Abstract
Background: This study investigated real-world treatment patterns and overall survival (OS) in early non-small-cell lung cancer patients and the association between OS and time-to-adjuvant-treatment. Materials & methods: This retrospective study using Surveillance, Epidemiology and End Results data linked with Medicare claims included resected early non-small-cell lung cancer patients between 2010 and 2015. Unadjusted OS analyses used Kaplan–Meier curves; adjusted OS analyses used extended Cox proportional hazards models. Results: Only 54–71% of stage II–IIIA patients received any adjuvant treatment. Adjusted risk of death was higher when starting treatment outside 6–8weeks after surgery (p<0.05). Conclusion: Improved systemic therapy in the adjuvant chemotherapy setting is needed.
Plain language summary
Lung cancer is one of the deadliest cancers in the USA. Most lung cancers are a type called non-small-cell lung cancer (NSCLC). Patients with NSCLC that has not spread to other parts of the body generally have surgery and may receive treatment before surgery, after surgery or both to help fight the cancer. It is not clear how often people receive treatment before or after surgery. It is important to know how patients are being treated because it helps clinicians decide how to use the new treatments that are becoming available. This study used a large database of more than 7000 people aged 65years and older with lung cancer in the USA to understand how they are treated. More than a third of patients had stage IA NSCLC (39%), followed by stage IB (24%), stage II (20%), stage IIIA (15%) and stage IIIB (2%). Most people had surgery (64%) and some received another treatment after surgery (27%). That treatment was most often about 2months of chemotherapy, on average. The study also tried to understand how the timing of treatment may have been important for their survival. People who received treatment after surgery lived the longest if they received that treatment about 6–8weeks after the surgery. Overall, the study showed that a substantial proportion of people do not receive treatment for their NSCLC after surgery, even though treatment after surgery is recommended by medical guidelines. There is a need for more effective treatments for these patients, and when those treatments are given may be important for their survival.
Tweetable abstract
Only 54–71% of US Medicare patients with stage II-IIIA NSCLC receive any adjuvant treatment. Chances of survival are best when treatment is given 6–8 weeks after surgery. Improved systemic therapy is needed in the adjuvant chemotherapy setting for people with early NSCLC.
Lung cancer remains one of the leading causes of cancer-related mortality, and non-small-cell lung cancer (NSCLC) accounts for the majority (>75%) of all lung cancer cases in the USA [Citation1]. Patients with early NSCLC (eNSCLC), defined as stage I, stage II or resectable stage III disease, comprise more than half of all patients with NSCLC at diagnosis [Citation2]. The estimated 5-year survival for patients with eNSCLC ranges from approximately 92% for those with stage IA disease to 36% for those with stage IIIA disease [Citation3,Citation4].
Surgical resection is the primary treatment approach for operable patients with resectable eNSCLC, and clinical practice guidelines have historically recommended adjuvant chemotherapy as the standard of care for eNSCLC patients with metastatic lymph node disease [Citation5]. Adjuvant chemotherapy has shown modest overall survival (OS) benefits for patients with eNSCLC in clinical trials [Citation6], but recurrence rates remain high [Citation7–10]. Real-world use of chemotherapy in stage II–III disease has been shown to be low in a national hospital registry study, suggesting potential gaps in care for these patients [Citation11]. However, with recent US FDA approval of immunotherapy and targeted therapy for eNSCLC [Citation12,Citation13], a greater understanding of real-world treatment patterns and survival outcomes among eNSCLC patients would provide insight into how these new treatments would build upon the standard of care. Additionally, it would improve our ability to measure the future potential impact of newer treatments for eNSCLC on patient outcomes by establishing a benchmark in the literature. Real-world treatment patterns may be of particular interest for elderly patients due to generally advanced age and related clinical considerations. Furthermore, given that elderly patients may have lower tolerance to chemotherapy, a better understanding of outcomes associated with delays in the administration of adjuvant chemotherapy would have implications for clinical practice.
