Abstract
Transarterial chemoembolization (TACE) is the treatment of choice for intermediate-stage hepatocellular carcinoma (HCC). Recent data suggest that TACE may boost the efficacy of anti-PD-1 immunotherapy. The authors present the trial protocol for PETAL, a phase Ib study, which will assess the safety and bioactivity of pembrolizumab, an anti-PD-1 antibody, following TACE in HCC. After a run-in phase evaluating six patients to establish preliminary safety, up to 26 additional participants will be enrolled. Pembrolizumab will be administered three-times weekly for 1 year or until progression, starting 30–45 days after TACE. The primary objective is to determine safety and the secondary objective is to preliminarily evaluate efficacy. Radiological responses will be evaluated every four cycles.
Clinical Trial Registration: NCT03397654 (ClinicalTrials.gov)
Background & rationale
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer mortality worldwide [Citation1]. Due to the advanced stage at which HCC is often diagnosed, the majority of newly diagnosed patients cannot be offered curative treatment in the form of liver resection, ablation or transplantation [Citation2]. However, recent developments in locoregional therapies and systemic therapies have led to a significant improvement in patient prognosis [Citation3,Citation4].
Transarterial chemoembolization (TACE) involves intra-arterial delivery of cytotoxic agents to the tumor followed by direct occlusion of its arterial supply and is recommended for patients with unresectable, nonmetastatic HCC who have preserved liver function and acceptable performance status [Citation5]. Its use is supported by two primary randomized controlled trials that demonstrated a significant improvement in two-year overall survival [Citation5]. Unfortunately, even in well-selected candidates for TACE, treatment response is heterogeneous with a significant subset of patients not responding to the therapy [Citation6], while the disease of a subset of those who initially respond then goes on to progress within or outside the liver [Citation7]. Furthermore, overall survival (OS) following TACE has been reported to be only around 20 months, explaining the fact that TACE is usually administered with a palliative intent [Citation3,Citation8–10].
Immunotherapy has become the standard-of-care for systemic front-line treatment in patients who do not respond to or progress during TACE, as well as patients who initially present with advanced HCC, including those with vascular invasion, extrahepatic spread or extensive disease not amenable to locoregional treatments [Citation11]. Indeed, the combination of the anti-PD-L1 antibody atezolizumab combined with the anti-VEGF antibody bevacizumab has been found to significantly improve OS compared with sorafenib, the former standard-of-care, and this combination had been approved by several regulatory agencies worldwide [Citation12,Citation13]. Median OS achieved with atezolizumab plus bevacizumab was as high as 19.2 months, almost as high as the survival reported in TACE trials [Citation14]. Recently, two other global phase III studies evaluating different immunotherapy-based regimens in patients with advanced HCC reported positive results, underlining the role of immunotherapy in patients with HCC [Citation4,Citation15–17].
The role of immunotherapy in the setting of unresectable, nonmetastatic HCC suitable for TACE remains unclear. Studies have shown that the antitumor efficacy of TACE stems not only from its direct ischemic and cytotoxic effects but also from its capacity to reprogram the tumor microenvironment [Citation18,Citation19]. The cellular debris, cytokines and danger-associated molecular patterns (DAMPs) released locally as a result of TACE may prime the immune system [Citation20]. Indeed, observational studies evaluating innate and adaptive immune cell responses in peripheral blood have highlighted an increased CD4/CD8 ratio, a rise in Th17 cells and a reduction in Tregs [Citation18,Citation21], which all seem to be positive prognosticators for response to TACE [Citation18,Citation21–24]. More recently, analysis of resected specimens in patients who underwent TACE prior to resection or transplantation confirmed favorable peripheral immune cell responses compared with specimens from patients who had not undergone TACE [Citation25]. Indeed, in patients with TACE pretreatment, a significantly lower density of immune-exhausted effector cytotoxic and regulatory T cells and Tregs within the tumors was observed [Citation25]. Taken together, these results pave the way for studies evaluating the combination of TACE with immunotherapy in patients with intermediate-stage HCC [Citation25].
