Abstract
Background: The current work was designed to estimate the cost–effectiveness of trifluridine/tipiracil (T/T) versus best supportive care (BSC) for patients with advanced stage or metastatic gastroesophageal cancer (mGC) from a UK perspective. Materials & methods: A partitioned survival analysis was undertaken using data from the phase III TAGS trial. A jointly fitted lognormal model was selected for overall survival and individual generalized gamma models were chosen for progression-free survival and time-to-treatment-discontinuation. The primary outcome was the cost per quality-adjusted life year (QALY) gained. Sensitivity analyses were undertaken to investigate uncertainty. Results: Compared with BSC, T/T was associated with a cost per QALY gained of £37,907. Conclusion: T/T provides a cost-effective treatment option for mGC in the UK setting.
Gastroesophageal cancers are aggressive, rapidly progressing and are typically associated with poor prognosis, particularly as most patients are diagnosed at an advanced stage where curative surgery is not an option. For patients with advanced stage/metastatic gastroesophageal cancer (mGC), the standard of care typically includes the use of sequential lines of systemic anticancer therapies (mostly chemotherapy), however, after these options have been exhausted, there remains an unmet need [Citation1]. Historically, clinical practice in the third-line setting has relied upon best supportive care (BSC), meaning that while an estimated 70–85% of patients who progress after two lines of therapy would not receive further active treatment, 15–30% of patients receive further systemic anticancer therapy [Citation2].
Life expectancy is especially limited for mGC patients that have progressed throughout sequential lines of therapy, with median overall survival (OS) estimated to be approximately four months with a one-year OS of approximately 12% for those that have progressed after two lines of therapy and are managed with BSC [Citation3,Citation4]. The GLOBOCAN 2020 report found that stomach cancer was the fourth leading cause of cancer deaths worldwide [Citation5]. In patients for whom further treatment is suitable, the aims of such treatment typically offered at this advanced stage of disease focus on extending survival, delaying progression and maintaining health-related quality of life (HRQoL), per established guidelines [Citation1].
Trifluridine/tipiracil (T/T; Lonsurf®) is an oral cytotoxic chemotherapy comprised of an antineoplastic thymidine-based nucleoside analogue, trifluridine, and the thymidine phosphorylase inhibitor, tipiracil hydrochloride. Data concerning the safety and efficacy of T/T in patients with mGC are available from the global pivotal phase III TAGS study (NCT02500043) [Citation3], and previous studies have investigated its use in other solid tumors (including metastatic colorectal cancer [mCRC], as part of the RECOURSE study [NCT01607957]) [Citation6].
TAGS is a placebo-controlled, multicenter, international, 2:1 randomized controlled trial (RCT) of patients with metastatic gastric adenocarcinoma (including adenocarcinoma of the gastroesophageal junction; GEJ) who had undergone two or more previous chemotherapy regimens for advanced or metastatic disease. The study was designed to detect a hazard ratio (HR) for death of 0.70 for T/T versus placebo with 90% power at an overall one-sided type 1 error of 0.025 [Citation6]. On the basis of the 2:1 treatment allocation (T/T:placebo) with 500 patients, 384 deaths were targeted for the final OS analysis. Ultimately, 507 patients were enrolled (intention-to-treat population) and were randomized to receive either T/T (n = 337) or placebo (n = 170) [Citation6]. Of these patients, 503 received at least one dose of either T/T or placebo (safety population).
Findings from TAGS demonstrate that T/T provides a statistically significant improvement in OS (HR: 0.69; p < 0.001), so the primary end point was met. In addition, findings from TAGS also demonstrated benefits for T/T in terms of progression-free survival (PFS; HR: 0.57; p < 0.001) and time to Eastern Cooperative Oncology Group Performance Status deterioration (HR: 0.69; p < 0.001) [Citation3]. There was also a trend toward T/T reducing the risk of HRQoL deterioration compared with BSC in the TAGS study [Citation7]. These findings ultimately led to the granting of European and UK marketing authorizations for T/T in mGC [Citation8–10].
