Abstract
The first-in-class, small molecule HIF-2αinhibitor, belzutifan, has demonstrated promising antitumor activity in previously treated patients with clear cell renal cell carcinoma (RCC). HIF-2α also regulates VEGF expression and is involved in resistance to anti-VEGF therapy. This study describes the rationale and design for a randomized, phase III study evaluating efficacy and safety of belzutifan plus the tyrosine kinase inhibitor (TKI) lenvatinib versus the TKI cabozantinib in patients with advanced RCC progressing after anti-PD-1/PD-L1 therapy in the first- or second-line setting or as adjuvant therapy. Considering the unmet need for effective and tolerable treatment of advanced RCC following immune checkpoint inhibitors, belzutifan plus lenvatinib may have a positive benefit/risk profile.
Clinical Trial Registration: NCT04586231 (ClinicalTrials.gov)
Tweetable abstract
Thephase III LITESPARK-011 study will evaluate efficacy and safety of the HIF-2α inhibitor belzutifan plus lenvatinib versus cabozantinib in patients with advanced RCC progressing after anti-PD-1/PD-L1 therapy.
Renal cell carcinoma (RCC) accounts for approximately 85% of all renal cancers [Citation1], and 2010–2016 US data indicate that about one-third of patients present with regional or distant metastatic disease [Citation2]. Immune checkpoint inhibitors targeted to programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1), administered with cytotoxic T-lymphocyte antigen 4 (CTLA-4) or VEGF-targeted therapies, are widely used first-line treatment for advanced clear cell RCC [Citation1,Citation3,Citation4]. These therapies, evaluated in phase III studies, include nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor), evaluated in CheckMate 214 [Citation5,Citation6]; avelumab (PD-L1 inhibitor) and axitinib (VEGF inhibitor), evaluated in JAVELIN Renal 101 [Citation7,Citation8]; pembrolizumab (PD-L1 inhibitor) and axitinib, evaluated in KEYNOTE-426 [Citation9,Citation10]; pembrolizumab and lenvatinib (tyrosine kinase inhibitor [TKI]), evaluated in CLEAR/KEYNOTE-581 [Citation11]; and nivolumab and cabozantinib (TKI inhibitor), evaluated in CheckMate 9ER [Citation12]. The primary analyses of these studies demonstrated median progression-free survivals (PFSs) from 11.6 to 23.9months and objective response rates (ORR) ranging from 42 to 71% [Citation6,Citation7,Citation9,Citation11,Citation12]. Median overall survival (OS) was not reached in these primary analyses [Citation6,Citation7,Citation9,Citation11,Citation12], nor in available extended follow-ups ranging from a median of 19.3 to 32.4months [Citation5,Citation8,Citation10]. PFS and ORR remained promising [Citation5,Citation8,Citation10].
No standard-of-care has been established based on randomized and controlled phase III studies for patients with advanced clear cell RCC that has progressed after anti-PD-1/PD-L1-based therapy [Citation1,Citation3,Citation4]. Resistance to VEGF-targeted therapies invariably develops, despite initial responses [Citation13]. Currently, the TKI cabozantinib and the PD-1 inhibitor nivolumab are preferred treatments after first-line combination therapy, and the TKI lenvatinib plus the mammalian target of rapamycin inhibitor everolimus is an alternative option [Citation1,Citation3,Citation4]. Cabozantinib was compared with everolimus in a phase III study (METEOR) that included 658 patients who had progressed after previous TKI therapy [Citation14]. Statistically significant improvements in PFS (7.4 vs 3.9months), OS (21.4 vs 16.5months) and ORR (17 vs 3%) were shown for cabozantinib compared with everolimus [Citation14]. In the cabozantinib arm, 71% of participants experienced a grade 3 or 4 adverse event (AE), the most common of which were hypertension (15%), diarrhea (13%), and fatigue (11%) [Citation14]. Nivolumab was compared with everolimus in a phase III study (CheckMate 025) of 821 patients with advanced or metastatic RCC who had progressed on antiangiogenic therapy [Citation15]. Statistically significant improvements in OS (25.0 vs 19.6months) and ORR (25 vs 5%) were observed for nivolumab compared with everolimus [Citation15]. In the nivolumab arm, 19% of participants experienced a grade 3 or 4 AE, the most common of which were fatigue and anemia (2% each) [Citation15]. In short, the majority of patients (75% or more) do not have an objective response to the current primary recommended therapies after first-line treatment, and there is an urgent unmet need for more efficacious treatment regimens.
