Abstract
Despite improvements made with checkpoint inhibitor (CPI) therapy, a need for new approaches to improve outcomes for patients with unresectable or metastatic melanoma remains. EVX-01, a personalized neoepitope vaccine, combined with pembrolizumab treatment, holds the potential to fulfill this need. Here we present the rationale and novel design behind the KEYNOTE – D36 trial: an open label, single arm, phase II trial aiming to establish the clinical proof of concept and evaluate the safety of EVX-01 in combination with pembrolizumab in CPI naive patients with unresectable or metastatic melanoma. The primary objective is to evaluate if EVX-01 improves best overall response after initial stable disease or partial response to pembrolizumab treatment, in patients with advanced melanoma. The novel end points ensure a decisive readout which may prove helpful before making major investments in phase III trials with limited phase I data.
Clinical Trial Registration: NCT05309421 (ClinicalTrials.gov)
Plain language summary
Drugs targeting the immune system have improved the outcomes for patients with advanced melanoma. However, a significant proportion of patients do not benefit and there is a need for better therapeutic agents to be used alone or in combination with immune modulating agents. This article summarizes the rationale and design of a new trial with a personalized vaccine (EVX-01) that may improve outcomes for patients with advanced melanoma (unresectable stage III or IV melanoma). The EVX-01 vaccine aims to stimulate the patient’s immune system to generate T cells that target specific molecules that can only be found on the surface of the individual patients’ cancer cells (i.e. neoepitopes), resulting in cancer cell death. The trial will investigate if the personalized EVX-01 vaccine together with checkpoint inhibitor therapy works better for patients with advanced melanoma, than checkpoint inhibitor therapy alone.
Melanoma is a leading cause of death from skin cancer and has a high mortality rate. Melanoma is characterized by a high potential for dissemination and metastasis. Additionally, melanoma belongs to a subset of malignancies with a high mutational burden. The treatment with novel check point inhibitors (CPIs) has improved the overall survival (OS) rate of patients with advanced or metastatic melanoma (MM) [Citation1]. However, a large proportion of patients have no benefit at all (primary resistance) or progress after a RECIST response [Citation1–3].
In the KEYNOTE-001 and KEYNOTE-006 trials, approximately half of the patients with unresectable or metastatic melanoma who initiated treatment with pembrolizumab, progressed during the first 12 weeks of treatment. Furthermore, only a subset of patients with stable disease (SD) or partial response (PR) at 12 weeks of treatment achieved a complete response (CR) thereafter [Citation4] and some of the patients with an initial SD and PR later progress. These data underline the unmet need for novel therapeutic modalities for patients with unresectable or metastatic melanoma treated with CPI [Citation2].
It is widely acknowledged, that the specificity and success of immunotherapy largely depends on the ability of T cells to recognize tumor-specific antigens, in particular, neoantigens. Emerging evidence, however, suggests that the treatment benefit achieved with CPI stems from the tumoricidal activity of T cells targeting neoepitopes displayed exclusively on the surface of the patient’s cancer cells [Citation5,Citation6]. Moreover, success of the durable response following adoptive cell therapy is thought to be dependent on maintaining the neoantigen-specific T-cell pool [Citation7,Citation8]. Thus, the administration of a personalized cancer vaccine that will generate and maintain a pool of neoepitope-specific T-cells together with CPI therapy may create a promising avenue to address the unmet clinical need in melanoma patients. This is supported by recent results from several clinical trials in the field of personalized cancer vaccines [Citation9–13], which highlight the potential of this therapeutic drug class and justify further development of cancer vaccines, particularly for combination with CPI therapy.
EVX-01
EVX-01 is a personalized peptide-based therapeutic vaccine in development, that consists of multiple peptides, each representing a neoepitope only found in the individual patient’s tumor, combined with a liposomal adjuvant (CAF09b), into a single vaccine formulation. EVX-01 has been able to induce long lasting neoepitope-specific T-cell responses in a phase I clinical trial [Citation14].
Identification of immunogenic T-cell epitopes exclusively present in the malignant tissue (and not in healthy tissue) is critical to the concept of personalized cancer vaccines. These epitopes, referred to as neoepitopes, arise from tumor specific mutations. As a limited number of somatic mutations are shared among tumors from different patients, designing a personalized vaccine specific for each patient’s tumor is desirable. Importantly, the high tumor specificity of the cancer neoantigens must be confirmed to minimize the potential risk of autoimmunity and induction of central and peripheral tolerance.
