endocrine therapy (), which was defined as the earliest endocrine therapy with a start date within 365 days of the latest breast cancer surgery record. Subsequent therapies following the first-line treatment were defined as the second-line, third-line and so on. Each regimen was considered to end if a new breast cancer treatment record not identified in the previous treatment was identified or there was a gap period of at least 120 days from the last breast cancer treatment to the following breast cancer treatment. The treatment duration for each line was calculated as the difference between the corresponding treatment end and start dates +1 day. The treatment duration was censored at the end of follow-up or the study period. Any treatment for breast cancer received within 30 days of the earlier treatment initiation was considered part of one regimen.
Table 1. Definition of the first-line treatment for each patient with advanced breast cancer.
Measures
Prescription patterns of palbociclib
The proportion of treatment lines (among the first-, second-, third- and fourth- or later-line treatments) prescribed with palbociclib was calculated for the entire study period and for every 6 months between December 2017 and December 2020; the only exception was the first block that included 7 months from December 2017 to June 2018. The proportion of endocrine therapies (Supplementary Table 1) prescribed in combination with palbociclib was calculated separately for each treatment line (up to the third line). The proportion of palbociclib treatment continuation assessed at 6 months was calculated for each treatment line (up to the third line).
Daily dose & dose reduction of palbociclib
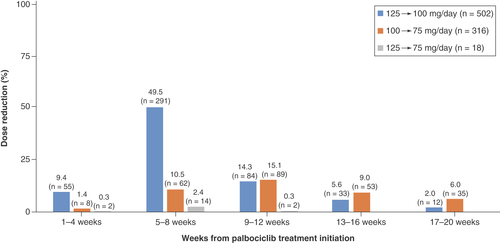
Initial and final palbociclib daily doses were identified for each treatment line among all patients. The timing of palbociclib dose reduction was identified separately during weeks 1–4, 5–8, 9–12, 13–16 and 17–20 after the initiation of palbociclib treatment for each reduction pattern of from 125 to 100, 100 to 75 and 125 to 75 mg/day. Doses were reduced several times for each patient.
Timing & number of blood tests performed after palbociclib initiation
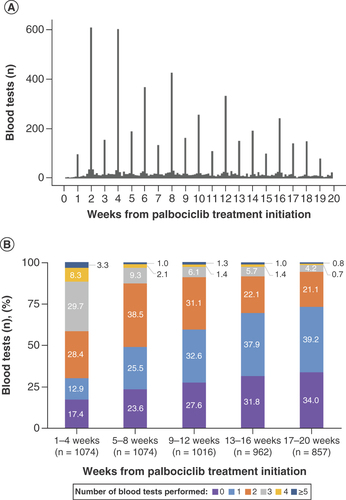
The timing and frequency of blood tests (Supplementary Table 2) were identified for each patient separately during weeks 1–4, 5–8, 9–12, 13–16 and 17–20 of palbociclib treatment initiation.
Statistical analysis
Descriptive statistics were used for all variables examined in this study, with mean and standard deviation (SD) for continuous variables and frequencies and percentages for categorical variables. If a patient received multiple lines of palbociclib treatments, only the earliest line was analyzed. The proportion of palbociclib treatment continued at 6 months was calculated using Kaplan–Meier estimation. No imputation was made for missing data. All statistical analyses were descriptive, and no p-value threshold was used in this study. All statistical analyses were performed using SAS v9.4 (SAS Institute, NC, USA) and R v3.4.0 or later (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline demographics & clinical characteristics of patients initiating palbociclib
Of the 185,865 patients with HR+/HER2- ABC identified in the database, 1074 patients treated with palbociclib were identified from September 2017 to December 2020 and were included in the analysis (). Palbociclib was predominantly prescribed to females (99.3%). The median (minimum, maximum [min, max]) age at palbociclib treatment initiation was 64.0 (32.0, 92.0) years, and most treatments started in 2018 (41.2%). Goserelin or leuprorelin was concomitantly prescribed with palbociclib in 13.6% of patients. The median (min, max) follow-up period from the initiation of palbociclib treatment to the end of the available data period was 15.9 (3.0, 38.6) months.
Table 2. Baseline demographic and clinical characteristics of patients initiating palbociclib treatments.