This study investigated real-world treatment patterns in a US Medicare population of patients with eNSCLC who had undergone surgical resection. The Medicare population is comprised of adults aged ≥65years who receive primary reimbursement of healthcare services through the national US Medicare program. The study also assessed the time from surgery to adjuvant treatment initiation and the association between adjuvant treatment timing and OS.
Materials & methods
This retrospective observational study used the 2010–2015 Surveillance, Epidemiology and End Results (SEER) registry data linked with 2010–2016 Medicare beneficiary claims data. The SEER-Medicare database comprises information from the SEER cancer registries (demographic, clinical and cause of death information) reported by healthcare providers in the USA and accounts for approximately 35% of the US population [Citation14]. Compared with the total US population, the population covered by the SEER program is more racially and ethnically diverse and includes more people who are economically disadvantaged. Among patients in the SEER registry, about 60% are linked to insurance claims for US Medicare beneficiaries with cancer (adults aged ≥65years; National Cancer Institute; healthcaredelivery.cancer.gov/seermedicare/overview/) [Citation14].
Study population
Eligible patients had to have a first and only NSCLC diagnosis (International Classification of Diseases for Oncology [ICD-O] histology codes 8000–8040, 8046–9989) identified between 2010 and 2015 from SEER data. This coding was based on SEER guidance for histology codes (seer.cancer.gov/icd-o-3/sitetype.icdo3.20220429.pdf) and has been used in previous studies to identify patients with NSCLC [Citation15]. Patients with a diagnosis of small-cell lung cancer (ICD-O histology codes 8041–8045), large-cell carcinoma (ICD-O histology codes 8012–8014) or neuroendocrine/carcinoid tumors (ICD-O histology codes 8240–8246, 8249) were excluded.
Eligible patients also had to have stage IA–IIIB cancer from SEER data, be at least 65years of age at the time of diagnosis and have continuous Medicare Parts A and B for at least 7months before and at least 12months after the index date and at least 6months after surgery to ensure adequate capture of baseline patient characteristics and outcomes of interest. Medicare Part D enrollment was required during the month prior to the index date and for 12months after the index date or death, whichever occurred first, to identify treatment. The index date was defined as the initial NSCLC diagnosis date, which was identified using the SEER registry reported diagnosis month and the earliest Medicare claim date with a lung cancer diagnosis code occurring in the SEER reported diagnosis month. If no diagnosis date was available from Medicare claims, the day of diagnosis was set as the 15th day of the diagnosis month from SEER. Medicare Parts A and B together provide health insurance coverage for inpatient and outpatient care, including surgery, examinations, laboratory tests and other services. Medicare Part D provides coverage for prescription medications.
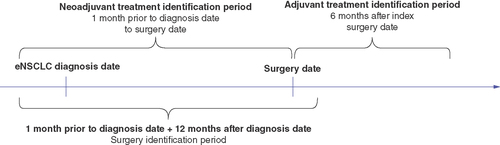
In addition, patients were included if they had surgery procedure codes in the claims data during the surgery identification period, which began 1month prior to the index date and ended 12months after the index date or death, whichever occurred first (). The index surgery date was defined as the first surgery date during the surgery identification period ().
eNSCLC: Early NSCLC.
Patients were excluded if they had missing or inconsistent data on diagnosis month or staging information or other missing or inconsistent data from SEER. Patients enrolled in a health maintenance organization plan or with military coverage were excluded due to the potential for missing claims data, as Medicare files do not include insurance claims data on managed care and Veterans Administration enrollees.
Variables & outcomes
The demographic and clinical characteristics based on SEER registry data included age at diagnosis, sex, race/ethnicity, region, urban or rural residence, NSCLC diagnostic confirmation (microscopically confirmed or unconfirmed), derived American Joint Committee on Cancer (AJCC7th edition) stage [Citation16] and histology. Other characteristics derived from Medicare claims included Charlson Comorbidity Index (CCI) score [Citation17], smoking history, pneumonitis, interstitial lung disease and chronic obstructive pulmonary disease.
