Abstract
Background: Chimeric antigen receptor T-cell (CAR-T) therapy represents a new frontier in multiple myeloma. It is important to understand critical success factors (CSFs) that may optimize its use in this therapeutic area. Methods: We estimated the CAR-T process using time-driven activity-based costing. Information was obtained through interviews at four US oncology centers and with payer representatives, and through publicly available data. Results: The CAR-T process comprises 13 steps which take 177 days; it was estimated to include 46 professionals and ten care settings. CSFs included proactive collaboration, streamlined reimbursement and CAR-T administration in alternative settings when possible. Implementing CSFs may reduce episode time and costs by 14.4 and 13.2%, respectively. Conclusion: Our research provides a blueprint for improving efficiencies in CAR-T therapy, thereby increasing its sustainability for multiple myeloma.
Plain language summary
Patients with multiple myeloma can now be treated with chimeric antigen receptor T-cell (CAR-T) therapy. We studied how CAR-T therapy is used for multiple myeloma. We also studied things that could help make this therapy easier for doctors to use. The CAR-T process takes 13 steps and 177 days. It begins with the choice to use the therapy and ends about 100 days after it is used. The process uses 46 different healthcare professionals and ten different locations. We found several possible changes that can improve this process. Of these changes, three stand out. First, improved teamwork between members of the care team can help them prepare for and resolve possible problems. Second, reducing insurance red tape will make it easier to provide CAR-T therapy to patients. Third, allowing use of CAR-T therapy in places other than hospitals can help more patients receive this therapy. If applied, these three things may lower the time needed to treat patients by 14.4% and may reduce costs by 13.2%.
Approximately 34,920 Americans will be newly diagnosed with multiple myeloma (MM) during 2021, and 12,410 will die from the disease [Citation1]. Since 1974, therapeutic options have expanded from steroids, chemotherapy and radiation to include immunomodulatory drugs, proteasome inhibitors, antibodies and BCMA-targeted therapies [Citation2]. Although MM is not curable [Citation3], the introduction of these novel agents has led to an increase in age-adjusted 5-year relative survival rates, from 25% in 1975 to 55.9% in 2019; the advent of hematopoietic stem-cell transplantation further increased median survival by 6 years [Citation4]. Despite these treatment advances, many patients require multiple lines of therapy, with 22–43% of patients with MM receiving three or more lines of therapy [Citation5–7].
Chimeric antigen receptor T-cell (CAR-T) therapy, an innovative immunological blood cancer treatment that has been referenced as the ‘fifth pillar’ of oncology treatment [Citation8], genetically modifies a patient’s T-cells to express chimeric antigen receptors on their surface and then infuses the modified cells back into the patient to target and destroy tumor cells [Citation9]. CAR-T products are approved to treat various B-cell lymphomas [Citation10]. Recently, the CAR-T product idecabtagene vicleucel (ide-cel) was approved by the US FDA for the treatment of relapsed or refractory MM in adult patients who have not responded to at least four prior treatment regimens (including an immunomodulatory agent, a proteasome inhibitor and an anti-CD38 monoclonal antibody) [Citation11]; ciltacabtagene autoleucel (cilta-cel) is another CAR-T asset that has been granted breakthrough designation and priority review by the FDA, with a decision on approval expected in early 2022 [Citation12–14]. Clinical studies have demonstrated the efficacy and safety of ide-cel and cilta-cel among heavily pretreated patients with advanced MM [Citation15–17].
While it is a powerful and innovative addition to the therapeutic options for MM, CAR-T treatment is a complex and time-intensive process that requires the relatively few qualified oncology centers in the USA to integrate logistical support and close communication across several healthcare providers and care settings (e.g., inpatient and outpatient) [Citation18]. To further complicate matters, current US reimbursement frameworks are not optimized for CAR-T therapy and have substantially different models for inpatient- versus outpatient-based care (available CAR-T products are often administered in the inpatient setting due to known sequelae such as cytokine release syndrome [CRS] and neurotoxicity). In one instance, the US Centers for Medicare & Medicaid Services issued a decision that resulted in a substantial difference in reimbursement for CAR-T therapy (USD$186,500 for inpatient-based care vs USD$400,000–500,000 for outpatient-based care) [Citation19]. Moreover, in the USA patients insured by private (i.e., commercial) insurance tend to switch insurance programs at high rates due to changes in employment/eligibility, which has resulted in relatively short-term (i.e., 12-month) reimbursement schemes that do not align well with the anticipated long-term benefits of CAR-T therapy [Citation20,Citation21].
Efficacy data from over 20 clinical trials reported to date are sufficiently promising to result in speculation that CAR-T therapy may ultimately be considered a potentially curative treatment for at least some patients [Citation22,Citation23]. However, without increased capacity, critical delays may occur between making the decision to use CAR-T therapy and administering it. Relatedly, barring the introduction of new reimbursement practices that appropriately acknowledge its long-term benefits, improvements to existing processes will likely further improve the value of CAR-T therapy, which in turn would increase the ability of physicians to use these therapies where clinically appropriate [Citation24,Citation25]. Given the recent expansion of CAR-T products to MM (with others anticipated shortly), it is time for relevant stakeholders – healthcare payers and providers and CAR-T manufacturers – to determine how best to evolve their processes to optimize the impact and patient benefit from these innovative therapies. Such evaluations are challenging, as individual stakeholders typically do not examine their care processes with the necessary detail and methodology required to conduct this analysis. Moreover, such analyses are often conducted from the perspective of a single stakeholder, curtailing the ability to draw broader conclusions and offer comprehensive yet workable solutions.
We describe herein such a comprehensive assessment that includes the use of time-driven activity-based costing (TDABC), a proven health economics methodology that provides a detailed accounting of the resources, time and costs required to render care across a discrete process (in this instance, CAR-T therapy for MM) for the purpose of identifying inefficiencies [Citation26–30]. Our assessment also includes information from interviews with healthcare professionals and financial administrators from four US oncology centers experienced in the use of CAR-T therapies, as well as several US payers. The goal of our study was to fully describe resources required for the current CAR-T process for MM and to identify critical success factors (CSFs) which, if implemented, could maximize efficiencies and facilitate the use of CAR-T therapies for the treatment of MM.
Materials & methods
Overview
Both components of our study – the development and estimation of the CAR-T process and the identification of CSFs – were informed through qualitative interviews with practicing healthcare providers and administrators with relevant CAR-T experience from four US oncology centers as well as representatives from selected US payers. TDABC was used to estimate two process maps: one thought to be representative of the expected standard-of-care for CAR-T for the treatment of MM, and one that identifies potential time or cost savings through application of the CSFs.
Site recruitment
Eligible US oncology centers had substantial experience with CAR-T therapy in clinical trials and practice (antigen targets including CD19, CD19/CD22 and BCMA) and anticipated the use of relevant CAR-T products for the treatment of MM (no MM-specific product was approved by the FDA at the time of study initiation). Participating centers made available personnel with experience in the clinical delivery and financial management/impact (e.g., reimbursement, facility profitability) of CAR-T therapies. Other criteria used to assess centers for participation included: years of experience in oncology and bone marrow transplant; number of patients previously treated with CAR-T therapies; experience in adapting standard operating procedures/treatment flows to accommodate the different demands of CAR-T therapies (at their advent, as well as due to the COVID-19 pandemic); and experience using CAR-T therapies in outpatient settings.