Prescription patterns of palbociclib
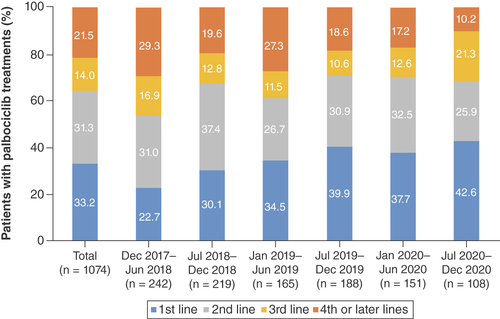
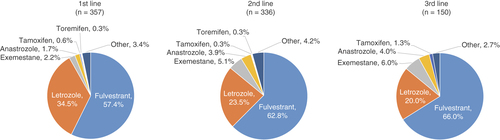
The overall proportions of patients prescribed palbociclib in the first-, second-, third- and fourth- or later-line treatments were 33.2, 31.3, 14.0 and 21.5%, respectively (). Palbociclib was initially prescribed more commonly as second- and later-line treatments; over time, the first-line treatment steadily increased from 22.7% (during December 2017–June 2018) to 42.6% (during July–December 2020). Concomitantly, the fourth- or later-line treatments decreased during the corresponding time period (from 29.3 to 10.2%). Fulvestrant was most commonly prescribed in combination with palbociclib regardless of treatment lines (57.4, 62.8 and 66.0% in the first-, second- and third-line, respectively), followed by letrozole (34.5, 23.5 and 20.0% in the first-, second- and third-line, respectively; ).
There was one patient who was prescribed palbociclib before December 2017 and was included in the total figure only.
This figure shows the results of the number of patients who were prescribed endocrine therapies in combination with palbociclib in the first three treatment lines (n = 843 out of 1074 patients). The results in the fourth or later treatment lines are not shown. The ‘other’ include patients prescribed ≥1 endocrine therapies and those prescribed medroxyprogesterone.
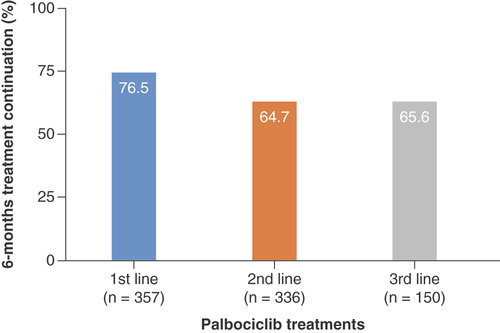
The proportions of palbociclib treatments that continued for at least 6 months were 76.5, 64.7 and 65.6% in the first-, second- and third-line treatments, respectively ().
This figure shows the results of 6-month palbociclib treatment continuation in the first three treatment lines (n = 843 out of 1074 patients). Results in the fourth or later treatment lines are not shown.
Palbociclib daily dose & trend in dose reduction
The majority of palbociclib doses started at 125 mg/day (82.0%; ). Among those, 33.6% retained the same dose until the end of palbociclib treatments, and 30.8 and 32.7% of patients were reduced to 100 and 75 mg/day, respectively. Among patients starting at a dose of 100 mg/day, 41.7% retained the same dose until the end of treatments, and 53.2% were reduced to 75 mg/day.
Table 3. Initial and final daily dose of palbociclib.
Dose reduction was identified in 588 patients within the first 20 weeks of palbociclib initiation (), and doses were reduced from 125 to 100 mg/day for over a half of patients within 8 weeks of palbociclib initiation (9.4 and 49.5% during weeks 1–4 and 5–8, respectively). The dose reduction from 100 to 75 mg/day peaked during weeks 9–12, and a two-level dose reduction from 125 to 75 mg/day was observed in 18 patients.
Frequency & number of blood tests performed during palbociclib treatments
Overall, there was a weekly and more notably biweekly pattern of blood tests performed during palbociclib treatments, with the largest number of blood tests conducted on day 14, followed by day 28 (A); however, the number declined subsequently. Within 1–4 weeks of palbociclib initiation, 69.7% of patients underwent ≥2 blood tests and the percentage declined in subsequent weeks to 30.2 and 26.8% during weeks 13–16 and 17–20, respectively (B). Of the total number of patients, 17.4% did not undergo blood tests during weeks 1–4 of palbociclib initiation, and this percentage increased thereafter to 31.8 and 34.0% during weeks 13–16 and 17–20, respectively.