Treatment patterns were analyzed for the overall study population and by disease stage for each treatment cohort. Treatment categories included surgery only, neoadjuvant treatment only, adjuvant treatment only and neoadjuvant and adjuvant treatment. The time period used to identify adjuvant treatment in the database started from the index surgery date (plus 1day) up to 6months after the index surgery date (6months after surgery, inclusive). The time period used to identify neoadjuvant treatment went from 1month before the index diagnosis date up to the index surgery date (6months before the index surgery, not including the surgery date). Surgical procedures and treatments in the identification periods are provided in Supplementary Table 1.
Each treatment cohort was defined according to the following criteria. The surgery-only cohort excluded patients with any nonsurgical treatment (radiation, stereotactic body radiation therapy, chemotherapy, immunotherapy, targeted therapy, ablation) anytime during the neoadjuvant or adjuvant treatment identification periods. The adjuvant treatment cohort included patients with any nonsurgical treatment during the adjuvant treatment identification period and excluded patients with any nonsurgical treatment during the neoadjuvant treatment identification period. The neoadjuvant treatment cohort included patients with any nonsurgical treatment based on claims data during the neoadjuvant treatment identification period and excluded patients with any nonsurgical treatment during the adjuvant treatment identification period. The neoadjuvant plus adjuvant treatment cohort included patients with any nonsurgical treatment during the neoadjuvant and adjuvant treatment identification periods.
Treatment durations for neoadjuvant and adjuvant treatments were calculated as the difference in days between the start and end dates for patients who received chemotherapy during the neoadjuvant or adjuvant treatment period. The neoadjuvant and adjuvant treatment start dates were based on the first identified treatment date during the neoadjuvant and adjuvant treatment identification period, respectively. End dates were identified as the last fill date of a regimen that had been discontinued (where a gap of more than 120days was considered discontinuation), disenrollment, death or the end of the study period, whichever came earliest.
The OS for patients receiving adjuvant treatment was calculated from the start of adjuvant treatment until death from any cause. OS was not evaluated for patients who did not receive any adjuvant treatment. The association between OS and time to adjuvant treatment, which was calculated as the time from surgery to initiation of adjuvant treatment, was assessed. Patients were categorized by the time from the surgery index date to initiation of adjuvant treatment as <3weeks, 3 to <6weeks, 6 to <8weeks, 8 to <16weeks or ≥16weeks.
Statistical analysis
Descriptive statistics were used to assess patient demographic and clinical characteristics and treatment patterns. Median (interquartile range [IQR]) was reported for continuous variables, and counts and percentages were reported for binary and categorical variables. The impact of the timing of adjuvant treatment initiation on OS was evaluated in time-to-event analyses. Unadjusted OS was described with Kaplan–Meier curves. Multivariate models controlling for observable differences between groups were used for the adjusted OS analysis. Extended Cox proportional hazards models (accounting for variables that did not meet the proportional hazards assumption) were used to control for demographic and clinical characteristics and adjuvant treatment (chemotherapy, radiation, systemic treatment). The models were adjusted for age at diagnosis, sex, race, region, urban/rural status, baseline smoking history, baseline CCI score, stage, grade, histology, diagnostic confirmation method, positive lymph node resection, postoperative complications, first-line adjuvant treatment type (platinum-based chemotherapy vs other), US Census Tract median income and US Census Tract proportion of patients with completed high school education. Postoperative complications included atrial arrhythmia, chest tube for ≥7days, air leak/bronchopleural fistula ≥5days, atelectasis, respiratory failure, pneumonia, hemorrhage, myocardial infarction, empyema, acute respiratory distress syndrome, chylothorax, bronchopleural fistulas, pleural effusion, pulmonary embolus, pneumothorax requiring chest tube reinsertion, ventilator support and tracheotomy. The 6- to <8-week subgroup was used as the reference group based on prior studies [Citation18,Citation19] and clinical practice in the USA. To address nonproportional hazards, the follow-up period was divided into three intervals (<12, 12–30 and >30months), and one hazard ratio was estimated for each time interval. A sensitivity analysis was conducted using the primary model with the adjuvant treatment initiation timing variable included as a continuous variable. The nonlinearity was modeled with 4-knot restricted cubic splines. All analyses were conducted using RStudio (www.rstudio.com).