Increased pressure on healthcare budgets necessitates evaluations of drugs that rely not only on their safety and efficacy but also their cost–effectiveness (i.e., their value for money). In June 2021, the Scottish Medicines Consortium (SMC) published its guidance recommending T/T as monotherapy for the treatment of adult patients with mGC, including adenocarcinoma of the GEJ, who have been previously treated with two systemic treatment regimens for advanced disease [Citation11]. However, a limited description of the key features of the cost-effectiveness analysis developed as part of the SMC’s health technology assessment (HTA) is publicly available. The current analysis was designed to estimate the cost-effectiveness of T/T (+BSC) in mGC compared with the standard of care (i.e., BSC alone), from a National Health Service (NHS) perspective.
Methods
Model overview
A three-state partitioned survival analysis (PartSA) model was constructed to assess the cost–effectiveness of T/T + BSC versus BSC alone. This model structure has been used extensively in economic evaluations of cancer drugs, particularly those used to treat advanced-stage disease (including a previous cost–effectiveness analysis of T/T in mCRC) [Citation12–14]. shows a schematic of the model.
OS: Overall survival; PD: Progressed disease; PF: Progression-free; PFS: Progression-free survival; t: Time.
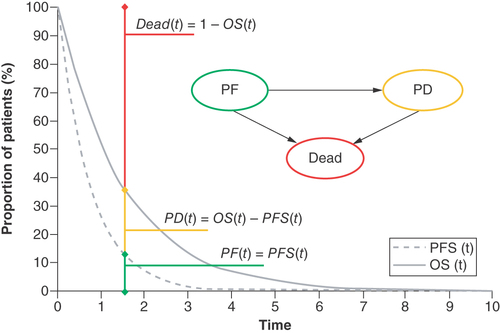
The model includes three health states: progression-free disease, progressed disease and death. Transitions between health states were not explicitly modeled using transition probabilities, but instead were inferred by calculating the area under the (extrapolated) OS and PFS curves. An additional time-to-treatment-discontinuation (TTD) curve was also fitted to more accurately estimate the costs related to the acquisition of T/T (not shown in ).
The model was constructed from an NHS perspective in accordance with guidance from the SMC (and also aligned with the reference case stipulated by the National Institute for Health and Care Excellence; NICE) [Citation15,Citation16]. As such, the main outcome of interest from the model was the incremental cost–effectiveness ratio (ICER; i.e., the cost per quality-adjusted life year [QALY] gained). Aligned with published UK HTA guidance, costs and QALYs were discounted at 3.5% per year [Citation15,Citation16]. A lifetime horizon of ten years was adopted to capture the estimated life expectancy of patients at this advanced stage of disease (i.e., 10 years was considered sufficiently long to capture any important differences in costs and outcomes across both treatment arms). A cycle length of 1 week was used for ease of incorporating relevant costs and to avoid the need to specify a half-cycle correction owing to this cycle length being sufficiently short.
The TAGS study enrolled patients with at least two prior lines of therapy, with no specific exclusion criteria according to the specific agents used in prior lines [Citation3]. However, patients seldom undergo more than two lines of chemotherapy given the lack of effective options available in clinical practice. As such, the cost–effectiveness analysis focused on a subgroup analysis of patients that received either T/T or placebo in the TAGS study as their third line of therapy. Please refer to the pivotal trial publication by Shitara et al. for further information concerning the TAGS study, including statistical analysis methodology [Citation3].
Efficacy
Parametric models for the outcomes of OS, PFS and TTD were fitted to the patient-level data from the TAGS study. Options were explored including models fitted separately to each arm (independent models) or with a covariate for treatment assignment (joint models). All models were fitted according to guidance from the NICE Decision Support Unit Technical Support Document 14 [Citation17]. Background mortality was captured within the final survival extrapolations to ensure that estimates were plausible (i.e., that the projected hazard of death was never lower than that of the age- and sex-adjusted general population).
For OS, a dependent lognormal model was selected, whereas for PFS, independent generalized gamma models were selected for each arm (owing to the shape of the PFS curves precluding the selection of a jointly fitted model). A generalized gamma model was also selected for the outcome of TTD (for the T/T arm only). These models were selected on the basis of visual inspection of the fits to the Kaplan-Meier estimates, consideration of supporting hazard-based plots (e.g., log-cumulative hazard plot), goodness-of-fit statistics (Akaike and Bayesian information criteria [AIC and BIC, respectively]) and the long-term clinical plausibility of each extrapolation.