Discussion
This study revealed treatment patterns of palbociclib and CBC monitoring among Japanese patients with HR+/HER2- ABC using a large medical claims database. Overall, palbociclib was prescribed broadly as the first- to fourth- or later-line treatment in Japan from December 2017 to December 2020, with more patients receiving palbociclib as the first-line treatment in the later years. Fulvestrant was most commonly combined with palbociclib from the first through third-line treatments. The majority of patients started palbociclib at a dose of 125 mg/day, and approximately 34% retained the same dose, whereas daily doses were reduced to 100 or 75 mg/day in the remaining majority. Most dose reductions were observed within 8 weeks of initiating palbociclib treatment. CBC was closely monitored; however, we found there were some patients who did not undergo blood tests from an early stage of palbociclib initiation.
Palbociclib was more commonly prescribed as the second- and fourth- or later-line treatment than as the first-line treatment just after its launch. However, as time progressed, palbociclib was prescribed more commonly as the first-line treatments and less commonly as the fourth- or later-line treatment. A similar trend was observed in a real-world study conducted in the USA, which reported that approximately 40% of patients were prescribed palbociclib as fourth- or later-line treatments just after its launch, and the proportion subsequently declined [Citation21]. The key difference in the treatment approach in Japan and the USA was that, from an early stage, palbociclib was more commonly prescribed as first-line treatment (~36%) in the USA compared with Japan where only 23% of patients received it as the first-line treatment. These findings suggested that palbociclib was introduced mainly into later line treatment in Japan, and later was gradually spread to earlier, more appropriate, treatment lines.
Fulvestrant was the most commonly prescribed endocrine therapy in combination with palbociclib in the first through third treatment lines in Japan. This result differed from that in the real-world study from the USA, where AIs were prescribed more commonly as the first-line treatment in combination with palbociclib, and fulvestrant was commonly used as the second-line treatment [Citation22]. This between-country difference is presumed to be a result of how palbociclib is labeled. In the USA, palbociclib is indicated for the treatment of HR+/HER2- advanced or metastatic breast cancer in combination with AI as the initial endocrine-based therapy in postmenopausal women and in men, or with fulvestrant in patients with disease progression following endocrine therapy [Citation5]. Conversely, the Japanese package insert does not recommend specific treatment line or concomitant therapy [Citation16]. The demographic and clinical characteristics of patients initiating palbociclib in Japan could be another reason for selecting fulvestrant as the first line in our study population. Here, approximately a half of the patients (45.7%) had a gap period of <12 month between the end of an adjuvant therapy and the initiation of first-line treatment, and 56.2% of these patients received AI as adjuvant therapy. Only 6.3% of patients had a gap period of ≥12 month. As the former set of patients was the target population of the PALOMA-3 study, in which the combination partner of palbociclib was fulvestrant, these backgrounds may have influenced fulvestrant selection in the first-line treatment in our study, and also indicate that the combination partner was selected according to evidence presented in clinical trials. A recent phase II study (PARSIFAL) showed no statistically significant differences in PFS between patients treated with palbociclib plus letrozole or palbociclib plus fulvestrant as the first-line treatment [Citation23]. Thus, there is still limited information available regarding the effects of different endocrine therapies combined with palbociclib as the first-line treatment, and further real-world assessments are of clinical value.
The 6-month palbociclib treatment continuation was high in the first-line setting. Previous reports from the real-world settings in the USA demonstrated a longer median PFS (11.6–20.7 months) or higher 12-month progression-free rates (89.6%) in patients treated with palbociclib in combination with endocrine therapy in the first-line treatments compared with later-line treatments [Citation22,Citation24–26]. Given the varying palbociclib treatment patterns between the USA and Japan as described above and the different timing of palbociclib approval, future real-world assessments of outcome measures including time-to-treatment failure and subsequent therapies after palbociclib treatments are essential.
Most patients (82%) started palbociclib at 125 mg/day, while approximately 17% started at 100 or 75 mg/day, the results generally in line with other real-world studies [Citation21,Citation22]. A previous study reported a reduced starting dose in patients aged ≥80 years, with an Eastern Cooperative Oncology Group performance status ≥2, and with a low geriatric score (G8 score ≤14) [Citation27]. It is possible that for similar reasons patients started at lower dose in our study. In a pooled analysis of clinical trials, the incidence of myelosuppression during palbociclib treatment was higher in patients aged ≥75 years than in younger patients aged 65–<75 years; however, there was no difference in the incidence of grade ≥3 myelosuppression across ages [Citation28]. As a limited number of older patients was included in the clinical trials, further real-world evidence is awaited for this under-represented groups.