Results
A total of 7142 patients with eNSCLC with surgical resection were included in the analysis (patient attrition is provided in Supplementary Figure 1). The median age was 73years, and 56% were women (). Most patients were White (86%) and from metropolitan areas (84%), and 37% had AJCC stage II, IIIA or IIIB disease. The majority of patients had microscopically confirmed diagnosis (98%). Based on the first surgery, lobectomy was the most common extent of surgery, and the most common surgery type was thoracotomy. Approximately a third (34%) of the patients had a baseline CCI score ≥3, a third had chronic obstructive pulmonary disease (36%) and 22% had a history of smoking; few patients had pneumonitis (<1%) or interstitial lung disease (<1%).
Table 1. Characteristics of the overall and treatment cohorts with early non-small-cell lung cancer.
Treatment patterns
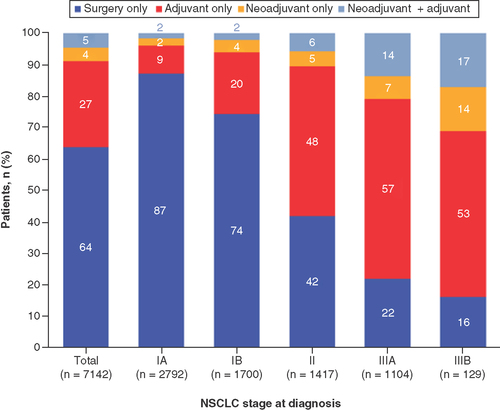
Overall, most patients had surgery only (64%), followed by adjuvant treatment only (27%), while 297 (4%) had neoadjuvant treatment and 335 (5%) had neoadjuvant plus adjuvant treatment (). Characteristics were generally similar across treatment subgroups, but with more advanced disease observed among those in the adjuvant and neoadjuvant plus adjuvant subgroups (36% and 52% were AJCC stage IIIA or IIIB, respectively; ).
Among patients who had received perioperative systemic therapy, adjuvant treatment was more common than neoadjuvant treatment; this was consistent across disease stages. Neoadjuvant and adjuvant treatment rates increased with stage. Of note, only 54% and 71% of patients with stage II or IIIA disease, respectively, received any adjuvant treatment either alone or in combination with neoadjuvant treatment.
Among patients with adjuvant or neoadjuvant plus adjuvant treatment, about 30% of patients were identified based on generic codes for chemotherapy administration. The median time from surgery to adjuvant treatment was 1.6months (IQR: 1.2–2.1months). The median adjuvant treatment duration was 2.1months (IQR: 1.4–2.5months), and the most common adjuvant treatment was carboplatin plus paclitaxel (32%) followed by carboplatin plus pemetrexed (16%). The most common adjuvant treatments among patients receiving any adjuvant treatment are provided in Supplementary Table 2. The breakdown of treatment types among patients receiving neoadjuvant or adjuvant therapies is provided in .
Table 2. Neoadjuvant and/or adjuvant therapy received.
Analysis of OS & timing of adjuvant treatment
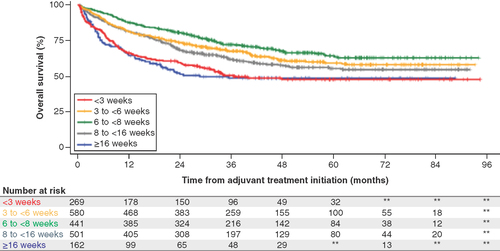
The median follow-up for the 7142 included patients was 39months (IQR: 25–42 months) and the 5-year survival rate was 68% (95% CI: 67, 69). Median survival rates were unavailable, since the median survival had not yet been reached by these patients. Unadjusted analysis of the impact of the timing of adjuvant treatment initiation on OS appeared to show better survival in patients receiving adjuvant treatment in the period 6–8weeks after surgery (). Adjuvant treatment initiation at <3weeks and >16weeks from resection had the worst OS and highest hazard ratio for death ( & ). After 36months, most patients were censored and thus the plateau of the survival curves on the right side may be partially due to not capturing the death due to censorship.