LITESPARK-011 study
Herein we describe the rationale and design for the phase III LITESPARK-011 study (NCT04586231), which will evaluate the efficacy and safety of the HIF-2αinhibitor belzutifan (MK-6482) plus the TKI lenvatinib versus cabozantinib alone in patients with advanced clear cell RCC who experienced disease progression on or after anti-PD-1/PD-L1 therapy.
Background & rationale
Approximately 90% of clear cell RCCs result in the loss of function of the Von Hippel-Lindau (VHL) tumor suppressor gene, leading to accumulating HIF-2α and promoting tumor growth [Citation16]. HIF-2α induces genes associated with angiogenesis, invasion, metastasis, resistance to the immune system, and the cell survival mechanism [Citation13]. A phase I, dose-escalation study of the first-in-class oral HIF-2α inhibitor PT2385 in 51 patients with advanced clear cell RCC previously treated with VEGF inhibitor had a manageable safety profile, with 41% of patients experiencing grade 3 or 4 treatment-emergent AEs. The most common grade 3 AEs were anemia (10%), hypoxia (10%) and hypophosphatemia (8%); grade 4 events were lymphocyte count decreased (n = 2) and hypercalcemia (n = 1) and pulmonary embolism (n = 1)[Citation17]. One patient had a complete response (2%), and 6 patients (12%) had a partial response [Citation17]. Plasma erythropoietin levels were decreased at all doses evaluated (100–1800mg, twice daily) [Citation17]. Although promising, PT2385 had highly variable pharmacokinetics (PK), leaving the potential for underexposure of patients, and thus next-generation inhibitors of HIF-2α were explored [Citation13].
Belzutifan (MK-6482) is an orally available, smallmolecule inhibitor of HIF-2α that selectively disrupts the heterodimerization of HIF-2α with HIF-1β [Citation13]. Belzutifan was also evaluated in a phase I/II dose-escalation/expansion study LITESPARK-001 (also known as PT2977-101; ClinicalTrials.gov identifier, NCT02974738) [Citation18]. The dose-escalation phase was evaluated in patients with solid tumors (n=43); the expansion phase was evaluated in previously treated advanced clear cell RCC (n=52). The maximum tolerated dose was not reached at doses up to 240mg per day, including 240mg once daily and 120mg twice daily. In the dose escalation phase, patients with advanced clear cell RCC received the recommended phase II dose of 120mg belzutifan orally once daily [Citation18]; this dose choice was supported because exposure to belzutifan did not markedly increase at doses higher than 120mg once daily [Citation19]. Belzutifan exposure at all dose levels was associated with reductions in erythropoietin [Citation18], a sensitive pharmacodynamic marker of HIF-2α inhibition [Citation20,Citation21]. An RCC cohort (n = 55) consisted of threepatients with clear cell RCC in the dose escalation phase treated with belzutifan 120 mg once daily and all 53 patients in the dose expansion phase. In the RCC cohort, the most common grade 3 AEs were anemia (27%) and hypoxia (16%); twopatients experienced four grade 4 AEs (sepsis [n = 2], hypercalcemia [n = 1], and respiratory failure [n = 1]), and fourpatients experienced grade 5 AEs (disease progression, malignant neoplasm progression, acute kidney injury, and cardiac arrest). No grade 4 or 5 AEs were considered related to treatment. These toxicities are expected with treatment using an HIF-2α inhibitor [Citation20–22]; anemia was treated with erythropoiesis-stimulating agent and/or blood transfusion, and hypoxia was managed with supplemental oxygen and treating other concomitant confounding conditions. The ORR in theRCC cohort was 25% (all partial responses), and median PFS was 14.5months [Citation18]. Belzutifan demonstrated preliminary antitumor activity in heavily pretreated patients [Citation18], suggesting that HIF-2α inhibition may offer an effective treatment for advanced clear cell RCC.