Dose reductions from 125 to 100 or 75 mg/day were observed in 30.8 and 32.7% of patients, respectively, which was in line with previous findings in clinical trials among Japanese patients (from 125 to 100 mg/day, 34.4 and 44% in the subgroup analyses of the PALOMA-2 [Citation13] and PALOMA-3 [Citation14] studies; from 125 to 75 mg/day, 28.1 and 15%, respectively). In approximately 10% of our patients, doses were reduced from 125 to 100 mg/day within the first 4 weeks of palbociclib initiation, and in over a half of patients, doses were reduced to 100 mg/day within 8 weeks. Conversely, the median time to the first dose reduction was 70 days in a pooled analysis of the PALOMA study [Citation29]. These results suggest that dose reductions occurred relatively early in Japanese clinical practice. Additionally, the palbociclib doses were reduced directly from 125 to 75 mg/day in 18 patients in our study, which is a two-level dose reduction outside the description of prescribing information. This finding raises concerns because palbociclib can only be reduced to a minimum of 75 mg/day [Citation6]. The early dose reductions or two-level dose reductions may lead to premature termination of palbociclib treatment and further limit treatment opportunities.
A frequency examination of blood tests indicated that the tests were performed regularly; although not described in the results, 6.8% of patients were prescribed granulocyte colony stimulating factor during palbociclib treatments, which was also in line with the PALOMA-3 (11%) [Citation30] and PALOMA-2 (10.7%) [Citation31] studies. Although, granulocyte colony stimulating factor prescription records cannot be matched with disease conditions (i.e., neutropenia) in the database, these results indicate that CBC is appropriately monitored in a Japanese clinical setting. However, some patients had no blood test records, and it is advisable to evaluate absolute neutrophil counts at least at the beginning of each cycle before starting palbociclib treatment.
This study has several limitations. Findings of this study may not be generalizable to all patients in Japan because the database contained information from participating facilities. As we used a hospital-based medical claims database, it is possible that patients medical history before their registration in the database and after changing hospitals/clinics were not captured in the database. For instance, blood tests performed outside the institutions where palbociclib was prescribed could not be evaluated in this study. Our analysis included only 68 patients (6.3%) with a ≥12-month gap period after adjuvant endocrine therapy. Thus, some patients medical history may be incomplete, and loss to follow-up is inevitable. Misclassification of treatment lines may exist in some cases, especially in patients who switched treatment during adjuvant therapy. This is because, treatment objectives are not recorded in the database, and it is therefore difficult to clearly distinguish patients who switched treatment as adjuvant therapy, from those who switched to first-line treatment due to recurrence during adjuvant therapy. As annotated in ; however, we have taken measures to minimize misclassification. Finally, as the database does not contain information on HR+/HER2- diagnosis, we identified patients based on prescription records of endocrine therapy and therapies targeting HER2, with the advanced stage identified based on treatments provided. Considering these limitations, we suggest that our findings need to be interpreted carefully.
Conclusion
This study revealed that palbociclib was prescribed at various treatment lines, with the first-line palbociclib treatment increasing over time. Fulvestrant was most commonly combined with palbociclib regardless of treatment lines. Attention should be paid to early or two-level dose reductions, because such reductions may lead to early discontinuation of palbociclib and restrict treatment opportunities. CBC was regularly monitored in Japanese clinical practice; however, our findings recommend that clinicians should exercise cautions to monitor CBC before starting palbociclib therapy and at the beginning of each cycle as part of safety risk management.
Palbociclib was approved and launched in 2017 in Japan for the treatment of inoperable or recurrent breast cancer in combination with endocrine therapy; however, data on real-world palbociclib treatment patterns and complete blood count (CBC) monitoring are still limited. Here, for the first time, we report the real-world treatment patterns of palbociclib and CBC monitoring using a hospital-based medical claims database in Japan.
Deidentified data of patients with advanced breast cancer who received palbociclib from 2017 to 2020 were extracted from a claims database managed by Medical Data Vision.
We identified 1074 patients treated with palbociclib. Palbociclib was predominantly prescribed to females (99.3%). The median (min, max) age at palbociclib treatment initiation was 64.0 (32.0, 92.0) years.
Palbociclib was initially prescribed more commonly as second- and later-line treatments; over time, the first-line treatment steadily increased from 22.7% in 2017/2018 to 42.6% in 2020. Concomitantly, the fourth- or later-line treatments decreased from 29.3 to 10.2%.