In the process of generating a personalized neoantigen vaccine, a major challenge relates to identifying the most potent neoantigen epitopes in a timely manner [Citation15]. For that, advanced computational tools have been developed by Evaxion Biotech for in silico neoantigen selection and accurate prediction of major histocompatibility complex (MHC) binding peptides specific to the patient’s human leukocyte antigen (HLA) type.
PIONEER™
Evaxion Biotech has developed an artificial intelligence (AI)-based system enabling high-throughput analysis of tumor genomic data. The system identifies cancer-specific neopeptides, predicts the immunogenicity and effect of targeting each neopeptide, and subsequently selects the most effective set of predicted cancer-specific neoepitopes. The in silico computational platform PIONEER™ allows processing of next-generation sequencing (NGS) data and selecting patient-specific immunogenic targets within 1 day, which facilitates rapid manufacturing of a personalized peptide-based vaccine. Such rapid manufacturing has already been achieved in a phase I trial (NCT03715985) [Citation14].
PIONEER has been developed to simulate key biological steps within cancer cells relevant for generating effective neoepitopes:
Identification of somatic mutations: cancer-specific somatic mutations are identified by comparing DNA sequencing data from the tumor sample(s) and normal tissue sample using an AI-based somatic variant caller.
Expression of somatic mutations: only a subset of the cancer-specific mutations is found in genes that are expressed in the tumor cells. The expression levels of each somatic mutation are determined by analyzing tumor RNA sequencing data.
Translation to neopeptide sequences: not all cancer-specific mutations result in altered protein sequences. Some mutations may be found in regions that do not code for protein sequences or they may simply be synonymous mutations (where the DNA sequence is altered, but the encoded amino acid is the same). The coding regions around non-synonymous mutations are translated into amino acid sequences, generating cancer-specific neopeptide sequences.
Presentation on MHC class I and class II: to induce an immune response, neopeptides must contain subsequences that are bound by MHC molecules and presented on the cell surface. The identified neopeptides are given as input to an AI-based MHC ligand prediction tool, along with the patient’s HLA type, to identify neopeptides containing MHC ligands bound by the patient’s MHC molecules specifically.
T-cell immunogenicity: neoepitopes presented by MHC class I and class II must be recognized by T-cells to trigger an immune response and tumor cell death. While being presented as MHC ligands is a prerequisite for generating an immune response, not all MHC ligands are recognized by T-cells. The likelihood of a given mutated MHC ligand eliciting a T-cell response is thus also predicted and included as a neoepitope selection parameter.
Clonality of neoepitopes: tumors are extremely heterogenous, meaning that not all tumor cells necessarily encode and express the same neoepitopes. Targeting clonal neoepitopes, defined as neoepitopes arising from clonal mutations that are present in all cancer cells, theoretically allows for systemic eradication of the whole tumor, as well as potential metastases in the patient. The clonal status of a neoepitope is estimated by analyzing the DNA sequencing data. For patients where DNA sequencing data from multiple tumor biopsies is available, information from each biopsy is used to improve the clonality estimate.
Phase I trial
PIONEER is also utilized in an ongoing phase I trial (NCT03715985) with EVX-01. Experience during this trial has substantiated the manufacturing feasibility and successful vaccination of patients diagnosed with unresectable or metastatic melanoma. The phase I trial enrolled two cohorts of patients: one cohort consisted of treatment naive patients (cohort A), the other cohort consisted of patients treated with anti-PD1 for more than 4 months and with SD at the time of enrollment (cohort B). Preliminary results from a cohort of five patients have recently been published [Citation14].
KEYNOTE – D36
Herein, we describe the novel design of the phase II trial KEYNOTE – D36: an open label, single arm trial evaluating the efficacy and safety of EVX-01 in combination with pembrolizumab in CPI treatment naive adults with unresectable or metastatic melanoma (NCT05309421).
To ensure the feasibility of the trial and facilitate reaching a pass/fail decision early, a single arm design where all patients receive pembrolizumab monotherapy as a run-in, followed by combination of EVX-01 and pembrolizumab after week 12 has been developed. Since the outcomes with CPI treatment in the target patient population are well characterized, a single arm trial that compares against historical outcomes and with well-defined and pre-specified success criteria is considered suitable to establish the clinical proof of concept that the combination therapy of CPI and EVX-01 improves outcomes compared with therapy with CPI alone. Meeting the primary end point, will not only establish clinical proof of concept for the combination of EVX-01 with pembrolizumab, but will also validate the PIONEER platform and quickly establish a solid foundation for conducting a larger, confirmatory, randomized clinical trial.