Payer recruitment
Payers were recruited from a pool of experienced senior-level medical directors, each with at least 10 years working for US commercial and/or government insurance plans in administrative capacities. Payers were pre-screened for their knowledge and familiarity with plans’ policy development and anticipated roles in future market access decision-making, and each director confirmed their involvement in coverage policy development and benefit administration for approved CAR-T therapies prior to study participation.
Process map
TDABC is a health economics methodology that guides enumeration of the time and resources required to provide patient care throughout a specified care process. Its purpose is to identify potential process inefficiencies and to estimate how process changes might address any inefficiencies identified. This method has been used in oncology [Citation31–37], orthopedics [Citation38–40], emergency department procedures [Citation41], other surgery types [Citation42–44] and other care processes [Citation45–49]. Unlike more traditional approaches to estimating the costs of care (e.g., relative value units, cost-to-charge ratios), TDABC is a bottom-up approach which is limited to enumerating resources, time and costs associated with the direct care related to the process under evaluation [Citation27,Citation30].
TDABC methods require the development of a process map which delineates the discrete steps that collectively comprise the care process. The CAR-T process was assumed to begin on the date of the office visit during which a patient with relapsed/refractory MM was identified as a potential candidate for CAR-T, and to end 100 days after CAR-T infusion. For each step, we elicited from site participants a complete listing of the resources – personnel, building space(s), equipment – required to render care. As our objective was to identify opportunities for process improvement, we focused on areas that were potentially amenable to process change that did not alter the quality and safety of patient care. For example, selecting relevant personnel to manage patient intake (e.g., medical technician vs nurse), choosing the setting in which to place a catheter (e.g., operating theater vs catheterization laboratory) and other aspects of patient care (e.g., infrared thermometer vs electronic thermometer with disposable sleeves) are all areas where changing a given resource to improve process and/or decrease cost was not expected to materially impact the quality and safety of patient care, and therefore all were included in the process map. Conversely, medications were excluded from the process map because they were assumed to be invariant and informed by patient need, physician experience and available evidence, and to be required regardless of potential improvement in care process/cost considerations. Accordingly, the study targeted personnel, equipment and the physical plant.
In a series of five 90-min interviews, site participants were asked to identify relevant steps in the process, along with corresponding resources and time estimates. Once enumerated, each step was summarized and reviewed with the group to ensure accuracy and attain consensus (i.e., when all agreed on all resources and timings required within each step of the overall CAR-T process). While consensus was sought for each resource within each step, we solicited ranges for the corresponding time estimates. The reason for this was that while broad consensus was easily and rapidly reached on enumeration of steps, individual centers’ experiences (including geographic region and overarching patient volume) resulted in heterogeneity around corresponding resource and time estimates. The time ranges allowed the map to represent real-world experience around the CAR-T process. Unit costs for each resource were obtained from publicly available sources that were deemed representative of the US healthcare system. For disposable/single-use items (e.g., syringes, phlebotomy tubes), relevant acquisition costs were assigned based on identified retail prices obtained through a review of relevant internet searches. For personnel, the national median estimates of total annual compensation (i.e., salary plus benefits such as retirement and health insurance) specific to each job description were used [Citation50]. For all other resources (e.g., computed tomography machine, examination room), acquisition costs were estimated using relevant internet searches, supplemented by peer-reviewed literature (see Supplementary Material). All costs were estimated from the perspective of US oncology centers and were adjusted to 2020 US dollars ($).
Once time and cost estimates were finalized, capacity cost rates were calculated using TDABC methodology [Citation27,Citation47]. These rates represented the cost per minute associated with each relevant resource and were derived based on time available to spend on patient care over 1 year (e.g., for personnel, this would be estimated as 365 days less weekend, holiday, sick and vacation days, with the resulting value further adjusted to account for lunch, breaks and meetings; for equipment, this included depreciation and maintenance time). A full list of resources, along with corresponding time and cost estimates and the ensuing capacity cost rates, can be found in the Supplementary Material. The cost of each step within the CAR-T process was estimated by multiplying the time required for each relevant resource by its corresponding capacity cost rate and then summing the resulting products; episode cost was estimated by summing the total costs of each step throughout the CAR-T process. Estimates of time for each step also were tallied. Because some steps require a few hours and others are spread out over several days, we calculated the time required to render the relevant care and the number of days over which the care was rendered.
Identification of CSFs
Semi-structured 1-h interviews were conducted with each payer and with representatives of each participating oncology center tasked with managing financial aspects of CAR-T therapy (including physicians who also were tasked with CAR-T administration and related patient care, where applicable). During each interview, participants were asked to share their perspectives on several topics, including the value of CAR-T therapies, lessons learned and the future of CAR-T therapy. Participating payers were asked about key factors considered in coverage policy development and decisions, and deviations from standard coverage policy development for CAR-T therapy. Participants from the oncology sites were asked about organizational drivers to create a CAR-T program, obstacles that were overcome in creating their program, and the roles of various individuals involved with program implementation. Responses from these interviews were then reviewed and consolidated into themes to identify existing challenges, lessons learned and factors required to ensure successful delivery of CAR-T.
Relevant CAR-T CSFs were reviewed with all participants; each step of the process map was reviewed to identify potential inefficiencies and process bottlenecks. Once an issue was identified, experts discussed potential solutions, including intended and unintended effects of changes to the process. Upon reaching consensus that a modification to the existing process may improve efficiencies or reduce costs without negatively impacting patient care, the process map was modified to reflect its implementation. This process was then repeated until all relevant recommendations upon which consensus was reached were incorporated, thereby creating an optimized CAR-T therapy process map.
Results
Site recruitment
Of 17 US sites identified, four met all selection criteria and agreed to participate. One site was an academic healthcare center (University of Pennsylvania, PA); two were oncology centers with academic associations (Intermountain Health, Salt Lake City, UT and Karmanos Cancer Center, Detroit, MI); and one was a community-based oncology center (Hematology Oncology, Inc., Cincinnati, OH). The sites collectively contributed 12 research participants, including five hematologist-oncologists (four of whom were integral in the financial administration of their CAR-T therapy programs), three cell therapy co-ordinators (all registered nurses or advanced practice nurses), one administrative director of cell therapy, one oncology nurse practitioner and two financial administrators for their respective cell therapy programs.
All site participants had extensive experience in oncology, bone marrow transplant and CAR-T therapy (). For CAR-T therapy experience, site hematologist-oncologists cumulatively treated 15–100 CAR-T-eligible patients annually; practitioners collectively had 3–33 years in clinical practice, 3–31 years of experience in oncology, 3–31 years in clinical trials and 3–11 years of experience in CAR-T care for CD19 or BCMA. Cell therapy co-ordinators averaged 13.3 years of oncology experience and 4–8 years of experience in CAR-T therapy. Each site administrator had 13–20 years of administrative oncology experience and had been involved in their respective CAR-T programs since their inceptions.