Health-related quality of life
HRQoL was assessed in TAGS using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30® questionnaire, a cancer-specific preference-based measure of patient HRQoL [Citation18]. Patients completed the EORTC QLQ-C30 within 7 days before randomization, before dose administration on day 1 of treatment cycles ≥2, and at the safety follow-up 30 days after the last dose of treatment (if not performed within the prior 4 weeks) [Citation7]. To obtain EQ-5D utility weights to populate the model, scores from the EORTC QLQ-C30 were mapped using a published algorithm by Kontodimopoulos et al., the only candidate mapping algorithm identified that was estimated in a gastric cancer population [Citation19]. The resultant utility values applied within the model were 0.764 for progression-free disease and 0.652 for progressed disease. Equal utility values were applied for both treatment arms (T/T and BSC) in the model. In addition to health state utility values, one-off treatment-specific utility decrements associated with grade 3 and 4 adverse events (AEs) were also included in the model.
Costs
T/T is provided to NHS Scotland with a commercially sensitive patient access scheme (PAS) discount, the details of which are confidential and cannot be reported here; however, the results presented in this study capture the cost of T/T including this PAS discount. T/T is dosed at 35 mg/m2 of body surface area (BSA) twice daily on days 1–5 and 8–12 of each 28-day treatment cycle. As T/T is taken orally, no cost was applied for its administration within the model. No cost was applied in the model for placebo, given that the placebo arm was assumed to serve as a proxy for BSC given in practice.
Medical resource use (MRU) costs associated with T/T included consultant appointments, CT scans and blood tests (full blood count as well as liver and renal function tests) for patients without disease progression. After progression and discontinuation of treatment, or for patients receiving BSC, only consultant appointments were included. Additional costs were applied after progression for patients in both treatment arms to capture the limited, but nonzero use of subsequent treatments including surgery, radiotherapy and systemic anticancer therapies. Subsequent therapy costs were slightly higher for the BSC arm, reflecting more patients that went on to undergo surgery, radiotherapy or further systemic anticancer therapy.
Unit MRU costs were taken from the national reference costs database and the Personal Social Services Research Unit [Citation20,Citation21]. Frequencies of MRU were derived from a combination of NICE TA378 (ramucirumab for treating mGC or GEJ adenocarcinoma previously treated with chemotherapy) and clinical expert opinion [Citation22]. A one-off terminal care cost taken from Round et al. and inflated to present values was applied in the model, using a reported cost for colorectal cancer patients, which was used as a proxy for mGC patients owing to a lack of more specific data available for this population [Citation21,Citation23].
Treatment-emergent grade 3 or 4 AEs were included in the model, provided they occurred in at least 5% of patients in either treatment arm in the TAGS study. The total losses in QALYs attributable to grade 3 or 4 AEs explicitly captured within the model were -0.00532 for the T/T arm and 0.00168 for the BSC arm. Unit costs associated with the resolution of each included AE were reflected in the model, leading to total costs of £306.26 and £86.86 for T/T versus BSC. A summary of the cost and utility input parameters used to populate the model is provided in Supplementary Table 1.
Model outputs
In addition to the headline model results (focused on the estimation of the deterministic ICER), several sensitivity analyses were performed. Owing to the commercially sensitive PAS discount for T/T, results are limited to the presentation of the ICER only. Probabilistic sensitivity analysis (PSA) was undertaken to explore the impact of parameter uncertainty on model results. Deterministic one-way sensitivity analysis (OWSA) was undertaken to also explore the impact of parameter uncertainty, as well as to identify key drivers of results. Additional deterministic scenario analyses were also undertaken to test alternative model settings and assumptions.
Results
Compared with BSC, T/T was associated with an ICER of £37,907 per QALY gained. This result, in combination with the results of various sensitivity analyses, formed the basis on which the SMC recommended the use of T/T in this patient population. A PSA was conducted by running 10,000 probabilistic iterations. The cost-effectiveness acceptability curve (CEAC; see Supplementary Materials) illustrates that at a willingness-to-pay threshold of £50,000 per QALY gained (a threshold used historically for end-of-life medicines), T/T is associated with a 79.1% probability of being a cost-effective treatment option.