Lenvatinib inhibits the kinase activities of VEGF receptors VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4) [Citation23,Citation24]. In addition, lenvatinib inhibits FGF receptors FGFR1, 2, 3, and 4; PDGFRα; KIT; and RET [Citation23,Citation24]. This inhibition results in arrest of the neo-vessel assembly and maturation, decreasing vascular permeability of the tumor microenvironment [Citation24]. Lenvatinib is currently approved in combination with everolimus for the treatment of advanced RCC after one antiangiogenic therapy, and in combination with pembrolizumab for the first-line treatment of advanced RCC [Citation23]. Lenvatinib, everolimus, and the combination was evaluated in a phase II, randomized, open-label study of 153 patients with advanced RCC [Citation25]. Per blinded, independent radiologic review, the combination of lenvatinib and everolimus significantly improved median PFS compared with everolimus (12.8 vs 5.6months) but not lenvatinib (9months) [Citation26]; ORRs were 35, 0 and 39%, respectively [Citation26]. Participants who received lenvatinib plus everolimus (71%) or lenvatinib alone (79%) reported more grade 3 or 4 AEs compared with everolimus alone (50%); the most common grade 3/4 AEs in recipients of lenvatinib plus everolimus and lenvatinib alone were constipation, diarrhea, proteinuria, hypertension, and fatigue or asthenia [Citation25]. A phase Ib/II study of lenvatinib plus pembrolizumab in 104 participants with advanced RCC who progressed during or after immune checkpoint inhibitor therapy had an ORR of 51%; the most common treatment-related AEs in the entire study were fatigue, diarrhea, proteinuria, hypertension, nausea, dysphonia, stomatitis and arthralgia [Citation27]. Thus, lenvatinib in combination with another targeted therapy appears to increase ORR compared with currently recommended agents [Citation27,Citation28].
Given that HIF-2α activation represents a resistance pathway for anti-VEGF therapy, it is hypothesized that combining belzutifan and lenvatinib will lead to repression of HIF-2α-regulated VEGF production at the level of transcription by belzutifan, and inhibition of VEGF production downstream of HIF-1α by lenvatinib at the growth factor level [Citation29]. An analysis of the phase II study of belzutifan plus cabozantinib in patients with advanced clear cell RCC demonstrated an ORR of 31% with a median PFS of 13.8months in 52 patients who had ≥1 dose of treatment[Citation30]. For all patients (N=52), the most common (≥10%) grade 3 treatment-related AEs were hypertension (27%), anemia (15%) and fatigue (12%); two patients experienced grade 3 hypoxia (4%) [Citation30]. Nograde 4 or 5 treatment-related AEs were reported [Citation30]. Therefore, the combination ofbelzutifan pluslenvatinib is an attractive therapeutic intervention for patients with advanced RCC.
Design
This is a phase III, open-label, multicenter, international, randomized, active-controlled study in patients with advanced clear cell RCC. The study will enroll approximately 708 adults with advanced clear cell RCC who experienced disease progression on or after an anti-PD-1/PD-L1 therapy as either first- or second-line treatment for locally advanced/metastatic RCC or as adjuvant treatment with progression on or within 6months of the last dose. The immediately preceding line of treatment must have been an anti-PD-1/PD-L1 therapy, with no more than twoprior systemic regimens permitted. Also, no more than one anti-PD-1/PD-L1 therapy for adjuvant or locally advanced/metastatic clear cell RCC is allowed.
Patients
Eligible patients () will be randomly assigned 1:1 to receive either 120mg oral belzutifan plus 20mg oral lenvatinib once daily or 60mg oral cabozantinib once daily; 354 patients will be included in each treatment arm (). At randomization, patients will be stratified based on International Metastatic RCC Database Consortium (IMDC) prognostic scores (0 vs 1–2 vs 3–6) [Citation31,Citation32], line of treatment in which anti-PD-1/PD-L1 therapy was given (1 [adjuvant, neoadjuvant/adjuvant, or first-line] vs 2 [second-line]), and geographic region (North America, Western Europe or rest of world). Study treatment will continue until documented disease progression, start of a new anticancer treatment, unacceptable toxicityor patient withdrawal.
aIncluding 1 anti-PD-1/PD-L1-containing adjuvant or neoadjuvant/adjuvant regimens with progression on or within 6months from the last dose of that regimen OR 1 or 2 regimens for locoregional/advanced disease.
DOR: Duration-of-response; IMDC: International Metastatic Renal Cell Carcinoma Database Consortium; KPS: Karnofsky Performance Status Scale; ORR: Objective response rate; OS: Overall survival; PD-1: Programmed death 1; PD-L1: Programmed death ligand 1; PFS: Progression-free survival; Q8W: Every 8 weeks; Q12W: Every 12 weeks; QD: Once daily; R: Randomization; RCC: Renal cell carcinoma; RECIST 1.1: Response Evaluation Criteria in Solid Tumors, version 1.1; ROW: Rest of world.