Table 1. Mean years of experience by interviewee position and type of experience.
Payer recruitment
Three US payer representatives agreed to participate, two of whom were employed by health plans ranked among the top 50 largest US private insurers in terms of number of covered lives (one representing a national commercial payer; the other, a regional integrated delivery network). The third payer participant was a Medicare Administrative Contractor medical director with responsibility for coverage and reimbursement policy management. The payer representative participants collectively had more than 12 years of experience in coverage and reimbursement policy management for CAR-T therapies.
Current CAR-T process map
Through discussions with research participants from the four oncology centers, the current CAR-T process was found to comprise 13 steps over a 177-day period. These steps broadly fall into one of four components: screening (steps 1–4; 30.5–52.4 h), CAR T-cell preparation and administration (steps 5–9; 71.5–192.8 h), acute management (step 10; 156.7–424.8 h) and long-term management (steps 11–13; 4.0–13.1 h; ). Care estimated throughout the CAR-T process required 46 unique healthcare professionals across ten unique care settings, accounting for approximately 472.9 h of direct patient care.
CAR: Chimeric antigen receptor; CRS: Cytokine release syndrome; h: Hour; HGG: Hypogammaglobulinemia; MAS: Macrophage activation syndrome.
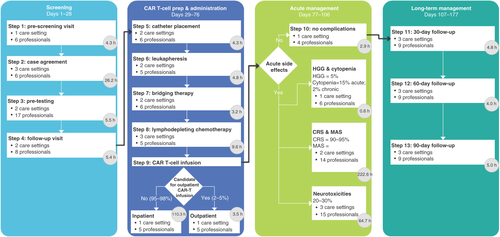
The time for each component varied according to the steps involved, with some steps occurring over several days. Steps are reported here as the number of direct contact hours estimated. The four steps in the screening component included an initial assessment of the patient’s candidacy for CAR-T therapy, including bloodwork (step 1; 2.7–6.0 h), obtaining necessary approvals from insurance payers to ensure reimbursement for the therapy (step 2; 22.5–30.0 h), additional testing such as bone marrow biopsy, CT scan, pulmonary function testing or cardiogram (step 3; 3.1–7.9 h) and a review of findings from steps 1–3 and confirmation of the suggested therapeutic approach with the patient (step 4; 2.3–8.6 h). The next component involved catheter placement for leukapheresis (step 5; 2.2–6.4 h), leukapheresis (step 6; 3.9–5.6 h), administration of bridging chemotherapy (step 7; 1.8–4.6 h), lymphodepleting chemotherapy (step 8; 6.5–12.8 h) and administration of the CAR-T therapy (step 9; 57.1–163.4 h; all patients were assumed to receive CAR-T in hospital). The acute management component (step 10) covered the 30-day period following CAR-T administration, representing the period when relevant sequelae were expected to occur. Because sequelae do not occur in a mutually exclusive manner, this single step was designed to capture all relevant sequelae, including the potential for an individual patient to experience multiple events (e.g., CRS and neurotoxicity). Treatment processes for each sequela were captured, with times and costs weighted according to the estimated proportion of the CAR-T-treated population expected to experience a given event (e.g., researchers expected some degree of CRS in 90–95% of treated patients) [Citation11,Citation13]. The final component comprised outpatient follow-up visits at 30 (step 11; 1.1–4.1 h of direct patient care), 60 (step 12; 1.1–4.0 h) and 90 days (step 13; 1.8–5.0 h) following CAR-T administration (i.e., approximately one visit per month), during which post-treatment staging occurs (e.g., one visit included a bone marrow biopsy).
Acute management comprised the largest proportion of direct patient care – 61.5% of total hours – due to the large proportions of patients assumed to experience acute side effects that required hospitalization (e.g., CRS, neurotoxicities). CAR-T preparation and administration comprised 28.0% of total hours of direct patient care; this was largely attributable to the expectation that CAR-T therapy would be infused exclusively in inpatient settings, necessitating a hospital admission. Screening and long-term management comprised 8.8 and 1.8% of hours in direct patient care, respectively; for the former, more than one-half of the time was for obtaining payer agreement for reimbursement.
Exclusive of medications and the CAR T-cell product, mean TDABC-estimated total costs associated with the episode were USD$45,126 (range: USD$24,863–65,389) and included USD$37,045 (range: USD$17,779–56,310) in personnel (82.1% of total estimated costs), USD$6890 (range: USD$6487–7293) in equipment (15.3%) and USD$1191 (range: USD$597–1785) for the physical plant (2.6%; ). The reader is reminded that TDABC is intended to identify opportunities for process improvement and should not be considered reflective/inclusive of total real-world costs incurred by sites in providing CAR-T therapy.
Table 2. Current presumed standard-of-care process map summaryTable Footnote†.
Costs associated with acute management were USD$19,080 (range: USD$7342–30,819), representing 42.3% of the total episode cost, and were largely due to costs required to provide care for CRS and neurotoxicities. Costs of the screening component were USD$12,477 (range: USD$9022–15,932), or 27.6% of the total costs of care, and were focused on case agreement and patient follow-up to ready them for CAR-T administration. Costs of CAR-T preparation and administration were USD$12,339 (range: USD$7570–17,108), representing 27.3% of total costs, and were mostly attributable to the assumption that administration of CAR-T therapy would require a hospital admission.
Substantial variation in personnel costs was identified in step 2 (case agreement; range: USD$3716–6230) and step 9 (CAR T-cell infusion; range: USD$2722–10,845). For step 2, variation was attributed to the payer and facility experience with CAR-T therapy and the need for individual contracts that led to increased time from the contracting/legal specialists and the chief financial officer (CFO) at the site. For step 9, variation was driven by the time patients were assumed to spend in the hospital during and immediately following CAR-T infusion, with the expected length of stay ranging from 2 to 5 days for observation. Similarly, costs associated with the acute management of adverse events such as CRS and neurotoxicities varied widely – from USD$5592 to USD$24,986 and from USD$1566 to USD$5184, respectively. These ranges were driven by acuity of the event(s) experienced.
CSF interviews
The administrative interviews with three payer representatives and seven financial representatives from participating sites identified six CSFs for CAR T-cell therapy programs in the treatment of MM (), each of which is detailed below.
Table 3. Summary of critical success factors and perspectives, by stakeholder type.
Therapeutic efficacy of CAR-T therapy
Facility stakeholders agreed that CAR-T therapy is clinically effective. Payer representatives also agreed that CAR-T therapy was effective but indicated that its effectiveness in real-world settings versus its efficacy in clinical trials was uncertain. To the degree that this uncertainty remains over time, payer representatives thought it may influence successful implementation of broader CAR-T programs.
Importance of prior experience with CAR-T therapies
Facility stakeholders articulated the importance of experience in the administration of innovative therapies such as CAR-T. Consensus feedback indicated that the sites’ experiences with CAR-T therapy informed the evolution of care through process improvements (e.g., enhanced care team co-ordination, proactive engagement with manufacturers and payers) and was a key driver of both realized patient outcomes and perceived efficiencies. These stakeholders also noted that their staffs’ added expertise in delivering stem-cell therapies and other advanced interventions favorably contributed to current processes.