A tornado plot showing the results of the OWSA is presented in the Supplementary Materials. The most influential parameters on the ICER identified by the OWSA were related to MRU, though the key drivers of results are expected to be survival, HRQoL and BSA (due to T/T being dosed according to BSA). Parameters relating to survival and HRQoL were not included in this analysis as they are correlated. Instead, the uncertainty associated with estimates of survival and HRQoL was captured in scenario analysis. The results of the scenario analyses undertaken are presented in . The scenarios associated with the largest increase in the ICER were those relating to the restriction of the time horizon, the chosen survival curve(s) and alternative health state utility values.
Table 1. Key scenario analysis results.
Discussion
This study demonstrates that T/T provides a cost-effective option for patients with mGC, including adenocarcinoma of the GEJ, as a third-line treatment. While the SMC has no stated willingness-to-pay threshold, previous research has found that ICERs of around £50,000 have been accepted in previous assessments for treatments that meet SMC’s end-of-life and orphan equivalent criteria, including the previous assessment of T/T for patients with mCRC [Citation11,Citation24].
The cost-effectiveness model adopts a simple structure, allowing for a transparent presentation of the clinical results from the pivotal phase III TAGS study [Citation3]. The model made full use of all relevant data collected as part of the TAGS study, including data concerning the safety and efficacy of T/T, HRQoL and TTD. TAGS is a well-conducted RCT, with a comparator relevant to decision-making in UK practice. It is noted that data from other studies may later become available and could influence cost-effectiveness results, as discussed by Thokala et al. in the context of “living HTA” [Citation25]. However, the data from the TAGS study are mature, meaning that extrapolations were not majorly affected by limited follow-up due to administrative censoring.
The current analysis was not without limitations, of which two were deemed of the greatest importance. First, although the TAGS study population was mostly European, some patients were from the USA or Japan. The generalizability of international RCTs to UK practice is often a limitation of trial-based economic evaluations, particularly in the context of those developed to inform HTA. Nevertheless, a clear majority of patients in TAGS treated in the third line were from Europe (90%), so results from this study may be considered reasonably generalizable to a UK population. In addition, the TAGS study is one of the largest, positive phase III clinical trials in a European refractory mGC population, so the generalizability of the study population represents a tradeoff versus the relatively large sample size (compared with other studies in similar patient populations).
In addition to possible differences in outcomes by region, it is important to acknowledge another limitation of this analysis, in that the TAGS patient population comprised patients with different previous treatment experiences, including therapies that were previously given. From a UK perspective, the second-line agent ramucirumab (Cyramza®, Eli Lilly) is not available in NHS practice, so it remains unclear how influential prior use of ramucirumab may have been on overall prognosis or capacity to benefit from treatment with T/T. However, exploratory analyses (not reported here) did not suggest any marked differences in findings by including or excluding patients who received prior ramucirumab specifically.
This analysis presents the first findings from a UK-based cost–effectiveness analysis of T/T in mGC, with model inputs derived directly from analyses of individual patient data collected as part of the TAGS study. This cost-effectiveness analysis, therefore, provides specific estimates of the likely effect of T/T on model-relevant outcomes that are consistent with the TAGS study, such as reflecting changes in HRQoL, PFS and OS. However, several previous studies have also considered the cost-effectiveness of T/T in mGC in other settings.
Gourzoulidis et al. conducted a similar cost–effectiveness analysis from a Greek perspective, concluding that T/T offers a cost-effective treatment option for eligible third-line patients in Greece, with an ICER of €47,114 (approximately, £41,673) per QALY gained (exchanges rate from Euro to Great British Pounds based on spot rate for currency exchange on February 10, 2023) [Citation26]. Conversely, Zhou et al. conducted a cost-effectiveness analysis from a US perspective, finding that T/T was associated with an ICER of US$986,333 (approximately £815,076 using the spot rate as described previously) per QALY gained [Citation27]. Of note, the authors of this study estimated a QALY gain for T/T of 0.06, which is seemingly contradictory to the statistically significant improvements in both OS and PFS found in the pivotal TAGS study, so the findings of this study should be interpreted with caution. It should also be noted that this study used a Markov model (as opposed to a PartSA model), with transition probabilities estimated based on median OS and PFS from TAGS, and given the exponential model was rejected in this analysis owing to poor visual and statistical goodness-of-fit, it is likely that the Zhou et al. Markov model does not provide a good fit to the TAGS data.