The demonstrated proinflammatory effect of TACE on the HCC microenvironment raises the question of whether TACE can prime the efficacy of subsequent immunotherapy [Citation25]. Specifically, targeting of the PD-1 pathway has become a focus of recent studies [Citation26]. A key question is to understand whether the ischemic and cytotoxic damage imposed by TACE to the tumor may facilitate priming of the antigen-specific branch of the immune system to a broad range of previously inaccessible neoepitopes, potentially enabling enhanced activity of PD-1/PD-L1 inhibitors. Mechanistic evidence suggests PD-L1 expression to be under the transcriptional control of the Hif-1a [Citation27], which further strengthens the rationale of combining TACE with PD-1/PD-L1 inhibitors in light of the documented role of TACE in activating a sustained hypoxic response and promoting the release of circulating VEGF levels and other proangiogenic cytokines.
Currently, there is no standard-of-care treatment combining locoregional and systemic treatments. However, guided by the overarching hypothesis that the locoregional immunogenic effects of TACE may boost the efficacy of PD-1-targeted immunotherapy, this combination appears promising. Here, the design and rationale of PETAL, a two-part multicenter phase Ib study to assess the safety and bioactivity of pembrolizumab following TACE in HCC are described.
Objectives
The primary study end point is to determine the safety and tolerability of pembrolizumab following TACE in patients with intermediate-stage HCC as assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4 [Citation28]. The secondary study end point is to evaluate the preliminary efficacy of pembrolizumab following TACE in intermediate-stage HCC using progression-free survival (PFS) rates every 12 weeks (4 cycles) using the modified response evaluation criteria in solid tumors (mRECIST) and RECIST v1.1 criteria [Citation29]. Exploratory end points include the identification of biomarkers of immune response to pembrolizumab. For this purpose, blood, urine and stool samples will be collected and analyzed using high-throughput proteomic and genomic analysis. Functional characterization of circulating lymphocyte populations will also be performed. Finally, quality of life will be assessed using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and QLQ-HCC18 criteria of the EORTC Quality of Life group [Citation30].
Trial design
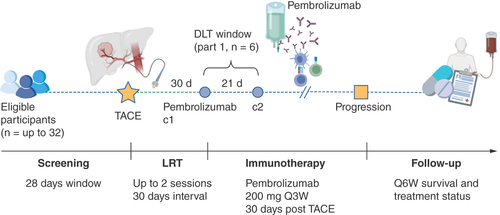
PETAL is an open-label, single-arm, sequential-phase study of sequential TACE followed by pembrolizumab in patients aged ≥18 years with HCC, aiming to recruit up to 32 evaluable participants. Pembrolizumab is a humanized monoclonal whole antibody specific for PD-1 of the IgG4/kappa isotype designed to directly block the interaction between PD-1 and its ligands, PD-L1 and PD-L2.
There are currently no clinical data illustrating the safety and tolerability of pembrolizumab following TACE. Hepatotoxicity is the main concern when combining TACE with anti-PD-1 immunotherapy. TACE is expected to produce low-grade hepatitis, although clinical studies have shown that the peak of cytokine and transaminase release happens approximately 7 days after TACE and subsequently resolves spontaneously [Citation31]. Immune-related hepatitis occurs in 1–6% of patients receiving anti-PD-1 monotherapy, with the onset usually 8–12 weeks after initiation of treatment, with reports of toxicity as early as 8 days [Citation32]. This potential interaction informs the timing of the two treatments and the monitoring requirements for dose-limiting toxicities (DLTs) in this protocol.