Fulvestrant was most commonly prescribed in combination with palbociclib regardless of treatment lines (57.4, 62.8 and 66.0% in the first-, second- and third-line, respectively).
Most patients (82.0%) initiated palbociclib at 125 mg/day; however, doses were reduced from 125 to 100 mg/day for over a half of patients within 8 weeks of palbociclib initiation. A two-level dose reduction from 125 to 75 mg/day was observed in 18 patients.
Attention should be paid to early or two-level dose reductions, because such reductions may lead to early discontinuation of palbociclib and restrict treatment opportunities.
CBC was regularly monitored in Japanese clinical practice; however, our findings recommend cautions when conducting blood tests before starting palbociclib and at the beginning of each cycle as part of safety risk management.
Author contributions
The authors certify that each co-author listed on this page participated sufficiently in the work to take responsibility for the content, and that all those who qualify are listed. Individual contributions are listed below. M Sawaki: conception, analysis and interpretation of data, drafting and revising the manuscript. Y Muramatsu: conception of the work, analysis and interpretation of data, drafting and revising the manuscript. K Togo: data acquisition, analysis and interpretation of data, drafting and revising the manuscript. T Laurent: analysis of data, revising the manuscript. H Iwata: conception of the work, revising the manuscript. In addition to the above, all authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial & competing interests disclosure
This work was supported by Pfizer Japan (Tokyo, Japan). The sponsor was involved in the study design, analysis, interpretation of data, the writing of the report, decision to publish, and preparation of the manuscript. Y Muramatsu and K Togo are employees of Pfizer Japan (Tokyo, Japan) and shareholders of Pfizer Inc. T Laurent is an employee of Clinical Study Support, Inc. (Nagoya, Japan) and received funding to conduct statistical analysis for this study. H Iwata has received honoraria and research funding from AstraZeneca and Pfizer and fees for promotional materials from AstraZeneca. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Statistical analysis support was provided by T Hirano at Clinical Study Support, and medical writing and editorial support were provided by R Hagihara and M Kawaguchi at Clinical Study Support, and both were funded by Pfizer Japan.
Ethical conduct of research
The database contains anonymously processed information under the amended Act on the Protection of Personal Information 2003. According to the Japanese Ethical Guidelines for Medical and Health Research involving human subjects, institutional review board approval and patient informed consent were not required for this observational study as it used secondary data without identifiable patient information.
Data sharing statement
The data that support the findings of this study are available for purchase from Medical Data Vision Co., Ltd. (MDV) and restrictions apply to the availability of these data due to contractual agreements between MDV and hospitals. For inquiries about access to the dataset used in this study, please contact MDV (website: (JP) https://www.mdv.co.jp/ (ENG) https://en.mdv.co.jp/; e-mail: [email protected]).
Supplementary materials
Download MS Word (21.6 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2021-1448
Additional information
Funding
References
- Cancer Statistics. Cancer Information Service, National Cancer Center, Japan. National Cancer Registry, Ministry of Health, Labour and Welfare (2021). https://ganjoho.jp/reg_stat/statistics/dl/index.html
- Harvey JM , ClarkGM, OsborneCK, AllredDC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol.17(5), 1474–1481 (1999).
- BQ6 . Should endocrine therapy or chemotherapy be the primary treatment for ER-positive HER2-negative metastatic/recurrent breast cancer? (2021). http://jbcs.gr.jp/guidline/2018/index/yakubutu/y2-bq-6/
- El Sayed R , ElJamal L, ElIskandarani S, KortJ, AbdelSalam M, AssiH. Endocrine and targeted therapy for hormone-receptor-positive, HER2-negative advanced breast cancer: insights to sequencing treatment and overcoming resistance based on clinical trials. Front. Oncol.9, 510 (2019).
- IBRANCE® (palbociclib) tablets, for oral use 2019. Prescribing information. Pfizer, NY, USA. (2022). www.accessdata.fda.gov/drugsatfda_docs/label/2019/212436lbl.pdf
- IBRANCE® (palbociclib) tablets, for oral use 2020 [interview form]. Pfizer, Shibuya, Japan [in Japanese] (2018). www.info.pmda.go.jp/go/interview/2/672212_4291051F1022_2_1F.pdf
- Finn RS , MartinM, RugoHSet al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med.375(20), 1925–1936 (2016).
- Rugo HS , FinnRS, DiérasVet al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat.174(3), 719–729 (2019).