After 36months, most patients were censored and thus the plateau of the survival curves on the right side may be partially due to not capturing the death due to censorship.
**Suppressed cell values (1–10 patients) or those that may be used to calculate suppressed cell values according to the Centers for Medicare & Medicaid Services Cell Size Suppression Policy (https://resdac.org/articles/cms-cell-size-suppression-policy).
All hazard ratios are compared with the 6- to <8-week reference group: risk of death within 12months (n=441), 12–30months (n=385) and >30months (n=272). Variables included age (<70, 70–74, 75–79, 80–84, ≥85), gender (male, female), race/ethnicity (White, non-White), region (West, South, Northeast, Midwest), urban/rural status (metropolitan, urban, rural), baseline smoking history (yes, no), baseline Charlson Comorbidity Index score (excluding cancer, 0, 1, 2, 3+), derived American Joint Committee on Cancer stage group (7th; stage IA, stage IB, stage II, stage IIIA, stage IIIB), grade (1, 2, 3, 4, missing), histology (adenocarcinoma, not otherwise specified, squamous cell carcinoma, other), method of diagnostic confirmation (positive histology, positive cytology, other), lymph node positivity (yes, no, no nodes examined), total number of postop complications (0, 1–2, 3+), Census Tract median income (≤US$40,502, $40,503–$56,178, $56,179–$79,560, >$79,560) and Census Tract proportion with college education (≤20, 21–28, 29–36, >36).
*p<0.05.
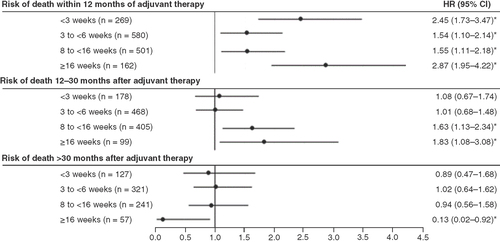
The OS model adjusting for demographic and clinical characteristics compared the risk of death in patients who initiated adjuvant treatment in the 6- to <8-week period after surgery versus each other period. After adjusting for confounding variables, the impact of time from surgery to adjuvant treatment initiation on the risk of death remained (). The adjusted risk of death within 12months of the initiation of adjuvant therapy was significantly higher in patients initiating treatment outside the 6- to <8-week period after surgery (). The same trends were observed for the risk of death within 12–30months, and the differences were significant only when the comparisons were made to patients initiating treatment more than 8weeks after surgery (). The results for adjusted risk of death after 30months of initiation of adjuvant therapy were mixed, with no significant differences in patients initiating treatment within 16weeks after surgery and significantly lower risk in patients initiating treatment more than 16weeks after surgery ().
Sensitivity analysis using the 4-knot restricted cubic splines showed consistent trends in the impact of adjuvant treatment initiation timing on risk of death (Supplementary Figure 2). Here the lowest risk of death within 30months was observed at approximately 50days, consistent with the 6- to <8-week window observed in the primary analysis.
Discussion
This retrospective observational study has provided an illustrative view of real-world treatment patterns from 2010 to 2015 among Medicare patients with resectable eNSCLC in the USA, before the approval and availability of immunotherapy. Despite clinical practice guidelines in the USA that define standards of care in stage II and III patients, a substantial proportion of patients with eNSCLC did not receive adjuvant systemic therapy. In this study, a significant number of patients with stage II (42%), IIIA (22%) and IIIB (16%) who underwent resection did not receive adjuvant or neoadjuvant systemic treatment (32% of stage II and III patients). This finding is consistent with a previous study showing that approximately 57% of stage II patients and 45% of stage IIIA patients received surgery only [Citation11].