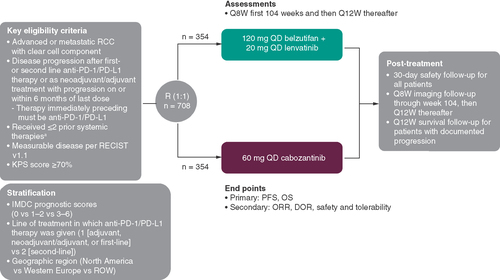
Table 1. Patient eligibility criteria.
Outcomes
The dual primary end points of this study are to evaluate the efficacy of belzutifan plus lenvatinib compared with cabozantinib for the treatment of advanced RCC as assessed by PFS as determined by blinded independent central review (BICR) per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1), and by OS. A meta-analysis of 31 studies (including 10,943 patients) evaluated the relationship between PFS and OS in metastatic RCC and concluded that in RCC, the treatment effects on PFS are strongly associated with treatment effects on OS [Citation33]. To ensure that PFS is rigorously evaluated, PFS will be determined by a BICR that will review all radiographic studies. The BICR will be blinded to study treatment. Safety and tolerability end points include but are not limited to the incidence of, causality, and outcome of AEs/serious AEs and changes in vital signs and laboratory values. AEs will be assessed as defined by Common Terminology Criteria for Adverse Events (CTCAE) v5.0. The open-label study design enables appropriate dose modifications for AEs in both study intervention treatment groups. Belzutifan, lenvatiniband cabozantinib each have unique safety profiles that may result in the participants experiencing different AEs that could disclose the treatment intervention received if the study were blinded.
Secondary end points include ORR and duration-of-response, as evaluated by RECIST 1.1, and safety and tolerability.
PK and pharmacodynamic end points include analysis of plasma concentrations of belzutifan. The PK of lenvatinib will not be analyzed in this study because a drug–drug interaction effect is not anticipated from belzutifan on lenvatinib PK, and lenvatinib dose modification will be based on observed toxicity. The effect of belzutifan on erythropoietin levels will be assessed using descriptive statistics.
Exploratory end points including health-related quality of life and disease-related symptoms will be investigated among all participants using the Functional Assessment of Cancer Therapy Kidney Cancer Symptom Index-Disease Related Symptoms (FKSI-DRS), European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30), and EuroQoL EQ-5D-5L questionnaires. Exploratory biomarker analyses include biospecimen (including blood components and tumor material) collection to support analysis of cellular components (e.g., protein, DNA, RNAand metabolites) and other circulating molecules.
Procedures
In the treatment phase, all patients will have weekly visits for the first 3weeks, then visits will be every 2weeks through week 9, and then every 4weeks thereafter. In addition to those visits, approximately the first 15 patients randomly assigned to each arm and treated (30 total) will have a visit at week 4, day 1 for safety assessments. The data from this visit will be reviewed by an external Data Monitoring Committee to provide recommendations on the conduct of the study should significant and/or new safety signals potentially associated with belzutifan plus lenvatinib be identified. These participants will be included in the intention-to-treat population for efficacy analyses.
Radiologic evaluation by computed tomography (CT) or MRI of the abdomen and pelvis with IV contrast (unless medically contraindicated) will occur every 8weeks for 80weeks, after which imaging will continue every 12weeks for patients who remain on the study drug until week 104. If the patient has a positive baseline bone scan at screening, after randomization bone imaging will be performed at week 16 and will continue every 16weeks through week 80, then every 24weeks to week 104 until disease progression is verified by BICR. A brain scan (MRIor CT) will be performed during screening for participants with brain metastases to ensure the participant’s condition is stable. After randomization, brain imaging will be performed as clinically indicated and to confirm complete response in patients with brain metastases at baseline.
AEs will be monitored during the study and graded in severity per CTCAE, version 5.0. AEs will be reported for 30days after cessation of study treatment. Serious AEs will be reported for 90days after cessation of study treatment or for 30days if the patient begins a new anticancer therapy regimen (whichever is earlier). A post-treatment safety follow-up visit will occur within 30days after discontinuation of study treatment.