Setting of care in which CAR-T therapy is administered
Current inpatient reimbursement frameworks incentivize administration and subsequent acute management of CAR-T therapy in outpatient settings. However, facility stakeholders identified the important need for flexibility in determining the optimal setting to administer CAR-T therapy, expressing the importance of having this decision be informed by specific patient characteristics and clinician expertise. The need for equitable reimbursement frameworks that allow for selection of the best care setting (inpatient or outpatient) for the patient was identified as a mechanism that may lead to improved clinical outcomes and more efficient use of healthcare resources. The ability to infuse CAR-T products in outpatient settings also would have the additional benefit of maintaining hospital capacity (in terms of beds and other necessary resources) to care for other patients, which is of particular interest and concern due to the ongoing COVID-19 pandemic [Citation51–54].
Adequacy of reimbursement for CAR-T therapies
Facility and payer representatives agreed that commercial insurers provided higher levels of reimbursement for CAR-T therapy compared with Medicare. Participants agreed that the decision to use CAR-T therapy was multifactorial and included non-clinical drivers such as perceived competitive pressures, institutional branding for innovation (including patient perceptions of a link between innovation and quality care) or service to small CAR-T-eligible populations. However, facility representatives agreed that over time, innovative reimbursement frameworks may be needed to assure sustainability of CAR-T programs.
Openness to non-fee-for-service CAR-T reimbursement models
Payer representatives indicated that the cost and perceived value of CAR-T therapies increased their receptiveness to mitigation methods, including outcomes-based contracting and capitation. Facility stakeholders noted that previously innovative payment frameworks, historically developed to render more equitable reimbursement to sites and incentivize cost savings, were now incentivizing the administration of CAR-T therapies and related patient management practices toward outpatient care settings. Similar to discussions around the adequacy of reimbursement, stakeholders discussed the need for innovative reimbursement mechanisms that provide adequate coverage for the adoption of innovative therapies.
Manufacturer-provided health economic data
Facility stakeholders and payer representatives agreed that robust health economic data would be not only helpful but essential in informing important treatment and reimbursement decisions. Robust health economic studies around CAR-T therapy were also identified by these stakeholders as important in informing and developing equitable reimbursement frameworks. Stakeholders agreed that manufacturers were best positioned to generate relevant evidence to support and inform the CAR-T market.
CAR-T process map with CSFs incorporated
After reviewing the process map developed to capture expected standard-of-care for an episode of CAR-T therapy for MM and the final set of CSFs, the facility research participants recommended two key changes which, if implemented, could result in increased process efficiencies and corresponding reductions in the costs of rendering care. The first change – administering CAR-T products in outpatient settings where appropriate (step 9) – relied on CSFs related to the importance of prior experience with CAR-T therapies and flexibility in CAR-T therapy setting of care. Based on input from facility researchers, we re-estimated the map assuming that 50–90% of patients with MM would be eligible for CAR-T therapy in outpatient settings. Mean time and cost savings due to revisions in step 9 (CAR-T infusion), due largely to reduced time (and corresponding cost) associated with patient monitoring/waiting for onset of relevant sequelae, were estimated at 63.7 h (a 13.5% reduction relative to the initial process map) and USD$3564 (a 7.9% reduction), respectively.
The second recommended process change was to develop a uniform reimbursement form that would streamline case agreement (step 2) and was based on CSFs related to therapeutic efficacy of CAR-T therapy, adequacy of reimbursement for CAR-T therapies and openness to non-fee-for-service CAR-T reimbursement models. The form would capture relevant information sufficient to address most or all payer inquires and cover most or all patient documentation for reimbursement of care. With the uniform reimbursement form, more time and responsibilities would shift from CFOs and contracting personnel to financial co-ordinators and cell therapy co-ordinators. As familiarity with the form grew, discussions and negotiations required to qualify a patient for CAR-T therapy would become less arduous. Mean time and cost savings due to changes in step 2 (case agreement) were estimated at 4.6 h (a 1.0% reduction relative to the initial process map) and USD$2407 (a 5.3% reduction), respectively.
Implementing both recommended revisions was expected to result in a time saving of approximately 68.2 h in direct patient care (14.4% of the total hours required in the initial process map) and a corresponding reduction of USD$5972 in direct costs of care (13.2% of the total costs). Overall, 93% of expected time savings and 60% of expected cost savings were associated with allowing eligible patients to receive CAR-T therapy in outpatient settings ().
Table 4. Success factors incorporated process map summaryTable Footnote†.
Discussion
CAR-T therapy represents an innovative option for patients with MM. While its clinical benefits have been established, comparatively little has been done to quantify the CAR-T process or to identify opportunities for its optimization. This is particularly challenging for CAR-T therapies, as they do not fit neatly into existing provider workflows or reimbursement frameworks. While consensus was reached on the clinical process of CAR-T therapy (over the approximate 6-month period used herein), several instances of uncertainty or inefficiency that hinder adoption of this technology were also readily identified, as was the acknowledgment that some processes likely vary by center and product. Accordingly, while our research identified several opportunities for process improvement which, if implemented, will likely benefit various stakeholders (i.e., patients, providers, healthcare systems, payers, CAR-T manufacturers), we also identified an ongoing need for first, real-world evidence to reinforce and supplement clinical trial findings and reimbursement practices and second, greater collaboration between key stakeholders.
Exclusive of care associated with treatment of CAR-T therapy sequelae, one of the most time-consuming and costly steps associated with the current CAR-T process was the case agreement step (step 2), which involves obtaining reimbursement approvals and guarantees from payers. Case agreement comprised 63% of total time (and 40% of total costs) involved in screening CAR-T therapy candidates, largely due to the disproportionate time required by senior personnel (e.g., cell therapy co-ordinators, hematologists/oncologists, senior financial administrators and the CFO) to address various criteria and conditions set forth by payers. Interestingly, despite the substantial amount of time associated with patient screening, it was the one step in which no direct patient care was provided, but the number of senior-level individuals required to complete the activities in this step resulted in high institutional costs. It is possible that the time required to implement this step may decrease as individuals become accustomed to a new therapy. However, our panel recommended that this important precursor to therapy could be accomplished through a uniform treatment approval contract that should be developed collaboratively between providers, payers and manufacturers. Implementing this CSF within the process map results in substantial reductions in the time – and therefore cost – required to qualify a patient for CAR-T therapy. It also would result in less involvement (at least as a function of time spent on the process) from senior high-cost individuals, freeing their time to attend to other important matters.