The reason patients did not receive adjuvant therapy was not examined, since these data are unavailable in claims data. Regardless of the reason, these findings suggest a prominent unmet need for improved systemic therapy utilization in the neoadjuvant or adjuvant treatment setting for patients with eNSCLC. It may be important to identify barriers to adjuvant chemotherapy, and efforts should be made to ensure they do not prevent patients from receiving more effective immunotherapies given with adjuvant chemotherapy.
Adjuvant chemotherapy utilization may increase with newer therapeutic options with improved efficacy and toxicity profiles compared with conventional chemotherapy approved for eNSCLC (adjuvant atezolizumab and osimertinib). Significant disease-free survival was shown for stage II–IIIA patients with adjuvant atezolizumab in the IMpower010 trial (NCT02486718) [Citation12] and with adjuvant osimertinib in the ADAURA trial (NCT02511106) [Citation13]. Ongoing clinical trials for perioperative therapy may offer further treatment options for patients with eNSCLC, such as with atezolizumab (NCT02927301, NCT03456063), osimertinib (NCT03433469) and pembrolizumab (NCT04061590, NCT03425643).
This study provided important insights into OS in the context of the timing of adjuvant treatment initiation that may inform the timing of adjuvant treatment approaches and clinical trial design. OS was longest for patients who received adjuvant treatment 6 to <8weeks after surgery, and these findings were consistent after adjusting for potential confounding variables. These results are consistent with trends reported in other studies. Salazar et al. [Citation18] reported the lowest 5-year OS risk for patients receiving adjuvant treatment 50days after surgery (7.1weeks); 5-year OS was not expected to be worse when adjuvant treatment was received 57–127days compared with 39–56days after surgery. Wang et al. [Citation19] reported the highest 5-year survival rate (50%; median OS: 67.3months) among patients who received adjuvant treatment 46–60days after resection. Survival rate was reported to be worst when adjuvant chemotherapy was given more than 60days after resection. The relative ‘U-shaped’effect of adjuvant treatment timing observed in this study was similar to that reported by Wang et al., where survival rates were 41, 48, 50 and 35% in the postresection adjuvant treatment periods of <30, 30–45, 46–60 and >60days. The present study evaluated an elderly (Medicare) US population in a more recent period than that of Salazar et al. [Citation18] (hospital-based tumor registry, 2004–2012) or Wang et al. [Citation19] (Taiwan health system, 2004–2010), nonetheless highlighting a persistent unmet need for therapeutic advancement in this setting.
It is possible that other factors might have contributed in part to the worse survival in patients starting adjuvant treatment outside the 6- to <8-week window after surgery. Patients who started adjuvant therapy <3weeks after surgery likely had a more aggressive cancer and were a higher-risk population. From a clinical perspective, patients need time to recover after surgery, which may further explain poorer outcomes for those starting adjuvant treatment so soon (<3weeks) after surgery. The contribution of residual confounding to the poor outcomes associated with adjuvant therapy outside the 6- to 8-week window cannot be completely ruled out. While the adjusted OS analyses accounted for relevant variables available in this dataset, potential confounding from unobserved variables is an acknowledged limitation of database studies. Initiating adjuvant therapy ≥16weeks after surgery could be perceived as being too late to derive benefit. The authors also note that in the recently published IMpower010 study of atezolizumab after adjuvant chemotherapy, the observed time from surgery to adjuvant chemotherapy was approximately 1.8months, which is consistent with the 6- to <8-week window observed in this study [Citation20].