Part 1 of the study will consist of a safety run-in of up to six patients receiving TACE as the standard-of-care, who will be administered pembrolizumab at the clinically recommended dose of 200 mg every three weeks, at least 30 days after TACE. DLTs will be monitored over 21 days with weekly laboratory assessments in cycle 1 only. If two or fewer DLTs are observed, part 2 will proceed with an expansion phase including up to 26 patients who will receive TACE followed by 200 mg pembrolizumab after at least 30 days (if no DLTs are observed in part 1) or 45 days (if 1–2 DLTs are observed). All participants entered into the study will receive pembrolizumab 200 mg every 3 weeks ± 3 days until radiologically confirmed disease progression, unacceptable toxicity, withdrawal or a maximum of 1 year of treatment has been completed. The study is summarized as a flowchart in .
Methods
Eligibility criteria
PETAL will recruit adult patients with Barcelona Clinic Liver Cancer stage B HCC suitable for TACE as the standard-of-care who are not amenable to resection or eligible for liver transplantation and are naive to prior systemic anticancer treatment for HCC. A multidisciplinary tumor board will determine if TACE is the best available option for the patient and the study investigators will offer study participation to the patient. Eligibility criteria are provided in .
Table 1. Inclusion and exclusion criteria.
Study procedures
Screening will take place during the 28 days prior to TACE to confirm eligibility. Baseline imaging may be in the form of a chest CT and dual phase CT or a contrast-enhanced MRI scan of the abdomen. The same method used at baseline must be used for all subsequent time points. TACE will be performed with either selective or superselective embolization of the arterial vascular bed perfusing the tumor. The procedure will be standardized across sites and will consist of the conventional transcatheter arterial chemoembolization (conventional TACE) or drug-eluting beads TACE (DEB TACE) approach. Contrast-enhanced imaging will also be performed approximately 30 days after TACE as part of standard care. Participants who fail to achieve complete tumor devascularization after the first session of TACE as assessed by follow-up contrast-enhanced scan may be allowed up to one further TACE session as per European Association for the Study of the Liver (EASL)/EORTC clinical guidelines unless clinical or technical factors preclude repeat treatment [Citation33].
The interval will be fixed at either 30 or 45 days (±3 days in each case). Pending evaluation of drug-related adverse events (AEs) in the first subset of 6 subjects within the prespecified evaluation period of 21 days for DLTs, the treatment schedule of pembrolizumab may be changed to extend the pembrolizumab-to-TACE interval from 30 (+3 days) to at least 45 days (+3 days) of TACE in an expansion cohort of 26 patients maximum. Treatment will consist of pembrolizumab given at a fixed dose of 200 mg Q3W at least 30 or 45 days after TACE, until disease progression, occurrence of unacceptable toxicities or withdrawal, until 1 year of administration has been completed. Follow-up will occur within 3 days of the start of each cycle and will involve direct physical examination, recording of concomitant medications, assessment of Eastern Cooperative Oncology Group (ECOG) performance status and blood testing. RECIST v1.1 and mRECIST criteria will be applied by a central expert liver radiologist to maximize reproducibility concerning patient response to treatment and progression-free survival (PFS) [Citation34,Citation35]. Assessment of response by imaging will be performed around 30 days after TACE and after every fourth cycle (every 12 weeks) and at discontinuation of treatment if this has occurred for a reason other than disease progression. Optional samples, including tumor tissue, blood, urine and stool will be collected from all consenting patients at screening and at study termination, with blood and urine also being collected every 12 weeks, including at cycle 1.
Patients will be reviewed for AEs at each follow-up visit. All AEs that occur prior to the visit will be recorded. Participants with ongoing AEs at the visit will be followed up by the principal investigator or delegate until resolution or stabilization of the event.
Following study termination, participants will be assessed at an early and a late safety follow-up (SFU) visit. The early SFU should be conducted approximately 30 days (±7 days) after the last dose of pembrolizumab. A late SFU visit will occur 130 days after the last dose of the pembrolizumab (±7 days) by telephone call.
AE management & dose modifications
AEs associated with pembrolizumab exposure that are likely to have an immune mechanism and are of otherwise unknown etiology may be considered immune-related AEs (irAEs). AE severity will be evaluated according to the NCI CTCAE version 4.0, while the principal or delegates must endeavor to obtain sufficient information, including supplementary investigations, to determine the causality of AEs before labeling these irAEs. Summaries of recommendations to managing irAEs and guidelines for halting or discontinuing pembrolizumab in response to toxicities are provided in .