This trial is being performed in accordance with the requirements of applicable local regulatory authorities and International Council of Harmonisation GCP guidelines. Institutional review board/independent ethics committee approval of the protocol and all amendments is required prior to implementation.
Background & rationale
Most malignant tumors studied to date contain somatic mutations which hold the potential to be targeted by the immune system if they are expressed and presented on the surface of cancer cells. Thus, strategies to boost the generation of neoepitope-specific T-cells may help raise an adequate immune response against the tumor cells.
This clinical trial will use a personalized neoepitope vaccine, EVX-01, to potentiate the natural capacity to generate T-cells targeting neoepitopes in patients with melanoma. This strategy will be combined with pembrolizumab, to overcome immune suppressive factors in the tumor microenvironment. Inhibitors of the PD-1/PD-L1 pathway, like pembrolizumab, are suggested to unleash the activity of vaccination-induced neoepitope-specific T-cells within the tumor microenvironment, and thereby induce tumor regression.
It is therefore hypothesized that in patients with unresectable or metastatic melanoma, a combination immunotherapy of anti-PD1 and immunization with a personalized neoepitope vaccine, will lead to better outcomes than with anti-PD1 monotherapy, especially for those who do not achieve a RECIST [Citation16] PR or CR in the first 12 weeks with pembrolizumab monotherapy, i.e. those with SD or PD who achieve the worst outcomes with monotherapy.
Trial design & objectives
This single arm, multi-national clinical trial in patients with unresectable or metastatic melanoma aims to evaluate if EVX-01 improves the best overall response (BOR) compared with pembrolizumab alone in historical trials like KEYNOTE-01 and 06 [Citation4]. A complete list of trial end points is presented in Box 1.
Primary
Composite of a BOR of CR or PR for patients with SD at the time of first EVX-01 administration and a BOR of CR for patients with PR at the time of first EVX-01 administration, within 2 years of treatment with pembrolizumab, as per RECIST 1.1 criteria
Secondary
Overall response rate, defined as the proportion of the patients who have best response as CR or PR assessed 2 years after initiation of treatment with pembrolizumab, as per RECIST 1.1 criteria
PFS in patients with an assessment of SD, PR or CR at the time of first EVX-01 administration, assessed 2 years after initiation of treatment with pembrolizumab and defined as the time from the first EVX-01 administration to the first documented disease progression per RECIST 1.1. criteria or death due to any causes, whichever occurs first
OS assessed 2 years after initiation of treatment with pembrolizumab and defined as the time from initiation of treatment of pembrolizumab to death due to any cause
Number, type and severity of adverse events (AEs) and serious adverse events (SAEs)
Activation and level of neoepitope specific CD4+ and CD8+ T-cells before, during and after EVX-01 immunization
Percentage of patients in which EVX-01 is generated, produced and administered
The trial will enroll CPI treatment naive patients with unresectable or metastatic malignant melanoma (stage III and IV) not amenable to local therapy as defined per the American Joint Committee on Cancer (AJCC) 8th edition staging system [Citation17], and as summarized in Box 2.
Inclusion criteria:
Be at least 18 years of age on day of signing informed consent
Histologically confirmed, and not amenable to local therapy, metastatic or unresectable melanoma stage III or stage IV, as per AJCC 8th ed. staging system
Patient may not have a diagnosis of uveal or ocular melanoma
Patients must be treatment naive to CPI therapy
Patients must have testing for a BRAF mutation
○ Note: patients with BRAF V600E mutant melanoma may have received prior BRAF inhibitor therapy as first-line systemic therapy and be eligible for this study as second-line treatment. At the discretion of the investigator, patients with BRAF V600E mutant melanoma who have NOT received a BRAF inhibitor are also eligible for this study as first-line treatment if they meet the following additional criteria:
LDH < local ULN, ii. No clinically significant tumor related symptoms in the judgment of the investigator, and iii. Absence of rapidly progressing metastatic melanoma in the judgment of the investigator
Have measurable disease per RECIST 1.1 as assessed by the local site investigator within 4 weeks prior to the first visit. Lesions situated in a previously irradiated area are considered measurable if progression has been demonstrated in such lesions
Patients must be willing and able to provide fresh or frozen tumor tissue from an unresectable or metastatic site of disease for neoepitope and biomarker analyses. If a sufficient amount of tumor tissue from an unresectable or metastatic site is not available prior to the start of the screening phase, subjects must consent to allow the acquisition of additional tumor tissue. In addition, participants may provide additional biopsy at the time of discontinuation due to progression
ECOG performance status of 0 or 1.