The CAR-T infusion step, which included direct in-hospital surveillance from the point of infusion until the acute management component of the process (largely for monitoring patients for the incidence of unwanted sequelae; step 9), was the other most time-consuming and costly step in the process and was associated with 83% of total time involved in the CAR-T preparation and administration steps and 55% of its total cost. However, our process map indicates that administration in the outpatient setting results in substantial time and cost savings to the oncology center. While the ability to offer outpatient infusion will depend at least in part on the specific product (the safety profile of some CAR-T products may not readily allow for it) and patient profile, and will require the acknowledgment of its potential to increase the burden to the patient’s caregiver/family, it is of keen interest. Outpatient administration of CAR-T therapy has been undertaken in other hematological malignancies [Citation55], and evidence suggests that BCMA-targeted therapies may be more readily tolerable than their counterparts targeting CD19 [Citation56]; moreover, current best practices for outpatient administration have recently been summarized [Citation58]. Early clinical evidence from the CARTITUDE-2 trial of cilta-cel, which allowed for its administration in outpatient settings (pending the discretion of the principal investigator and sponsor endorsement based on a given subject’s clinical factors) may provide further support for outpatient administration [Citation57]. In clinical studies of cilta-cel, the median time to CRS onset was 7 days versus 2 days for ide-cel, which suggests that the former may more readily lend itself to outpatient administration than the latter [Citation59]; confirmation will be required following approval. Moreover, by reserving inpatient administration of CAR-T for patients who are not suited for outpatient administration, a shift to outpatient infusion for all other patients would ensure inpatient capacity would be reserved for other deserving patients in need. As >250 different CAR-T therapies are in varying stages of clinical development [Citation60,Citation61], hospitals will need to identify alternative settings in which to administer these life-extending treatments while reserving capacity to care for patients with other maladies.
Estimates based on the revised process map were conservative, as opportunities for continued process improvement through knowledge sharing within and across oncology centers were not considered. As experience grows with available CAR-T products, and as new products are introduced to market, it is likely that individual centers will incorporate best practice elements across oncology centers. In fact, while there was broad consensus on the steps required to provision CAR-T therapy, there was heterogeneity across participating sites in what was done within each step, who performed tasks within each step and how long it took to complete each step. Accordingly, even within our relatively small sample there were opportunities for relevant professionals to learn from each other and potentially to evolve their processes based on feedback from other centers. Similarly, we did not incorporate other innovative aspects of care into the CAR-T therapy process map. For example, use of telehealth visits (which evidence suggests is an acceptable means to provide oncology care at a reduced cost) is a potential process improvement. One study that also used TDABC methods to estimate cost savings through use of telehealth visits during radiology oncological care suggested a potential saving of nearly USD$600 during the course of treatment [Citation35]. In addition to time and cost savings, patients may opt for telehealth visits for other reasons, including (but not necessarily limited to) a preference for the comfort or privacy of their own home and/or reduced transportation burden [Citation62]. While the researchers agreed there would be opportunities for telehealth visits throughout the CAR-T therapy process, especially for patients who respond well to treatment, the proportion of patients for whom telehealth visits would be suitable and when such visits would be appropriate throughout the process, is unclear.
The ability to use the process map to demonstrate the potential impact of implementing CSFs was limited by the perspective taken – in this case, the oncology center. One key CSF from the centers’ perspective is the modification of current practices to render reimbursement more equitably. Successful implementation of this CSF will require the input and participation of payers, centers and manufacturers. Participant researchers made clear that addressing this long-term concern would improve their ability to provide CAR-T therapies across all relevant indications, not just MM. Another CSF – manufacturer-generated real-world evidence on the effectiveness and safety of CAR-T products – will undoubtedly increase confidence in these therapies for physicians, payers and patients, and we note that such data are already being generated and demonstrate real-world outcomes comparable to those reported in clinical studies [Citation63–71]. In turn, these data should reinforce arguments in support of new reimbursement frameworks, ultimately expanding the use of CAR-T therapies and resulting in better patient outcomes. Alternative payment models are of particular interest in oncology, as current reimbursement models do not adequately recognize or appreciate that treatment benefits may span multiple years, or the impact of therapy on patient outcomes [Citation21,Citation72–76]. However, demonstrating the impact of such efforts was outside the scope of this study.
Like all research, our study has limitations that should be discussed. First is the ability of TDABC, or any costing method, to fully capture comprehensive and detailed time and cost estimates of a complex process such as CAR-T therapy. Other methods that are commonly used to estimate costs of provided care (as opposed to reimbursed amounts, which represent costs from a payer’s perspective) include institution charges and cost-to-charge ratios that attempt to incorporate institutional overhead in addition to all services provided to the patient. However, these methods are limited in their ability to identify process inefficiencies. Conversely, the TDABC methodology was developed to identify instances of inefficiency and opportunities for process improvement. TDABC has been used in several therapeutic areas, including oncology [Citation33,Citation36,Citation77–80]. Because the TDABC method follows the patient, behind-the-scenes processes (e.g., the work of a purchasing agent to obtain sufficient office and clinic supplies, or activities that occur during an inpatient admission related to hospitality, housekeeping and building maintenance) may be underestimated or not included. Due to this limitation in collecting all relevant overhead, the TDABC method results in an underestimation of actual cost to provide care. A review of TDABC studies showed the method to consistently produce cost estimates that were approximately 40–60% of those produced using other methods [Citation38,Citation81–84]. With that in mind, the corresponding mean estimate of the cost of CAR-T therapy to US oncology centers (exclusive of the cost of medications or the CAR-T product) using other methods could be between USD$75,210 (range: USD$41,438–108,982) and USD$112,815 (range: USD$62,157–163,473). We note that these estimates should not be interpreted as the actual cost reported by oncology centers, as those are estimated with different methodologies and for a different purpose. As our focus was on identifying areas of potential improvement and on estimating the benefits of such improvements to the center, the use of TDABC allowed us to enumerate current processes and demonstrated the potential impact of CSFs in terms of cost and time savings without sacrificing quality patient care.
Second, due to the COVID-19 pandemic, we were unable to convene participants in person for development of the CAR-T process map. Semistructured interviews and discussion groups, when held in person, can lead to a more free-flowing and engaged conversation between participants. Additionally, focused time away from daily responsibilities may have added to the content the experts generated. However, for most participants, virtual meetings have become more normative since the beginning of the pandemic. The virtual meetings were beneficial in allowing participants to meet without travel. Additionally, meetings could be held more often – albeit for shorter durations – than would likely have been possible had they been conducted face to face.
Despite these limitations and the differences between institutions in their care delivery processes, we encourage readers to compare their processes against those articulated in the process maps. As part of research participant feedback, one clinician logged their activities against the process map that was generated in collaboration with the other research participants. Despite the process map being an amalgamation and generalization of the CAR-T process between the represented institutions, the participant researcher found that it accurately reflected their daily activities. The effort by the participant researchers to identify and articulate the core activities essential to the CAR-T process should allow varied institutions interested in CAR-T therapy to use the process map as a baseline for quality care in CAR-T therapy.
Conclusion
While their clinical benefits have been established, existing process and reimbursement frameworks may not efficiently accommodate CAR-T therapies and may thus require modification. Identified CSFs for CAR-T therapies for MM included: the need for payers, manufacturers and providers to work collaboratively to streamline eligibility requirements and develop equitable reimbursement; the willingness of providers and facility administrators to foster communication within and across sites to evolve and optimize their processes; and a commitment by manufacturers to generate robust and compelling health economic and outcomes research in support of these products. Collectively, these CSFs are expected to reduce the time and cost required to administer CAR-T therapies while maintaining the quality of care. CAR-T therapy represents an innovative therapeutic option for many patients with MM, and all other relevant stakeholders – manufacturers, providers and administrators – must now evolve to keep pace with this life-changing therapy.