Patient-reported outcomes (PRO) questionnaires will be administered every 2weeks during treatment up to week 13, when they will shift to every 4weeks. Every effort will be made to administer PRO surveys on site before dosing and before other assessments and procedures. The electronic PRO assessments will continue for up to 2years from randomization (week 105) or until safety follow-up after end-of-treatment.
Blood for PK analyses will be drawn from participants randomly assigned to the belzutifan plus lenvatinib arm at weeks 1, 2, 3 and 5. Blood draws for pharmacodynamic analyses and experimental biomarker analyses will be drawn before dose at weeks 1, 3 and 5 and at time of discontinuation; serum biomarker analyses in patients randomly allocated to belzutifan plus lenvatinib will also have postdose collections at 1, 2 and 4h following week 1 dose.
Statistics
This study is ongoing and the planned sample size is approximately 708 patients. The efficacy analyses will be performed in the intention-to-treat population (all randomly assigned patients). The primary end points of PFS and OS will be evaluated using a stratified log-rank test and will be controlled for family wise type 1 error. The hazard ratio will be estimated using a stratified Cox proportional hazard model, and survival probabilities over time will be estimated within each treatment group using the Kaplan–Meier method. The secondary end point of ORR will be analyzed using the stratified Miettinen and Nurminen method [Citation34], with strata weighted by sample size.
The safety analysis will be performed for the all-patients-as-treated population (all randomly assigned patients who receive ≥1 dose of study treatment). The safety results will be summarized by treatment group and number and percentage of AEs. The Miettinen and Nurminen method will be used to perform analyses in which 95% CIs will be provided for between-treatment differences in the percentages of patients who experienced events.
Conclusion
Here we described a phase III, open-label, multicenter, randomized, active-controlled study (LITESPARK-011; NCT04586231) designed to compare the efficacy and safety of belzutifan plus lenvatinib with that of cabozantinib alone in patients with advanced clear cell RCC who experienced disease progression on or after anti-PD-1/PD-L1 therapy. Given the high risk of disease progression in patients with advanced clear cell RCC, there is an unmet medical need for more effective and tolerable treatment options; a positive benefit/risk profile is expected for belzutifan across various tumor types.
Effective treatment options are limited for patients with advanced clear cell renal cell carcinoma (RCC) whose disease progresses after anti-programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) therapy.
Rationale
By combining the HIF-2αinhibitor belzutifan and the VEGF inhibitor lenvatinib, it is hypothesized that VEGF production regulated by HIF-2α will be repressed at the level of transcription by belzutifan, and production of VEGF downstream of HIF-1α will be inhibited by lenvatinib at the growth factor receptor level.
In addition, as HIF-2α drives tumor cell expression of several oncogenes in clear cell RCC, VEGF being just one of them, the combination could inhibit multiple oncogenic signaling pathways involved in initiation, progressionand metastasis.
LITESPARK-011 study design & eligibility criteria
The LITESPARK-011 study is a randomized, phase III study evaluating efficacy and safety of belzutifan plus the VEGF inhibitor lenvatinib versus the tyrosine kinase inhibitor cabozantinib in patients with advanced clear cell RCC whose disease progresses after anti-PD-1/PD-L1 therapy.
Approximately 708 patients will be randomly assigned 1:1 to receive either 120 mg oral belzutifan plus 20 mg oral lenvatinib once daily or 60 mg oral cabozantinib once daily.
Randomization will be stratified based on International Metastatic Renal Cell Carcinoma Database Consortium prognostic scores (0 vs 1–2 vs 3–6), line of treatment in which anti-PD-1/PD-L1 therapy was given (1 [adjuvant, neoadjuvant/adjuvant, or first-line] vs 2 [second-line]), and geographic region (North America, Western Europeor rest of world).
Outcomes measures/end points
The dual primary end points are progression-free survival as determined by blinded independent central review per RECIST 1.1, and by overall survival; secondary end points include objective response rate, duration-of-response, and safety and tolerability.
Conclusion
Results from this study may indicate more effective treatments for advanced clear cell RCC following treatment with immune checkpoint inhibitors.