Takushima et al. estimated the cost-effectiveness of T/T versus nivolumab (Opdivo®, Bristol-Myers Squibb) from the perspective of the Japanese public healthcare payer [Citation28]. While it is not possible to compare results from Takushima and colleagues to this study (given the different comparator), the authors found that T/T was more cost-effective than nivolumab (nivolumab was associated with an ICER of over ¥32 million (equivalent to over £200,000, using the spot rate described previously) per QALY gained). Overall, published literature supports the findings of the present analysis, except the study by Zhou et al., which had a number of limitations both with respect to the generalizability of outcomes by differing perspectives (US vs UK), and decisions made with respect to the methods employed to undertake the economic analyses.
Conclusion
In conclusion, this analysis demonstrates that T/T offers a cost-effective third-line treatment option for patients with third-line mGC, including adenocarcinoma of the GEJ. Based on the findings from this study, combined with T/T having a minimally invasive route of administration allowing for treatment to be administered in the community, T/T provides a valuable treatment option for mGC patients at the end of life, addressing a high unmet need in this population for whom there are otherwise no effective treatment options available in practice.
The TAGS study assessed the use of trifluridine/tipiracil (T/T) in patients with metastatic gastric adenocarcinoma (including adenocarcinoma of the gastroesophageal junction) who had undergone two or more previous chemotherapy regimens for advanced or metastatic disease.
This study presents a cost–effectiveness analysis of T/T versus best supportive care from a UK healthcare payer perspective.
A three-state partitioned survival analysis was developed based on data collected as part of the TAGS study, including extrapolations of overall survival, progression-free survival and time to treatment discontinuation, as well as data concerning the occurrence of adverse events and changes in health-related quality of life.
The base-case analysis found that T/T is likely to be a cost-effective use of National Health Service resources, with an incremental cost-effectiveness ratio of £37,907.
Author contributions
L Hamerton, E Hook and A Bullement developed the original cost–effectiveness model used to inform this study and contributed to the drafting of the original manuscript. K Gomes provided data and information concerning clinical aspects of the model and manuscript, and contributed to the drafting of the manuscript. R Fougeray provided input to the statistical analyses presented in this study and the original analyses of the TAGS study data used to populate the model. M Vargas Gomes and O Hauch provided input to the final cost-effectiveness model analyses presented in the manuscript and contributed to the writing of the manuscript. All authors reviewed the draft versions of the manuscript and approved the final version ahead of submission.
Financial & competing interests disclosure
This work was funded by Les Laboratoires Servier, the manufacturer and marketing authorization holder for trifluridine/tipiracil (Lonsurf®). L Hamerton, K Gomes, R Fougeray, M Vargas Gomes and O Hauch are employees of Servier and/or its subsidiaries. E S Hook and A Bullement are employees of Delta Hat Ltd, an independent consultancy that received fees from Servier Affaires Medical for the development of the cost-effectiveness model. Medical writing and editorial support were provided by A Bullement and E S Hook of Delta Hat Ltd, and were funded by Servier Affaires Medical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
The authors certify that this manuscript reports the secondary analysis of clinical trial data that have been shared with them, and that the use of this shared data is in accordance with the terms (if any) agreed upon their receipt. The source of this data is: TAGS study, NCT02500043, Taiho Oncology, Inc.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2022-0662
Additional information
Funding
References
- Smyth E , VerheijM, AllumW, CunninghamD, CervantesA, ArnoldD. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.27, v38–v49 (2016).
- Servier data on file. Advisory board meeting minutes. (2019).
- Shitara K , DoiT, DvorkinMet al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.19(11), 1437–1448 (2018).
- Li J , QinS, XuJ, XiongJ, WuC, BaiYet al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol.34(13), 1448–1454 (2016).
- Sung H , FerlayJ, SiegelRLet al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249 (2021).
- Mayer RJ , Van CutsemE, FalconeAet al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med.372(20), 1909–1919 (2015).
- Tabernero J , AlsinaM, ShitaraKet al. Health-related quality of life associated with trifluridine/tipiracil in heavily pretreated metastatic gastric cancer: results from TAGS. Gastric Cancer23(4), 689–698 (2020).