The current chimeric antigen receptor T-cell (CAR-T) process for the treatment of multiple myeloma was estimated to span 177 days and to require 46 healthcare professionals and ten settings of care, which collectively accounted for approximately 472.9 h of direct care.
Time-driven activity-based costing is a useful method to estimate time and cost savings that may be realized through process change(s); however, the methodology is conservative and generally produces estimates that are approximately one-half of estimates from traditional methods (e.g., cost-to-charge ratios).
Payer representatives and providers and financial administrators from oncology centers experienced in CAR-T therapies identified several critical success factors associated with use of these therapies for the treatment of multiple myeloma, including: the need for payers, manufacturers and providers to work collaboratively to streamline eligibility requirements and develop equitable reimbursement frameworks; a willingness of providers and facility administrators to foster communication within and across sites to evolve and optimize their processes; and the need for manufacturers to continue to generate robust and compelling health economic and outcomes research on their CAR-T products.
Applying relevant critical success factors to the current CAR-T process map identified the potential to save 14.4% of hours in direct patient care and 13.2% in costs to the oncology center, relative to the current process.
Author contributions
The following authors made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafted the work or revising it critically for important intellectual content; provided final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: A Berger, T Henderson-Clark, C Crivera, A Deol, H Difilippo, E Faber Jr, J Fowler, A Garrett, D Hoda, B Hunter, C Jackson, A Lorden, L Meaux, D Porter, C Riccobono, R Richards, R Stewart, P Theocharous and E Weber.
Financial & competing interests disclosure
Funding for this research was provided by Janssen Scientific Affairs, LLC. A Berger, A Lorden and R Stewart are salaried employees of Evidera, a consulting company with current and ongoing relationships with pharmaceutical, biotech, and medical device companies. C Riccobono and A Garrett are employees and shareholders of Legend Biotech. C Crivera, J Fowler and C Jackson are employees and shareholders of Janssen. A Deol served in a consulting capacity to Kite/Gilead, Janssen, and Adicet. E Faber served in a consulting or advisory capacity for AbbVie, Adaptive, Amgen, Astra Zeneca, BMS, Cardinal Health, Celgene, GlaxoSmith Kline, Janssen, Juno, Karyopharm, Kite, Sanofi Genzyme and Takeda. B Hunter served in a consulting or advisory capacity for Kite, Speakers’ Bureau, Novartis, BMS, Janssen and ADC Therapeutics. D Porter identified the following as financial and conflict of interest disclosures: employment – Genentech, Roche (spouse employment, ended Sept 2020); stock and other ownership interests – Genentech, Roche (spouse former employment); consulting or advisory role – Novartis, Kite/Gilead, Incyte, Gerson Lehrman Group, Janssen (Johnson and Johnson), Jazz, Adepcet Bio, DeCART. BMS, Bluebird Bio, Kadmon; research funding – Novartis; patents, royalties, other intellectual property – Novartis, Tmunity (patent inventor for use of CAR T cells in CD19+ malignancies); other relationships – National Marrow Donor Program (Board of Directors, Chair Oct 2018–Oct 2020), American Board of Internal Medicine (Former, Hematology board exam writing committee member through Oct 2019), Wiley (Associate editor, Am. J. Hematol.), Elsevier (Deputy editor, Transplant Cell Ther. [ASTCT journal]). P Theocharous is a salaried employee of PPD, a consulting company with current and ongoing relationships with pharmaceutical, biotech and medical device companies. R Richards served in a consulting or advisory capacity to Kite, Novartis, BMS, Iovance, Janssen, Legend, Cardinal, Vineti, Autolus, Association of Community Cancer Center-CAR T in the community setting, ASTCT Admin SIG and ASTCT Liaison to Cell Therapy Committee. E Weber has held preceptorships with Novartis and Janssen and worked as a consultant to Novartis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Table S1. Capacity Cost Rates: Personnel
Download MS Excel (95.2 KB)Acknowledgments
The authors acknowledge E Faulkner and M Miller for their early contributions to this research; H Tighe for her administrative and logistic assistance throughout the conduct of this research; and L Hullinger, S Kaveney and C Riccobono (Medical Science Liaisons, Legend Biotech), without whom this research would not have been possible.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2022-0162
Additional information
Funding
References
- American Cancer Society . Key statistics about multiple myeloma (2021). www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html
- Multiple Myeloma Research Foundation . Standard treatments. https://themmrf.org/multiple-myeloma/treatment-options/standard-treatments/
- Ding L , HuY, HuangH. Novel progresses of chimeric antigen receptor (CAR) T cell therapy in multiple myeloma. Stem Cell Investig8, 1 (2021).
- Cancer Stat Facts . Myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html
- Song X , CongZ, WilsonK. Real-world treatment patterns, comorbidities, and disease-related complications in patients with multiple myeloma in the United States. Curr. Med. Res. Opin.32(1), 95–103 (2016).
- Willenbacher E , WegerR, RochauU, SiebertU, WillenbacherW, AustrianMyeloma Registry. Real-world use of 3rd line therapy for multiple myeloma in Austria: an Austrian Myeloma Registry (AMR) analysis of the therapeutic landscape and clinical outcomes prior to the use of next generation myeloma therapeutics. PLoS ONE11(3), e0147381–e0147381 (2016).
- Cid Ruzafa J , MerinopoulouE, BaggaleyRFet al. Patient population with multiple myeloma and transitions across different lines of therapy in the USA: an epidemiologic model. Pharmacoepidemiol. Drug Saf.25(8), 871–879 (2016).
- Valand HA , HudaF, TuRK. Chimeric antigen receptor T-cell therapy: what the neuroradiologist needs to know. AJNR Am. J. Neuroradiol.40(5), 766–768 (2019).
- Feins S , KongW, WilliamsEF, MiloneMC, FraiettaJA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am. J. Hematol.94(S1), S3–S9 (2019).
- Dana Farber Cancer Institute . Frequently asked questions about CAR T-cell therapy. www.dana-farber.org/cellular-therapies-program/car-t-cell-therapy/faq-about-car-t-cell-therapy/
- ABECMA® . Highlights of prescribing information [package insert]. Bristol Myers Squibb, NY, USA. https://packageinserts.bms.com/pi/pi_abecma.pdf
- NIH National Cancer Institute . FDA approves BCMA-targeted CAR T-cell therapy for multiple myeloma (2014). www.cancer.gov/news-events/cancer-currents-blog/2021/fda-ide-cel-car-t-multiple-myeloma
- Johnson & Johnson . Janssen presents initial results for BCMA CAR-T therapy JNJ-4528 showing early, deep and high responses in the treatment of relapsed or refractory multiple myeloma (2019). www.jnj.com/janssen-presents-initial-results-for-bcma-car-t-therapy-jnj-4528-showing-early-deep-and-high-responses-in-the-treatment-of-relapsed-or-refractory-multiple-myeloma
- Sternberg A . Cilta-Cel earns FDA priority review for relapsed/refractory multiple myeloma (2021). www.cancernetwork.com/view/cilta-cel-earns-fda-priority-review-for-relapsed-refractory-multiple-myeloma
- Roex G , TimmersM, WoutersKet al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J. Hematol. Oncol.13(1), 164 (2020).