Exclusion criteria:
Has received prior therapy with an anti-PD-1, anti-PD-L1 or anti PD L2 agent or with an agent directed to another stimulatory or co-inhibitory T-cell receptor (e.g., CTLA-4, OX 40, CD137).
Has received prior systemic anti-cancer therapy including investigational agents within 4 weeks prior to treatment.
Has received prior radiotherapy within 2 weeks of start of study treatment. Participants must have recovered from all radiation-related toxicities, not require corticosteroids and not have had radiation pneumonitis. A 1-week washout is permitted for palliative radiation (≤2 weeks of radiotherapy) to non-CNS disease.
Has received a live or live-attenuated vaccine within 30 days prior to the first dose of study intervention. Note: administration of killed vaccines are allowed.
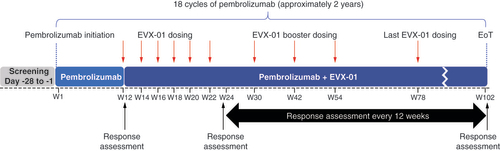
Enrolled patients will initiate treatment with pembrolizumab at trial week 1. Immunization with EVX-01 will start at week 12. EVX-01 will be administered intramuscularly, with six doses given 2 weeks apart and four booster doses at trial weeks 30, 42, 54 and 78. The dose of the neopeptides that compose EVX-01 will not exceed 2000 μg and 200 μg per neopeptide. Tumor response will be assessed at week 12 (time of first EVX-01 administration) and every 12 weeks throughout the trial and determined according to RECIST 1.1. [Citation16] and immune RECIST (iRECIST) [Citation18]. Patients with documented disease progression are still eligible to initiate or continue treatment with EVX-01 at the investigator’s discretion. Similarly, the decision to remain on pembrolizumab monotherapy or not for patients with progressive disease, will be up to the discretion of the investigator.
Pembrolizumab will be given up to 18 treatment cycles (~2 years of treatment). During this time, patients will be immunized with a total of ten EVX-01 doses. The visit frequency will be approximately every 2–3 weeks during the first 6 months, and every 6 weeks after 6 months of treatment with pembrolizumab. An overview of the trial design is presented in .
Patient safety will be monitored continuously, including safety signal detection, throughout the trial. Data Monitoring and Safety Committees will be appointed for the trial.
Induction of neoepitope specific T-cell responses (CD8+ and CD4+ reactive T-cells) in circulation and in tumor upon immunization with EVX-01 will be monitored over the time course of treatment. T-cell responses will be measured using patient-derived PBMCs from whole blood. Immune responses can be measured by methodologies like ELISPOT, intracellular cytokine staining followed by flow cytometry analysis and MHC I multimer staining.
Statistics
Sample size
The trial will enroll approximately 90 patients to achieve 33 patients with a tumor response assessment of either SD or PR at week 12 after initiating treatment with pembrolizumab.
Enrolling 33 patients with SD or PR will provide 80% power of showing a 50% improvement in the best overall response, assuming 70% of patients with SD at 12 weeks will improve to PR or CR, and 45% of patients with PR at 12 weeks will improve to CR. These values will be compared with performance goals of 46.7% improvement to PR or CR for SD patients at week 12 and 29.9% improvement to CR for patients with PR at week 12.
Primary analysis
The primary analysis will consider patients with SD and PR after 12 weeks of pembrolizumab treatment and evaluate if additional treatment with EVX-01 will increase the conversion of patients to PR or CR. Logistic regression with patient-specific (SD or PR) offset will be used to calculate the increased odds of response with EVX-01.
Safety
Summary statistics will be provided for the safety end points as appropriate. The number, type and severity of AEs will be described by organ class. Rates will be described overall (intention-to-treat population) and in patients receiving EVX-01 (as-treated population). The safety of the trial participants will be followed at frequent visits as stipulated in the protocol, medical monitoring by the sponsor as well as oversight by an independent data monitoring committee composed of clinical experts in melanoma research.