Table 2. General recommendations for managing immunotherapy-related adverse events.
Statistical analysis
As the primary end point of this study is safety, no power calculation for hypothesis testing was performed. To evaluate the primary outcome, the frequency of AEs in all participants who receive at least one dose of pembrolizumab will be assessed for severity (CTCAE version 4.0), expectedness, severity and causal relationship to the drug. In addition, AEs will be summarized by toxicity type, impact on pembrolizumab dosing and timing. For the secondary outcome, all participants who receive at least one dose of pembrolizumab and undergo disease re-evaluation will be included in the efficacy analysis. If patients remain disease-free until the end of follow-up, they will be censored at the date of the last imaging.
For all outcomes, frequencies and percentages will be used for categorical variables and means and standard deviations for normally distributed continuous variables. The secondary end point (PFS) will be estimated by the Kaplan-Meier method, calculated from the start of pembrolizumab administration until the date of progression or death from any cause. Uni- and multivariable Cox regression analyses will be used to evaluate baseline factors associated with PFS. For all estimates, 95% CIs will be calculated. Paired tests will be used to evaluate changes in quality of life. The collected biological samples will be used for future ethically approved research. For the translational/exploratory end points, appropriate descriptive measures as well as univariable and multivariable models for the predictive value of biomarkers for disease progression will be shown.
Sample size & power considerations
Since this is the first time that pembrolizumab will be given in addition to TACE, the study was designed as a safety-oriented trial. With the primary end points being safety, no power calculation for hypothesis testing is required to formally power the study: the upper 95% CI for toxicity events will inform the decision to proceed to a future, adequately powered phase II trial. Using the rule of three, if 0 events are observed in 26 participants, the upper 95% CI will be 15%.
Conclusion
Our growing understanding of how locoregional therapies, such as TACE, induce innate and adaptive immune cell responses against tumor antigens in HCC has provided momentum for the investigation of immune checkpoint inhibition in combination with TACE [Citation22,Citation25]. Indeed, TACE may induce immunogenic cell death resulting in the release of substances called alarmins, significantly activating immune cell activation and maturation [Citation36]. Furthermore, TACE-induced local release of DAMPs may additionally prime the immune system [Citation20].
This preclinical rationale is already supported by some early clinical data. A recent, retrospective, propensity-score matched study evaluated the effects of TACE within 60 days prior to nivolumab initiation compared with nivolumab monotherapy. Interestingly, patients with TACE pretreatment had a significantly longer PFS. Reassuringly, patients in the combination arm did not experience a significant increase in high-grade AEs [Citation37]. While these results appear promising, patient numbers were low and the study is prone to selection bias due to its retrospective design [Citation37]. Therefore, a prospective safety evaluation of this treatment combination is urgently needed.
This appears especially crucial in light of previous studies evaluating combination treatments with TACE. Unfortunately, the combination of TACE with the anti-VEGF antibody bevacizumab was not only ineffective in terms of OS or radiologic response, but also led to a significantly higher number of severe septic and vascular side effects, and the trial was stopped prematurely [Citation38]. In contrast, the combination of TACE with sorafenib was found to be safe in four trials, however, this combination did not improve outcomes in three of these randomized trials [Citation39–41]. In the most recent trial, patients in the combination treatment arm finally had an improved progression-free survival [Citation42], but it remains to be established if this approach is cost-effective and also associated with an acceptable quality of life [Citation43]. Very recently, another trial reported improved outcomes in patients receiving lenvatinib plus on-demand TACE compared with lenvatinib monotherapy. Importantly, the rate of grade 3–4 hepatotoxicity was elevated in the combination treatment arm [Citation44].