- Berdeja JG , MadduriD, UsmaniSZet al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet398(10297), 314–324 (2021).
- Munshi NC , AndersonLDJr, ShahNet al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med.384(8), 705–716 (2021).
- Geethakumari PR , DhakalB, RamasamyDP, KansagraAJ. CAR T-cell therapy: current practice and future solutions to optimize patient access. J. Clin. Pathways7, 54–62 (2021).
- Ladika S . Beware of the CAR-T hitches. Managed Care28(5), 35–37 (2019).
- Barlow JF , YangM, TeagardenJR. Are payers ready, willing, and able to provide access to new durable gene therapies?Value Health22(6), 642–647 (2019).
- Kocher B , RajkumarR. Setting the stage for the next 10 years of health care payment innovation. JAMA326(10), 905–906 (2021).
- Mikkilineni L , KochenderferJN. CAR T cell therapies for patients with multiple myeloma. Nat. Rev. Clin. Oncol.18(2), 71–84 (2021).
- Teoh PJ , ChngWJ. CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer J.11(4), 84 (2021).
- Manz CR , PorterDL, BekelmanJE. Innovation and access at the mercy of payment policy: the future of chimeric antigen receptor therapies. J. Clin. Oncol.38(5), 384–387 (2019).
- Zheng PP , KrosJM, LiJ. Approved CAR T cell therapies: ice bucket challenges on glaring safety risks and long-term impacts. Drug Discov. Today23(6), 1175–1182 (2018).
- Etges A , RuschelKB, PolanczykCA, UrmanRD. Advances in value-based healthcare by the application of time-driven activity-based costing for inpatient management: a systematic review. Value Health23(6), 812–823 (2020).
- Kaplan RS , AndersonSR. Time-driven activity-based costing. Harv. Bus. Rev.82(11), 131–138(2004).
- Keel G , SavageC, RafiqM, MazzocatoP. Time-driven activity-based costing in health care: a systematic review of the literature. Health Policy121(7), 755–763 (2017).
- Mcbain RK , JeromeG, WarshJet al. Rethinking the cost of healthcare in low-resource settings: the value of time-driven activity-based costing. BMJ Glob. Health1(3), e000134 (2016).
- Kaplan RS , AndersonSR. Time-driven activity-based costing (2003). https://papers.ssrn.com/sol3/papers.cfm?abstract_id=485443
- Dziemianowicz M , BurmeisterJ, DominelloM. Examining the financial impact of altered fractionation in breast cancer: an analysis using time-driven activity-based costing. Pract. Radiat. Oncol.11(4), 245–251 (2021).
- French KE , GuzmanAB, RubioAC, FrenzelJC, FeeleyTW. Value based care and bundled payments: anesthesia care costs for outpatient oncology surgery using time-driven activity-based costing. Healthcare (Amsterdam)4(3), 173–180 (2016).
- French KE , RecinosI, GuzmanABet al. Continuous quality improvement measured with time-driven activity-based costing in an outpatient cancer surgery center. J. Oncol. Pract.15(2), e162–e168 (2019).
- Laviana AA , IlgAM, VeruttipongDet al. Utilizing time-driven activity-based costing to understand the short- and long-term costs of treating localized, low-risk prostate cancer. Cancer122(3), 447–455 (2016).
- Parikh NR , ChangEM, KishanAU, KaprealianTB, SteinbergML, RaldowAC. Time-driven activity-based costing analysis of telemedicine services in radiation oncology. Int. J. Radiat. Oncol. Biol. Phys.108(2), 430–434 (2020).
- Pezzi TA , NingMS, ThakerNGet al. Evaluating single-institution resource costs of consolidative radiotherapy for oligometastatic non-small cell lung cancer using time-driven activity-based costing. Clin. Transl. Radiat. Oncol.23, 80–84 (2020).
- Suralik G , RudraS, DuttaSWet al. Time-driven activity-based costing of a novel form of CT-guided high-dose-rate brachytherapy intraoperative radiation therapy compared with conventional breast intraoperative radiation therapy for early stage breast cancer. Brachytherapy19(3), 348–354 (2020).
- Akhavan S , WardL, BozicKJ. Time-driven activity-based costing more accurately reflects costs in arthroplasty surgery. Clin. Orthop. Relat. Res.474(1), 8–15 (2016).
- Blaschke BL , ParikhHR, VangSX, CunninghamBP. Time-driven activity-based costing: a better way to understand the cost of caring for hip fractures. Geriatr. Orthop. Surg. Rehabil.11, 2151459320958202 (2020).
- Husted H , KristensenBB, AndreasenSE, SkovgaardNielsen C, TroelsenA, GromovK. Time-driven activity-based cost of outpatient total hip and knee arthroplasty in different set-ups. Acta Orthop.89(5), 515–521 (2018).
- Heaton HA , NestlerDM, BarryWJet al. A time-driven activity-based costing analysis of emergency department scribes. Mayo Clin. Proc. Innov. Qual. Outcomes3(1), 30–34 (2019).
- Erhun F , MistryB, PlatchekT, MilsteinA, NarayananVG, KaplanRS. Time-driven activity-based costing of multivessel coronary artery bypass grafting across national boundaries to identify improvement opportunities: study protocol. BMJ Open5(8), e008765 (2015).
- Martin JA , MayhewCR, MorrisAJ, BaderAM, TsaiMH, UrmanRD. Using time-driven activity-based costing as a key component of the value platform: a pilot analysis of colonoscopy, aortic valve replacement and carpal tunnel release procedures. J. Clin. Med. Res.10(4), 314–320 (2018).
- Mcclintock TR , ShahMA, ChangSL, HaleblianGE. Time-driven activity-based costing in urologic surgery cycles of care. Value Health22(7), 768–771 (2019).
- Anzai Y , HeilbrunME, HaasDet al. Dissecting costs of CT study: application of TDABC (time-driven activity-based costing) in a tertiary academic center. Acad. Radiol.24(2), 200–208 (2017).
- Keel G , MuhammadR, SavageCet al. Time-driven activity-based costing for patients with multiple chronic conditions: a mixed-method study to cost care in a multidisciplinary and integrated care delivery centre at a university-affiliated tertiary teaching hospital in Stockholm, Sweden. BMJ Open10(6), e032573 (2020).
- Öker F , ÖzyapıcıH. A new costing model in hospital management: time-driven activity-based costing system. Health Care Manag. (Frederick)32(1), 23–36 (2013).
- Ridderstrale M . Comparison between individually and group-based insulin pump initiation by time-driven activity-based costing. J. Diabetes Sci. Technol.11(4), 759–765 (2017).
- Yun BJ , PrabhakarAM, WarshJet al. Time-driven activity-based costing in emergency medicine. Ann. Emerg. Med.67(6), 765–772 (2016).