CMC
For each patient the selected patient-specific neoantigen peptides are synthesized with a high throughput synthesis platform, mixed into a peptide pool and aseptically filled under GMP conditions. Activities are performed in parallel when possible and feasible to ensure as fast turnaround time as currently possible. The EVX-01 vaccine is then prepared by mixing the peptide pool with adjuvant CAF®09b. CAF09b is a novel liposome-based vaccine adjuvant developed by Statens Serum Institut (SSI), Denmark, based on cationic surfactant dimethyl-dioctadecyl ammonium (DDA) combined with C-type lectin agonist monomycoloyl glycerol (MMG) and TLR3 agonist poly I:C.
Conclusion
CPIs have become the standard of care for unresectable and metastatic melanoma with major improvements in OS. Still, many patients do not confer any, or only a transient benefit from currently approved immunotherapies [Citation2,Citation3]. Thus, a need for more effective treatments remains for these patients.
EVX-01 uses the proprietary platform PIONEER for fast and accurate design of a neoepitope vaccine tailored to each individual patient. The vaccine, containing peptide sequences designed based on the patient’s individual tumor neoepitopes, is formulated with a novel adjuvant, CAF09b, to strengthen CD4+ and CD8+ T-cell antitumor immunity. CPI targeting PD-1 is administered both before, during and after vaccination to unleash the activity of vaccine-induced immune responses.
To clearly determine whether EVX-01 improves BOR after initial SD or PR to pembrolizumab treatment, in patients with advanced melanoma, a randomized trial will be needed. The global open label phase II, single arm trial presented here aims to establish the clinical proof of concept of treatment with EVX-01 and pembrolizumab in CPI naive adults with unresectable or metastatic melanoma. The trial will provide insights into the efficacy and safety of EVX-01 and pembrolizumab treatment compared with historical outcomes with pembrolizumab alone, as well as the immunologic response induced by EVX-01.
Background
Although check point inhibitor (CPI) therapy has improved the overall survival (OS) rate for patients with advanced melanoma, many patients still fail to achieve a complete response (CR).
A need for novel approaches to improve outcomes for patients with unresectable or metastatic melanoma remains.
The treatment benefit achieved with CPI likely stems from the tumoricidal activity of T-cells targeting neoepitopes displayed exclusively on the surface of the patient’s cancer cells.
Administration of a personalized cancer vaccine that will generate and maintain a pool of neoepitope-specific T-cells, together with CPI therapy, may thus be a promising avenue to address this unmet need.
EVX-01
EVX-01 is a personalized peptide-based therapeutic vaccine consisting of multiple peptides, each representing a neoepitope.
An artificial intelligence system (PIONEER™) is used to ensure fast and accurate design of the vaccine.
EVX-01 has been shown to induce long lasting neoepitope-specific T-cell responses in a phase I clinical trial.
KEYNOTE – D36
KEYNOTE – D36 is an open label, single arm, phase II trial evaluating the efficacy and safety of EVX-01 in combination with pembrolizumab in CPI treatment naive adults with unresectable or metastatic melanoma (NCT05309421).
The trial will enroll CPI treatment naive patients with unresectable or metastatic malignant melanoma (stage III and IV) not amenable to local therapy.
Patients will be treated with pembrolizumab for up to 18 treatment cycles (~2 years of treatment). After the initial 12 weeks of pembrolizumab treatment, patients will be immunized with a dose of EVX-01 every 2 weeks six-times (trial weeks 12–22), followed by four booster doses at trial weeks 30, 42, 54 and 78.
The primary objective of the trial is to evaluate if EVX-01 improves the best overall response (BOR) compared with historical data on patients on anti-PD1 treatment with pembrolizumab alone.
The primary end point is the composite of a BOR of CR or PR for patients with SD at the time of first EVX-01 administration and a BOR of CR for patients with PR at the time of first EVX-01 administration, within 2 years of treatment with pembrolizumab, as per RECIST 1.1 criteria.
Secondary end points include overall response rate, defined as the proportion of the patients who have best response as CR or PR assessed 2 years after initiation of treatment with pembrolizumab, as per RECIST 1.1 criteria, progression-free survival, OS, adverse event evaluations and assessment of immunologic response.
Author contributions
All authors fulfil the four authorship criteria.