When given as monotherapy, pembrolizumab has only shown efficacy in a subgroup of patients with HCC and other combinatorial treatments have exceeded its efficacy. Nevertheless, the combination of TACE with monoimmunotherapy has never been studied prospectively and its safety must be established before proceeding with the combinatorial regimen. Furthermore, this trial was designed prior to the publication of evidence for the combinatorial treatment of HCC and this is a potential limitation of the study design.
Anti-PD-1 monotherapies are usually well tolerated with a low rate of serious AEs and are associated with a better quality of life compared with sorafenib [Citation45]. However, given the fact that anti-PD-1 monotherapies failed to improve the prognosis of patients with advanced HCC [Citation45,Citation46], combination therapies using synergistic effects to enhance the antitumor immune response seem to be the most promising treatment option. Interestingly, bevacizumab, a drug with very limited intrinsic anti-HCC activity in combination with atezolizumab helped to almost double objective response rates compared with previous anti-PD-1 monotherapy trials [Citation45–48]. This success might be explained by bevacizumab’s efficacy in reversing VEGF-induced immunosuppression, through the impairment of dendritic and CD8+ T-cell function, upregulation of immune checkpoint molecules and the accumulation of immunosuppressive cell types, such as tumor-associated macrophages, myeloid-derived suppressor cells and Treg cells [Citation49]. However, concomitant anti-VEGF treatment increases the risk of bleeding, which is already substantial in a significant number of patients [Citation12].
Given the appealing pathophysiological rationale for the combination of TACE with immunotherapy and the potential chances of initiating immunotherapy in earlier tumor stages, this trial will be crucial to elucidate the safety and preliminary efficacy of this combination. The emerging interest in combining locoregional therapy to stimulate the immune system with immunotherapy is also reflected by several other clinical trials evaluating the combined use of TACE or, more recently, transarterial radioembolization with anti-PD-1 nivolumab [Citation3,Citation50].
In summary, PETAL is the first study to investigate the safety and preliminary efficacy of pembrolizumab with TACE in patients with unresectable, nonmetastatic HCC. The results of this study will lay the groundwork for elucidating the role of post-TACE anti-PD-1 immunotherapy in the management of unresectable HCC, with the potential to improve survival outcomes.
Background & rationale
Transarterial chemoembolization (TACE) is the standard-of-care for intermediate-stage hepatocellular carcinoma (HCC). However, a significant proportion of patients progress after or do not respond to TACE.
According to recent data, the immunomodulatory effects of TACE may have a synergistic effect with anti-PD-1 immunotherapy.
PETAL is a two-part, multicenter, phase Ib study that assesses the safety and bioactivity of pembrolizumab following TACE in HCC.
PETAL study design & eligibility criteria
PETAL will recruit adult patients suitable for TACE as the standard-of-care who are not amenable to resection or eligible for liver transplantation and are naive to prior systemic anticancer treatment for HCC.
In the safety run-in (part 1) of the study, 6 patients will be evaluated to establish preliminary safety. Following this, in part 2 of the study, a maximum of 26 further participants will be enrolled.
Pembrolizumab at a fixed dose of 200 mg will be administered every 21 days for up to a year or until disease progression starting 30–45 days after TACE in patients with complete tumor devascularization.
Radiological responses will be evaluated every four cycles of immunotherapy using modified response evaluation criteria in solid tumors (mRECIST) as well as RECIST v1.1.
Outcome measures
The primary objective is to determine the safety and tolerability of pembrolizumab following TACE.
The secondary objective is to preliminarily evaluate the efficacy of this combination as measured by progression-free survival rates.
Conclusion
The results of this study will help to establish the safety and preliminary efficacy of pembrolizumab following TACE with the aim to improve patient outcomes.
Author contributions
R Sharma and DJ Pinato were responsible for the study concept and design. P Fessas, B Scheiner and DJ Pinato drafted the manuscript. All authors were involved in the critical revision of the manuscript for important intellectual content. DJ Pinato obtained funding and supervised the study.