- Salary.com. (Accessed 13September2021). https://www.salary.com/
- Bella T . Alabama man dies after being turned away from 43 hospitals as COVID packs ICUs, family says. Washington Post (2021). www.washingtonpost.com/health/2021/09/12/alabama-ray-demonia-hospitals-icu/
- Czeisler ME , MarynakK, ClarkeKENet al. Delay or avoidance of medical care because of COVID-19-related concerns – United States, June 2020. MMWR Morb. Mortal. Wkly Rep.69(36), 1250–1257 (2020).
- Evans M . Hospitals swamped with delta cases struggle to care for critical patientsWall Street Journal. (2021). www.wsj.com/articles/hospitals-swamped-with-delta-cases-struggle-to-care-for-critical-patients-11630661403
- Scott D . Americans are dying because no hospital will take them (2021). www.vox.com/coronavirus-covid19/2021/9/14/22650733/us-covid-19-hospitals-full-texas-alabama
- Myers GD , VernerisMR, GoyA, MaziarzRT. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J. Immunother. Cancer9(4), e002056 (2021).
- Amorosi D . First FDA-approved CAR T-cell therapy for multiple myeloma hailed as ‘game changer’ (2021). www.healio.com/news/hematology-oncology/20210412/first-fdaapproved-car-tcell-therapy-for-multiple-myeloma-hailed-as-game-changer
- Agha ME , CohenAD, MadduriDet al. CARTITUDE-2: efficacy and safety of ciltacabtagene autoleucel (cilta-cel), a BCMA-directed CAR T-cell therapy, in patients with progressive multiple myeloma (MM) after one to three prior lines of therapy. J. Clin. Oncol.39(Suppl. 15), 8013 (2021).
- Alexander M , CulosK, RoddyJet al. Chimeric antigen receptor T Cell therapy: a comprehensive review of clinical efficacy, toxicity, and best practices for outpatient administration. Transplant. Cell. Ther.27(7), 558–570 (2021).
- OncLive . Dr Shah on the potential rationale for selecting ide-cel vs cilta-cel in multiple myeloma (2021). www.onclive.com/view/dr-shah-on-the-potential-rationale-for-selecting-ide-cel-vs-cilta-cel-in-multiple-myeloma
- Globe Newswire . Comprehensive Analysis of 250+ Key Companies Developing 250+ Novel CAR-T Therapies: DelveInsight (20 April 2021). www.globenewswire.com/news-release/2021/04/21/2213788/0/en/Comprehensive-Analysis-of-250-Key-Companies-Developing-250-Novel-CAR-T-Therapies-DelveInsight.html
- McKinsey . Driving the next wave of innovation in CAR T-cell therapies (2019). www.mckinsey.com/industries/life-sciences/our-insights/driving-the-next-wave-of-innovation-in-car-t-cell-therapies
- Jiang CY , StrohbehnGW, DedinskyRMet al. Teleoncology for veterans: high patient satisfaction coupled with positive financial and environmental impacts. JCO Oncol. Pract.17(9), e1362–e1374 (2021).
- Casadei B , ArgnaniL, GuadagnuoloSet al. Real world evidence of CAR T-cell therapies for the treatment of relapsed/refractory B-cell non-Hodgkin lymphoma: a monocentric experience. Cancers (Basel)13(19), 4789 (2021).
- Tang K , NastoupilLJ. Real-world experiences of CAR T-cell therapy for large B-cell lymphoma: how similar are they to the prospective studies?JIPO4, 150–159 (2021).
- Nailey C . Real-world experience of lymphoma patients receiving CAR T-cell therapy. Oncology Times41, 20 (2019).
- Lai LS , Blauer-PetersonC, DacostaByfield S, MalinJ. Real-world evidence (RWE) study of CAR-T agents in leukemia and lymphoma patients. J. Clin. Oncol.39, 15 (2021).
- Nastoupil LJ , JainMD, FengLet al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J. Clin. Oncol.38(27), 3119–3128 (2020).
- Jacobson CA , HunterBD, ReddRet al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J. Clin. Oncol.38(27), 3095–3106 (2020).
- Pasquini MC , HuZH, CurranKet al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv.4(21), 5414–5424 (2020).
- Sermer D , BatleviC, PalombaMLet al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv.4(19), 4669–4678 (2020).
- Yassine F , IqbalM, MurthyH, Kharfan-DabajaMA, ChavezJC. Real world experience of approved chimeric antigen receptor T-cell therapies outside of clinical trials. Curr. Res. Transl. Med.68(4), 159–170 (2020).
- Jorgensen J , HannaE, KefalasP. Outcomes-based reimbursement for gene therapies in practice: the experience of recently launched CAR-T cell therapies in major European countries. J. Mark. Access Health Policy8(1), 1715536 (2020).
- Kline RM , BlauS, BuescherNRet al. Secret sauce – how diverse practices succeed in centers for Medicare & Medicaid Services Oncology Care Model. JCO Oncol. Pract.17(12), 734–743 (2021).
- Kudrin A . Reimbursement challenges with cancer immunotherapeutics. Hum. Vaccin. Immunother.8(9), 1326–1334 (2012).
- Navathe AS , BoyleCW, EmanuelEJ. Alternative payment models – victims of their own success?JAMA324(3), 237–238 (2020).
- Navathe AS , LiaoJM, WangEet al. Association of patient outcomes with bundled payments among hospitalized patients attributed to accountable care organizations. JAMA Health Forum2(8), e212131 (2021).
- Alves RJV , EtgesA, NetoGB, PolanczykCA. Activity-based costing and time-driven activity-based costing for assessing the costs of cancer prevention, diagnosis, and treatment: a systematic review of the literature. Value Health Reg. Issues17, 142–147 (2018).
- Beriwal S , ChinoJ. Time-driven activity-based costing in oncology: a step in the right direction. Int. J. Radiat. Oncol. Biol. Phys.100(1), 95–96 (2018).
- Nabelsi V , PlouffeV. Breast cancer treatment pathway improvement using time-driven activity-based costing. Int. J. Health Plann. Manag.34(4), e1736–e1746 (2019).
- Schutzer ME , ArthurDW, AnscherMS. Time-driven activity-based costing: a comparative cost analysis of whole-breast radiotherapy versus balloon-based brachytherapy in the management of early-stage breast cancer. J. Oncol. Pract.12(5), e584–593 (2016).
- Hoozee S , HansenSC. A comparison of activity-based costing and time-driven activity-based costing. J. Manag. Account. Res.30, 143–167 (2018).
- Jayakumar P , TrianaB, BozicKJ. Editorial commentary: the value of time-driven, activity-based costing in health care delivery. Arthroscopy37(5), 1628–1631 (2021).
- Koolmees D , BernsteinDN, MakhniEC. Time-driven activity-based costing provides a lower and more accurate assessment of costs in the field of orthopaedic surgery compared with traditional accounting methods. Arthroscopy37(5), 1620–1627 (2021).
- McCreary DL , DugarteAJ, MarloweHK, VangS, PlowmanBL, CunninghamBP. Time-driven activity-based costing in trauma (Poster 156). J. Orthopaedic Trauma (2017). https://ota.org/sites/files/legacy_abstracts/ota17/OTA%20AM17%20Poster%20156.pdf