Patient age and comorbidities may impact the decision to administer adjuvant chemotherapy. The Lung Adjuvant Cisplatin Evaluation meta-analysis of adjuvant chemotherapy in NSCLC demonstrated no significant differences in severe toxicity (grade ≥4 adverse events 34–41%) or treatment-related deaths associated with age differences (0.7, 1.4and 1.9% and <65, 65–69 and ≥70years old, respectively; p=0.24) [Citation21]. However, elderly patients (≥70years) received significantly lower initial doses of cisplatin than younger patients (53% vs 71% receiving >95mg/m2; p<0.0001) [Citation21]. In contrast, the National Cancer Database report on the use of adjuvant chemotherapy following lung cancer resection in 19,691 patients revealed that the 6-month mortality rates associated with chemotherapy were 2.6, 3.1, 4.1, 5.3and 7.6% in patients aged ≤50, 51–60, 61–70, 71–80 and >80years, respectively (p<0.001) [Citation22]. In addition to age, the National Cancer Database data also showed that the Charlson–Deyo comorbidity score of 2 versus 0 (odds ratio: 1.52; 95% CI: 1.22, 1.89; p<0.001) was a significant independent predictor of adjuvant chemotherapy-associated mortality [Citation22]. In the present study, the median age was 73years (range: 79–77), and 34% of patients had a CCI score of ≥3. Both of these factors may contribute to the real-world data more meaningfully where performance status is balanced with stage-directed therapy outside of the context of the fittest patients (Eastern Cooperative Oncology Group performance status 0 or 1) seen in clinical trials, resulting in poorer OS in patients who receive chemotherapy <3weeks from resection and under the implementation or dosing of adjuvant chemotherapy.
These findings should be interpreted in the context of certain inherent strengths and limitations of the study. This study used a large administrative claims database restricted to the US Medicare patient population (adult beneficiaries aged ≥65years). Although the population is representative of the US Medicare population with cancer, the generalizability of these findings outside the US Medicare population may be limited due to factors related to healthcare system and policy structure affecting treatment practices and access. Although multivariate analyses were conducted to control for observable differences between groups, the availability of variables in the database may have limited the ability to account for all potential confounders.
Conclusion
This study has shown a substantial unmet need for systemic therapy in elderly patients with resectable eNSCLC in US clinical practice. Despite guidelines that define standards of care, real-world use of adjuvant systemic therapy for elderly patients with early disease appears to be inadequate, particularly for stage II or III disease. Survival outcomes may be improved with the increased use of chemotherapy if an optimal period for initiation of adjuvant therapy can be determined based on individual patient characteristics and considerations, such as age and comorbidities. Newer drugs, such as immune checkpoint inhibitors or targeted therapies, may improve the stage-directed incorporation of routine systemic therapy use in resectable eNSCLC.
This study investigated real-world treatment patterns and overall survival in early non-small-cell lung cancer (eNSCLC) patients and the association between overall survival and time-to-adjuvant-treatment.
This was a retrospective observational study using Surveillance, Epidemiology and End Results data linked with Medicare claims for resected eNSCLC patients between 2010 and 2015.
Overall, most patients had surgery only (64%), followed by adjuvant treatment only (27%), while 297 (4%) had neoadjuvant treatment and 335 (5%) had neoadjuvant plus adjuvant treatment.
Among patients who received perioperative systemic therapy, adjuvant treatment was more common than neoadjuvant treatment, which was consistent across disease stages.
Neoadjuvant and adjuvant treatment rates increased with stage.
Despite clinical practice guidelines in the USA that define standards of care in stage II and III patients, only 54% and 71% of patients with stage II or IIIA disease, respectively, received any adjuvant treatment.
A significant number of patients with stage II (42%), IIIA (22%) and IIIB (16%) who underwent resection did not receive adjuvant or neoadjuvant systemic treatment (32% of stage II and III patients).
The median time from surgery to adjuvant treatment was 1.6months, and the median adjuvant treatment duration was 2.1months.
The most common adjuvant treatment was carboplatin plus paclitaxel (32%) followed by carboplatin plus pemetrexed (16%).
The median follow-up for the 7142 included patients was 39months and the 5-year survival rate was 68% (95% CI: 67%, 69%).
The adjusted risk of death within 12months or 12–30months of starting adjuvant therapy was significantly higher in patients who started treatment outside the 6- to <8-week period after surgery.