Financial & competing interests disclosure
GV Long: consultant advisor for Agenus, Amgen, Array Biopharma, Boehringer Ingelheim, Bristol–Myers Squibb, Evaxion, Hexal AG (Sandoz Company), Highlight Therapeutics, Innovent Biologics, MSD, Novartis, OncoSec, Pierre Fabre, Provectus, Qbiotics, Regeneron. PF Ferrucci: advisory role for BMS, Novartis, MSD, Pierre Fabre and Roche. Research fund from BMS. A Khattak: no disclosures. TM Meniawy: advisory role for GlaxoSmithKline, AstraZeneca, Takeda, Novartis, Bristol–Myers Squibb and consultant for Eisai. PA Ott: research funding from and advisory role for Neon Therapeutics, Bristol–Myers Squibb, Merck Sharpe & Dohme, CytomX, Pfizer, Novartis, Celldex, Amgen, Array, AstraZeneca/MedImmune, Armo BioSciences, Xencor, Oncorus, Evaxion, Phio, and Roche/Genentech. M Chisamore: is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway (NJ, USA) and owns stock in Merck & Co., Inc., Rahway (NJ, USA). A Hyseni, T Trolle and E Heegard are employees of Evaxion Biotech A/S and own stock options in Evaxion Biotech A/S.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by LS Schmidt from Pharma IT Aps, funded by Evaxion Biotech A/S.
Ethical conduct of research
The authors state that this trial is being performed in accordance with the requirements of applicable local regulatory authorities and the International Council of Harmonisation GCP guidelines. Institutional review board/independent ethics committee approval of the protocol and all amendments is required prior to implementation.
Notes
AE: Adverse event; BOR: Best overall response; CR: Complete response; OS: Overall survival; PFS: Progression-free survival; PR: Partial response; RECIST: Response evaluation criteria in solid tumors; SAE: Serious adverse event; SD: Stable disease.
AJCC: American Joint Committee on Cancer; CNS: Central nervous system; CPI: Checkpoint inhibitor; ECOG: Eastern Cooperative Oncology Group; LDH: Lactate dehydrogenase; RECIST: Response evaluation criteria in solid tumors; ULN: Upper limit of normal.
References
- Hamid O , RobertC, DaudAet al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol.30(4), 582–588 (2019).
- Larkin J , Chiarion-SileniV, GonzalezRet al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med.381(16), 1535–1546 (2019).
- Robert C , SchachterJ, LongGvet al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med.372(26), 2521–2532 (2015).
- Hamid O , RobertC, DaudAet al. Long-term outcomes in patients with advanced melanoma who had initial stable disease with pembrolizumab in KEYNOTE-001 and KEYNOTE-006. Eur. J. Cancer157, 391–402 (2021).
- Gubin MM , ZhangX, SchusterHet al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature515(7528), 577–581 (2014).
- van Rooij N , van BuurenMM, PhilipsDet al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol.31(32), 439–442 (2013).
- Lu Y-C , YaoX, LiYFet al. Mutated PPP1R3B is recognized by T cells used to treat a melanoma patient who experienced a durable complete tumor regression. J. Immunol.190(12), 6034–6042 (2013).
- Zhou J , DudleyME, RosenbergSA, RobbinsPF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. Journal of Immunotherapy28(1), 53–62 (2005).
- Keskin DB , AnandappaAJ, SunJet al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature565(7738), 234–239 (2019).
- Ott PA , HuZ, KeskinDBet al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature547(7662), 217–221 (2017).
- Ralli M , BotticelliA, ViscontiICet al. Immunotherapy in the treatment of metastatic melanoma: current knowledge and future directions. J. Immunol. Res.2020, 9235638 (2020).
- Sahin U , DerhovanessianE, MillerMet al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature547(7662), 222–226 (2017).
- Ott PA , Hu-LieskovanS, ChmielowskiBet al. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell183(2), 347–362 (2020).
- Mørk SK , KadivarM, BolKFet al. Personalized therapy with peptide-based neoantigen vaccine (EVX-01) including a novel adjuvant, CAF®09b, in patients with metastatic melanoma. OncoImmunology11(1), 2023255 (2022).
- Guo Y , LeiK, TangL. Neoantigen vaccine delivery for personalized anticancer immunotherapy. Front. Immunol.9, 1499 (2018).
- Eisenhauer EA , TherasseP, BogaertsJet al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer45(2), 228–247 (2009).
- Amin MB , EdgeSB, GreeneFLet al. American Joint Committee on Cancer (AJCC). Cancer J. Clin.67(2), 93–99 (2017).
- Seymour L , BogaertsJ, PerroneAet al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol.18(3), e143–e152 (2017).