Author contributions
RJ Motzer: conception, design or planning of the study and drafting the manuscript. M Schmidinger: acquisition of the data, interpretation of the results and drafting the manuscript. M Eto: acquisition of the data. C Suarez: conception, design or planning of the study, analysis and acquisition of the data and interpretation of the results. R Figlin: conception, design or planning of the study, analysis of the data, interpretation of the results and drafting of the manuscript. Y Liu: conception, design or planning of the study and acquisition of the data. R Perini: conception, design or planning of the study, analysis and acquisition of the data and interpretation of the results. Y Zhang: conception, design or planning of the study, analysis of the data and interpretation of the result. DYC Heng: conception, design or planning of the study. All authors critically reviewed and/or revised the manuscript for important intellectual content and approved the final manuscript for submission.
Ethical conduct of research
The investigator or medically qualified designee (consistent with local requirements) will obtain documented consent from each potential participant or each participant’s legally acceptable representative prior to their participation in the study. MSD clinical trials are conducted in compliance with local and/or national regulations (including all applicable data protection laws and regulations), and International Council for Harmonisation Good Clinical Practice (ICH-GCP), and also in accordance with the ethical principles that have their origin in the Declaration of Helsinki.
Data sharing statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Acknowledgments
The authors thank the patients and their families and investigators and site personnel. Patients treated at Memorial Sloan Kettering Cancer Center are supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (NJ, USA) and Eisai Inc. (NJ, USA).
Financial & competing interests disclosure
RJ Motzer has received funding for this manuscript in the form of consultant/advisory board (grant support to employer, MSKCC) from Merck, grant support from Eisai, Exelixis, Pfizer, Genentech/Roche, Novartis and Aveo (grant support to employer, MSKCC) and consulting fees from Exelixis, Eisai, Pfizer, Genentech/Roche, EMD Serome, Calithera and Aveo (grant support to employer, MSKCC). M Schmidinger has received grants or contracts in the form of honoraria from Ipsen, Exelixis, EUSA, Eisai, MSD, Bristol Myers Squibb, Merck, Astellas, Janssen, Alkermes and Pfizer; has received consulting fees from advisory boards from Ipsen, Exelixis, EUSA, Eisai, MSD, Bristol Myers Squibb, Merck, Astellas, Janssen and Alkermes; has received honoraria from Ipsen, Exelixis, EUSA, Eisai, MSD, Bristol Myers Squibb, Merck and Janssen; has received meeting support from Ipsen, Bristol Myers Squibb, Pfizer and Roche; and has participated in a data safety monitoring board for Ipsen, Exelixis, Eisai, MSD, Merck and Bristol Myers Squib. M Eto received research funding (institution) from Sanofi, Bayer, Astellas, ONO and Takeda and has received honoraria from ONO, Takeda, Novartis, Pfizer, Bristol Myers Squibb, Janssen, MSD, Merck, AstraZeneca and Eisai. C Suarez received research grants (self) from Ipsen; consulting fees from Bristol Myers Squibb (institution), Pfizer, Roche, Astellas, Ipsen, Sanofi, Bayer and MSD; honoraria from Bristol Myers Squibb (institution), Pfizer, Roche, Astellas and Ipsen; payment for expert testimony from Bristol Myers Squibb (institution), Pfizer, Roche, Astellas and Ipsen; meeting support from Bristol Myers Squibb (institution), Pfizer and Roche. R Figlin received funding for this manuscript and research funding (institution) from Merck. Y Liu is an employee of Merck Sharp and Dohme, LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. R Perini is an employee of Merck Sharp and Dohme, LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and a stockholder of Merck & Co., Inc., Rahway, NJ, USA. Y Zhang is an employee of Merck Sharp and Dohme, LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. DYC Heng has received consulting fees and honoraria from Merck, Bristol Myers Squibb, Novartis, Pfizer, Eisai and Ipsen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing and/or editorial assistance was provided by JM Kulak and M Grzywacz of ApotheCom (PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (NJ, USA), and Eisai Inc. (NJ, USA).
Additional information
Funding
References
- National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology (NCCN guidelines): kidney cancer (version 2.2022) (2021). www.nccn.org/patients/guidelines/content/PDF/kidney-patient.pdfAccessed 26July2022).
- Howlader N , NooneAM, KrapchoMet al. SEER cancer statistics review, 1975–2016 (2020). https://seer.cancer.gov/archive/csr/1975_2016/ (Accessed 26July2022).
- Escudier B , PortaC, SchmidingerMet al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.30(5), 706–720 (2019).
- Powles T , AlbigesL, BexAet al. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann. Oncol.32(12), 1511–1519 (2021).
- Motzer RJ , RiniBI, McDermottDFet al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol.20(10), 1370–1385 (2019).