- EMA . Summary of product characteristics (SmPC): trifluridine/tipiracil (Lonsurf). (2016). www.ema.europa.eu/en/documents/product-information/lonsurf-epar-product-information_en.pdf
- Medicines & Healthcare Products Regulatory Agency . MHRA: Summary of Product Characteristics–Lonsurf 15 mg/6.14 mg film-coated tablets. (2021). https://mhraproducts4853.blob.core.windows.net/docs/43bcc3b79dd4ebf599d263e3a393f40689a6d972
- Medicines & Healthcare products Regulatory Agency . MHRA: Summary of Product Characteristics–Lonsurf 20 mg/8.19 mg film-coated tablets (2021). https://mhraproducts4853.blob.core.windows.net/docs/9ddf7b44d7c62c0e902f9bc955528c68bd8db0e0
- Medicines Advice: Trifluridine/Tipiracil (Lonsurf). Scottish Medicines Consortium, 1–13 (2021). www.scottishmedicines.org.uk/medicines-advice/trifluridinetipiracil-lonsurf-full-smc2329/
- Woods BS , SiderisE, PalmerS, LatimerN, SoaresM. Partitioned survival and state transition models for healthcare decision making in oncology: where are we now?Value Health23(12), 1613–1621 (2020).
- Bullement A , CranmerHL, ShieldsGE. A review of recent decision-analytic models used to evaluate the economic value of cancer treatments. Appl. Health Econ. Health Policy17(6), 771–780 (2019).
- Bullement A , UnderhillS, FougerayR, HatswellAJ. Cost-effectiveness of trifluridine/tipiracil for previously treated metastatic colorectal cancer in England and Wales. Clin. Colorectal Cancer17(1), e143–e151 (2018).
- Working with SMC–A Guide for Manufacturers . Scottish Medicines Consortium. (2021). www.scottishmedicines.org.uk/media/6164/working-with-smc.pdf
- PMG36: NICE Health Technology Evaluations: the Manual . National Institute for Health and Care Excellence (2022). www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation
- Latimer NR . NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations Alongside Clinical Trials–Extrapolation with Patient-Level Data.University of Sheffield, Sheffield, UK, 1–52 (2011). http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf
- Aaronson NK , AhmedzaiS, BergmanBet al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst.85(5), 365–376 (1993).
- Kontodimopoulos N , AletrasVH, PaliourasD, NiakasD. Mapping the cancer-specific EORTC QLQ-C30 to the preference-based EQ-5D, SF-6D, and 15D instruments. Value Health12(8), 1151–1157 (2009).
- National Health Service . Improvement 2017/18 NHS Reference Costs (2019). https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https:/improvement.nhs.uk/resources/reference-costs/
- Curtis L , BurnsA, Personal Social Services Research Unit. Unit Costs of Health and Social Care.University of Kent, Canterbury, Kent, UK, 1–201 (2018). www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018/
- TA378: Ramucirumab for Treating Advanced Gastric Cancer or Gastro–oesophageal Junction Adenocarcinoma Previously Treated with Chemotherapy. National Institute for Health and Care Excellence. (2016). www.nice.org.uk/guidance/ta378/history
- Round J , JonesL, MorrisS. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat. Med.29(10), 899–907 (2015).
- Medicines Advice: Autologous Anti-CD19-transduced CD3+ Cells (KTE-X19) 0.4 to 2×108 Cells Dispersion for Infusion (Tecartus®). Scottish Medicines Consortium. Glasgow, UK (2021). www.scottishmedicines.org.uk/media/6180/autologous-tecartus-final-july-2021-for-website.pdf
- Thokala P , SrivastavaT, SmithRet al. Living health technology assessment: issues, challenges and opportunities. Pharmacoeconomics41(3), 227–237 (2023).
- Gourzoulidis G , KoulentakiM, KoumarianouAet al. Cost-effectiveness of trifluridine/tipiracil as a third-line treatment of metastatic gastric cancer, including adenocarcinoma of the gastrohesophageal junction, among patients previously treated in Greece. Expert Rev. Pharmacoecon. Outcomes Res.22(2), 259–269 (2022).
- Zhou K , ZhouJ, ZhangM, LiaoW, LiQ. Cost-effectiveness of trifluridine/tipiracil (TAS102) for heavily pretreated metastatic gastric cancer. Clin. Transl. Oncol.22(3), 337–343 (2020).
- Takushima Y , IgarashiA, YoshiharaH, ShitaraK, DoiT. Cost-effectiveness of trifluridine/tipiracil against nivolumab for heavily pretreated metastatic gastric cancer in Japan. Jpn J. Clin. Oncol.51(9), 1383–1390 (2021).