- Turner NC , RoJ, AndréFet al. Palbociclib in hormone-receptor-positive advanced breast cancer. N. Engl. J. Med.373(3), 209–219 (2015).
- Cristofanilli M , TurnerNC, BondarenkoIet al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase III randomised controlled trial. Lancet Oncol.17(4), 425–439 (2016).
- Turner NC , SlamonDJ, RoJet al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med.379(20), 1926–1936 (2018).
- IBRANCE® Summary of product characteristics. Pfizer, Kent, UK (2018). www.medicines.org.uk/emc/product/4449/smpc
- Mukai H , ShimizuC, MasudaNet al. Palbociclib in combination with letrozole in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-2 subgroup analysis of Japanese patients. Int. J. Clin. Oncol.24(3), 274–287 (2019).
- Masuda N , InoueK, NakamuraRet al. Palbociclib in combination with fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-3 subgroup analysis of Japanese patients. Int. J. Clin. Onco.l24(3), 262–273 (2019).
- Pharmaceutical and Medical Devices Agency. New drugs approved in FY 2017 (2017). www.pmda.go.jp/files/000232769.pdf
- IBRANCE® (palbociclib) capsules, for oral use 2020 [package insert]: Highlights of prescribing information. Pfizer, Shibuya, Japan [in Japanese].https://pins.japic.or.jp/pdf/newPINS/00067167.pdf
- Loibl S , TurnerNC, RoJet al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist22(9), 1028–1038 (2017).
- Medical Data Vision Co., Ltd. Press release: updated database information 2019. [in Japanese]. www.mdv.co.jp/press/2019/detail_1201.html
- Japanese Society for Pharmacoepidemiology . Survey of Japanese databases in Japan available for clinical/pharmaco-epidemiology 2019. https://drive.google.com/file/d/1wlX96kYd-rPT_OgBRjjt-Yq1XHeL0qLN/view
- e-GOV Act on the Protection of Personal Information 2019. [in Japanese]. https://elaws.e-gov.go.jp/search/elawsSearch/elaws_search/lsg0500/detail?lawId=415AC0000000057#240
- Kish JK , WardMA, GarofaloDet al. Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res.20(1), 37 (2018).
- Taylor-Stokes G , MitraD, WallerJ, GibsonK, MilliganG, IyerS. Treatment patterns and clinical outcomes among patients receiving palbociclib in combination with an aromatase inhibitor or fulvestrant for HR +/HER2-negative advanced/metastatic breast cancer in real-world settings in the US: results from the IRIS study. Breast43, 22–27 (2019).
- Llombart-Cussac A , Pérez-GarcíaJM, BelletMet al. PARSIFAL: a randomized, multicenter, open-label, phase II trial to evaluate palbociclib in combination with fulvestrant or letrozole in endocrine-sensitive patients with estrogen receptor (ER)[+]/HER2[-] metastatic breast cancer. J. Clin. Oncol.38(Suppl. 15), 1007 (2020).
- Varella L , EziokwuAS, JiaXet al. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res. Treat.176(2), 429–434 (2019).
- Demichele A , CristofanilliM, BrufskyAet al. Overall survival for first-line palbociclib plus letrozole vs letrozole alone for HR +/HER2- metastatic breast cancer patients in US real-world clinical practice. Cancer Res.80, P1-19-02 (2020).
- Xi J , OzaA, ThomasSet al. Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J. Natl Compr. Canc. Netw.17(2), 141–147 (2019).
- Caillet P , PulidoM, BrainEet al. PALOMAGE, a French real-world cohort of elderly women beyond age 70 with advanced breast cancer receiving palbociclib: baseline characteristics and safety evaluation. J. Clin. Oncol.39(Suppl. 15), 1012–1012 (2021).
- Rugo HS , TurnerNC, FinnRSet al. Palbociclib plus endocrine therapy in older women with HR +/HER2- advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur. J. Cancer101, 123–133 (2018).
- Ettl J , ImSA, RoJet al. Hematologic adverse events following palbociclib dose reduction in patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: pooled analysis from randomized phase II and III studies. Breast Cancer Res.22(1), 27 (2020).
- Verma S , BartlettCH, SchnellPet al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist21(10), 1165–1175 (2016).
- Diéras V , HarbeckN, JoyAAet al. Palbociclib with letrozole in postmenopausal women with ER +/HER2- advanced breast cancer: hematologic safety analysis of the randomized PALOMA-2 trial. Oncologist24(12), 1514–1525 (2019).