Author contributions
JM Lee, R Wang, A Johnson, S Ogale, M Kent and JS Lee were responsible for conception and design; R Wang, M Kent and JS Lee were responsible for data analysis; all authors were responsible for drafting and revision of the manuscript.
Financial & competing interests disclosure
This study was sponsored by Genentech, Inc., a member of the Roche Group. The study sponsor participated in the study design; the collection, analysis and interpretation of data; the writing of the report; and the decision to submit the article for publication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Support for third-party writing assistance for this manuscript, furnished by J Frimpter, MPH, of Health Interactions, Inc., was provided by Genentech, Inc., a member of the Roche Group.
Ethical conduct of research
The authors state the institutional review board approval was waived for this study due to the use of anonymized secondary data.
Data sharing statement
The datasets analyzed for this study are available in the SEER repository (seer.cancer.gov).
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2022-0845
Additional information
Funding
References
- Howlader N , KrapchoM, MillerDet al. SEER Cancer Statistics Review, 1975–2016. National Cancer Institute, MD, USA (2020).
- Lu T , YangX, HuangYet al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res.11, 943–953 (2019).
- Goldstraw P , ChanskyK, CrowleyJet al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol.11, 39–51 (2016).
- Howlader N , ForjazG, MooradianMJet al. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med.383, 640–649 (2020).
- Pisters K , KrisMG, GasparLEet al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I–IIIA completely resected non-small-cell lung cancer: ASCO guideline rapid recommendation update. J. Clin. Oncol.40, 1127–1129 (2022).
- Pignon J-P , TribodetH, ScagliottiGVet al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J. Clin. Oncol.26, 3552–3559 (2008).
- NSCLC Meta-analysis Collaborative Group . Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet383, 1561–1571 (2014).
- Martin J , GinsbergRJ, VenkatramanESet al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J. Clin. Oncol.20, 1989–1995 (2002).
- Uramoto H , TanakaF. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res.3, 242–249 (2014).
- Sugimura H , NicholsFC, YangPet al. Survival after recurrent non small-cell lung cancer after complete pulmonary resection. Ann. Thorac. Surg.83, 409–417 (2007).
- MacLean M , LuoX, WangS, KernstineK, GerberDE, XieY. Outcomes of neoadjuvant and adjuvant chemotherapy in stage 2 and 3 non-small cell lung cancer: an analysis of the National Cancer Database. Oncotarget9, 24470–24479 (2018).
- Felip E , AltorkiN, ZhouCet al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet398, 1344–1357 (2021).
- Wu Y-L , TsuboiM, HeJet al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med.383, 1711–1723 (2020).
- Enewold L , ParsonsH, ZhaoLet al. Updated overview of the SEER-Medicare data: enhanced content and applications. J. Natl Cancer Inst. Monogr.2020(55), 3–13 (2020).
- Nadpara P , MadhavanSS, TworekC. Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: a population-based study. Cancer Epidemiol.39, 1136–1144 (2015).
- Goldstraw P , CrowleyJ, ChanskyKet al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J. Thorac. Oncol.2, 706–714 (2007).
- Quan H , LiB, CourisCMet al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol.173, 676–682 (2011).
- Salazar MC , RosenJE, WangZet al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol.3, 610–619 (2017).
- Wang B-Y , HuangJ-Y, HungW-Het al. Impact on survival on interval between surgery and adjuvant chemotherapy in completely resected stage IB–IIIA lung cancer. PLOS ONE11, e0163809 (2016).
- Altorki N , FelipE, ZhouCet al. IMpower010: characterization of stage IB–IIIA NSCLC patients by type and extent of therapy prior to adjuvant atezolizumab. Oral presentation at: World Conference on Lung Cancer.Virtual (September 2021).
- Früh M , RollandE, PignonJ-Pet al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J. Clin. Oncol.26, 3573–3581 (2008).
- Morgansztern D , SamsonPS, WaqarSNet al. Early mortality in patients undergoing adjuvant chemotherapy for non-small cell lung cancer. J. Thorac. Oncol.13, 543–549 (2018).