- Motzer RJ , TannirNM, McdermottDFet al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma.N. Engl. J. Med.378(14), 1277–1290 (2018).
- Motzer RJ , PenkovK, HaanenJet al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma.N. Engl. J. Med.380(12), 1103–1115 (2019).
- Choueiri TK , MotzerRJ, RiniBIet al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol.31(8), 1030–1039 (2020).
- Rini BI , PlimackER, StusVet al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma.N. Eng. J. Med.380(12), 1116–1127 (2019).
- Powles T , PlimackER, SoulièresDet al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol.21(12), 1563–1573 (2020).
- Motzer R , AlekseevB, RhaSYet al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma.N. Engl. J. Med.384(14), 1289–1300 (2021).
- Choueiri TK , PowlesT, BurottoMet al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma.N. Engl. J. Med.384(9), 829–841 (2021).
- Choueiri TK , KaelinWG. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat. Med.26(10), 1519–1530 (2020).
- Choueiri TK , EscudierB, PowlesTet al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol.17(7), 917–927 (2016).
- Motzer RJ , EscudierB, McDermottDFet al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med.373(19), 1803–1813 (2015).
- Linehan WM , RickettsCJ. The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat. Rev. Urol.16(9), 539–552 (2019).
- Courtney KD , InfanteJR, LamETet al. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2α antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J. Clin. Oncol.36(9), 867–874 (2018).
- Choueiri TK , BauerTM, PapadopoulosKPet al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat. Med.27(5), 802–805 (2021).
- Xu R , WangK, RizziJPet al. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a hypoxia-inducible factor 2α (HIF-2α) inhibitor for the treatment of clear cell renal cell carcinoma. J. Med. Chem.62(15), 6876–6893 (2019).
- Rankin EB , BijuMP, LiuQet al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J. Clin. Invest.117(4), 1068–1077 (2007).
- Scortegagna M , DingK, ZhangQet al. HIF-2α regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood105(8), 3133–3140 (2005).
- Dai Z , ZhuMM, PengYet al. Therapeutic targeting of vascular remodeling and right heart failure in pulmonary arterial hypertension with a HIF-2α inhibitor. Am. J. Respir. Crit. Care Med.198(11), 1423–1434 (2018).
- Lenvima® (lenvatinib) capsules, for oral use. 02/2020. Eisai Inc NJ, USA (2020).
- Capozzi M , DeDivitiis C, OttaianoAet al. Lenvatinib, a molecule with versatile application: from preclinical evidence to future development in anti-cancer treatment. Cancer Manag. Res.11, 3847–3860 (2019).
- Motzer RJ , HutsonTE, GlenHet al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol.16(15), 1473–1482 (2015).
- Motzer RJ , HutsonTE, RenM, DutcusC, LarkinJ. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol.17(1), e4–e5 (2016).
- Lee CH , ShahAY, HsiehJJet al. Phase II trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) for disease progression after PD-1/PD-L1 immune checkpoint inhibitor (ICI) in metastatic clear cell renal cell carcinoma (mccRCC). J. Clin. Oncol.38(Suppl. 15), 5008 (2020).
- Taylor MH , LeeCH, MakkerVet al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J. Clin. Oncol.38(11), 1154–1163 (2020).
- Zhao D , ZhaiB, HeCet al. Upregulation of HIF-2α induced by sorafenib contributes to the resistance by activating the TGF-α/EGFR pathway in hepatocellular carcinoma cells. Cell. Signal.26(5), 1039 (2014).
- McDermott DF , ChoueiriTK, TykodiSet al. Phase II study of belzutifan plus cabozantinib for previously treated advanced renal cell carcinoma (RCC): Update from cohort 2 of LITESPARK-003. Ann. Oncol.33, S1208–S1209 (2022).
- Heng DY , XieW, ReganMMet al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J. Clin. Oncol.27(34), 5794–5799 (2009).
- Heng DY , XieW, ReganMMet al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol.14(2), 141–148 (2013).
- Delea TE , KhuuA, HengDY, HaasT, SoulieresD. Association between treatment effects on disease progression end points and overall survival in clinical studies of patients with metastatic renal cell carcinoma. Br. J. Cancer107(7), 1059–1068 (2012).
- Miettinen O , NurminenM. Comparative analysis of two rates. Stat. Med.4(2), 213–226 (1985).