Financial & competing interests disclosure
The study was funded by Merck Sharpe and Dohme (MSD). The authors would like to acknowledge infrastructure support provided by the Imperial College Experimental Cancer Medicine Centre (ECMC), the Cancer Research UK Imperial Centre, the NIHR Imperial College Biomedical Research Centre and the NIHR Clinical Research Facility. A D’Alessio is supported by the NIHR Imperial BRC, by grant funding from the European Association for the Study of the Liver (Andrew Burroughs Fellowship) and Cancer Research UK (RCCPDB-Nov21/100008). B Scheiner is supported by a scientific grant from the Università degli Studi del Piemonte Orientale, Novara, Italy. A Cortellini is supported by the NIHR Imperial BRC. DJ Pinato is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG 25697). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Siegel RL , MillerKD, JemalA. Cancer statistics, 2016. CA Cancer J. Clin.66(1), 7–30 (2016).
- Bruix J , ReigM, ShermanM. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology150(4), 835–853 (2016).
- Rognoni C , CianiO, SommarivaSet al. Trans-arterial radioembolization in intermediate-advanced hepatocellular carcinoma: systematic review and meta-analyses. Oncotarget7(44), 72343–72355 (2016).
- Facciorusso A , AbdEl Aziz MA, SaccoR. Efficacy of regorafenib in hepatocellular carcinoma patients: a systematic review and meta-analysis. Cancers (Basel)12(1), 36 (2019).
- Forner A , GilabertM, BruixJ, RaoulJL. Treatment of intermediate-stage hepatocellular carcinoma. Nat. Rev. Clin. Oncol.11(9), 525–535 (2014).
- Piscaglia F , BolondiL. The intermediate hepatocellular carcinoma stage: should treatment be expanded?Dig. Liver Dis.42(Suppl. 3), S258–S263 (2010).
- Kudo M , MatsuiO, IzumiNet al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology87(Suppl. 1), 22–31 (2014).
- Llovet JM , BruixJ. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology37(2), 429–442 (2003).
- Hucke F , PinterM, GraziadeiIet al. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J. Hepatol.61(6), 1287–1296 (2014).
- Pinato DJ , HowellJ, RamaswamiR, SharmaR. Review article: delivering precision oncology in intermediate-stage liver cancer. Aliment. Pharmacol. Ther.45(12), 1514–1523 (2017).
- Reig M , FornerA, RimolaJet al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J. Hepatol.76(3), 681–693 (2022).
- Finn RS , QinS, IkedaMet al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med.382(20), 1894–1905 (2020).
- Cheng AL , QinS, IkedaMet al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol.76(4), 862–873 (2022).
- Finn RS , QinS, IkedaMet al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). 39(Suppl. 3), 267–267 (2021). doi: 10.1200/JCO.2021.39.3_suppl.267.
- Kelley RK , RimassaL, ChengA-Let al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol.23(8), 995–1008 (2022).
- Abou-Alfa GK , LauG, KudoMet al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence (2022).
- Yau T , KangYK, KimTYet al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol.6(11), e204564 (2020).
- Liao J , XiaoJ, ZhouY, LiuZ, WangC. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol. Med. Rep.12(4), 6065–6071 (2015).
- Guan HT , WangJ, YangM, SongL, TongXQ, ZouYH. Changes in immunological function after treatment with transarterial chemoembolization plus radiofrequency ablation in hepatocellular carcinoma patients. Chin. Med. J. (Engl.)126(19), 3651–3655 (2013).
- Pol J , VacchelliE, ArandaFet al. Trial Watch: immunogenic cell death inducers for anticancer chemotherapy. 4(4), (2015). doi: 10.1080/2162402X.2015.1008866.
- Liao Y , WangB, HuangZLet al. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma: a prospective study. PLOS ONE8(4), e60444 (2013).
- Zerbini A , PilliM, PennaAet al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res.66(2), 1139–1146 (2006).
- Hiroishi K , EguchiJ, BabaTet al. Strong CD8+ T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J. Gastroenterol.45(4), 451–458 (2010).
- Pinato DJ , KaramanakosG, ArizumiTet al. Dynamic changes of the inflammation-based index predict mortality following chemoembolisation for hepatocellular carcinoma: a prospective study. Aliment. Pharmacol. Ther.40(11–12), 1270–1281 (2014).
- Pinato DJ , MurraySM, FornerAet al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J. Immunother. Cancer9(9), e003311 (2021).
- Chao Y , WuCY, KuoCYet al. Cytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolization. Hepatol. Int.7(3), 883–892 (2013).
- Chen J , JiangCC, JinL, ZhangXD. Regulation of PD-L1: a novel role of pro-survival signaling in cancer. Ann. Oncol.27(3), 409–416 (2016).
- National Cancer Institute, Division of Cancer Treatment & Diagnosis, Cancer Therapy Evaluation Program . Common Terminology Criteria for Adverse Events (CTCAE). (2010).
- Eisenhauer EA , TherasseP, BogaertsJet al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer45(2), 228–247 (2009).
- EORTC.org . EORTC–Quality of Life. https://qol.eortc.org/questionnaires/
- Kim MJ , JangJW, OhBSet al. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine64(2), 516–522 (2013).
- Grover S , RahmaOE, HashemiN, LimRM. Gastrointestinal and hepatic toxicities of checkpoint inhibitors: algorithms for management. Am. Soc. Clin. Oncol. Educ. Book (38), 13–19 (2018).
- Llovet JM , DucreuxM, LencioniRet al. EASL-EORTC Clinical Practice Guidelines: management of hepatocellular carcinoma. J. Hepatol.56(4), 908–943 (2012).
- Lencioni R , LlovetJM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis.30(1), 52–60 (2010).
- Eisenhauer EA , TherasseP, BogaertsJet al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer45(2), 228–247 (2009).
- Yang D , HanZ, OppenheimJJ. Alarmins and immunity. Immunol. Rev.280(1), 41–56 (2017).
- Marinelli B , KimE, D’AlessioAet al. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study. J. Immunother. Cancer10(6), e004205 (2022).
- Pinter M , UlbrichG, SieghartWet al. Hepatocellular carcinoma: A phase II randomized controlled double-blind trial of transarterial chemoembolization in combination with biweekly intravenous administration of bevacizumab or a placebo. Radiology277(3), 903–912 (2015).
- Kudo M , ImanakaK, ChidaNet al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur. J. Cancer47(14), 2117–2127 (2011).
- Pinato DJ , SharmaR, AllaraEet al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J. Hepatol.66(2), 338–346 (2017).
- Meyer T , FoxR, MaYTet al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol.2(8), 565–575 (2017).
- Kudo M , UeshimaK, IkedaMet al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut69(8), 1492–1501 (2020).
- Radu P , DufourJF. Changing tactics in intermediate HCC: TACE plus sorafenib. Gut69(8), 1374–1376 (2020).
- Peng Z , FanW, ZhuBet al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J. Clin. Oncol.41(1), 117–127 (2022).
- Yau T , ParkJW, FinnRSet al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol.30, v874–v875 (2019).
- Finn RS , RyooBY, MerlePet al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol.38(3), 193–202 (2020).
- Pinter M , UlbrichG, SieghartWet al. Hepatocellular carcinoma: a phase II randomized controlled double-blind trial of transarterial chemoembolization in combination with biweekly intravenous administration of bevacizumab or a placebo. Radiology277(3), 903–912 (2015).
- Finn RS , QinS, IkedaMet al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med.382(20), 1894–1905 (2020).
- Pinter M , JainRK, DudaDG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: a review. JAMA Oncol.7(1), 113–123 (2021).
- Viveiros P , RiazA, LewandowskiRJ, MahalingamD. Current state of liver-directed therapies and combinatory approaches with systemic therapy in hepatocellular carcinoma (HCC). Cancers11(8), 1085 (2019).