Abstract
Aims: To describe, in patients with advanced/metastatic non-small-cell lung cancer, the relationship between baseline immunosuppressive drug (ISD)/corticosteroid (CS) use, as well as the incidence of mild/moderate adverse events (AEs), and the clinical effectiveness of PD (L)-1 blockade. Patients & methods: This was a retrospective cohort study of patients with no evidence (n = 131) or positive evidence (n = 269) of ISD/CS use. Results: Duration of treatment, time to next treatment, progression-free survival and overall survival were significantly reduced for patients with evidence of prior ISD/CS use. Occurrence of mild/moderate AEs did not affect any clinical outcomes. Conclusion: Prior ISD/CS use was associated with a poorer prognosis in advanced/metastatic non-small-cell lung cancer patients treated with PD-(L)1 inhibitors, but the occurrence of AEs had no effect.
Plain language summary
What is the article about?
Patients with advanced/metastatic non-small-cell lung cancer (aNSCLC) are often treated with a class of drugs known as checkpoint inhibitors. There have been previous reports that treatment with corticosteroids and other drugs that suppress the immune system in the period leading up to treatment with checkpoint inhibitors may result in poorer outcomes, but most of these reports focus on serious adverse events leading to hospitalizations or emergency room visits that result from treatment. This study aimed to determine whether treatment with corticosteroids in these patients had any impact on the occurrence of mild or moderate adverse events and long-term treatment outcomes.
What were the results?
By looking back at deidentified medical insurance claims from patients with aNSCLC, we found that patients who were treated with corticosteroids or other immunosuppressive drugs (vs those who did not receive these drugs) in the months leading up to treatment with checkpoint inhibitors had poorer treatment outcomes (e.g., shorter overall survival).
What do the results of the study mean?
This study investigated the real-world outcomes in aNSCLC patients treated with checkpoint inhibitors and found that the use of corticosteroids or other immunosuppressive drugs may have an adverse effect. However, we are unable to rule out the possibility that there was an underlying difference between these two sets of patients that caused the difference in treatment outcomes. Further studies with larger sample sizes are needed.
Tweetable abstract
A retrospective cohort analysis showed that, in patients with advanced/metastatic non-small-cell lung cancer, baseline corticosteroid use in the months prior to PD-(L)1 blockade treatment is associated with poorer outcomes.
Lung cancer is the leading cause of cancer death in the USA for both men and women [Citation1]. While the estimated 235,760 new cases in 2021 represented 12.4% of all new cancer cases, lung cancer comprised over one-fifth of cancer deaths in that year [Citation1]. Since 2015, there have been significant and accelerating gains in survival, largely attributable to improved treatment [Citation1]. In 2015, the US FDA first approved nivolumab, a drug targeting PD-1, for patients with advanced or metastatic squamous non-small-cell lung cancer (NSCLC) whose disease had progressed after other treatments and who had tumors expressing the biomarker PD-L1 [Citation2]. Soon after that, pembrolizumab (a PD-1 inhibitor) and atezolizumab (a PD-L1 inhibitor) were approved for use as second-line therapy in advanced or metastatic NSCLC (aNSCLC) [Citation2]. Subsequent approvals of these drugs as monotherapy or in combination with chemotherapy in the first line, as well as the more recent approval of the PD-1 inhibitor cemiplimab as monotherapy in 2021, have led to the recommendation of PD-(L)1 blockade therapy as standard-of-care for first-line treatment of aNSCLC [Citation3].
Although enhanced antitumor immune activity associated with PD-(L)1 blockade can lead to durable clinical benefit in many patients and serious adverse events (AEs) are uncommon [Citation4,Citation5], a high proportion (>80%) of patients in clinical trials experienced non-serious AEs [Citation5–7] which may impact quality of life. The most common included fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, diarrhea and constipation [Citation5–7]. Immune-related AEs specific to PD-(L)1 blockade include rash, pruritus, diarrhea, renal failure and thyroid disease, among other conditions of the skin, endocrine, digestive, respiratory and urinary systems [Citation8]. Corticosteroids (CSs) are standard treatment for AEs associated with PD-(L)1 blockade therapy [Citation9], and their use in this context during therapy has not been found to be associated with impaired drug efficacy [Citation10–12].
However, as early as 2017, it was reported that CS use at initiation of treatment may negatively impact outcomes among aNSCLC patients receiving PD-(L)1 blockade [Citation13]. Since then, several retrospective studies have repeated the observation that early use of steroids – usually defined as ≥10 mg prednisone equivalent within a month of PD-(L)1 blockade initiation – is associated with poor disease control, shorter progression-free survival (PFS) and overall survival (OS) [Citation10–12,Citation14,Citation15] and receipt of fewer cycles of therapy [Citation14].
In addition to AE management, CSs are routinely used by patients with aNSCLC for the control of brain metastases, fatigue, dyspnea, chronic obstructive pulmonary disease (COPD) or other respiratory conditions, particularly common in patients with history of smoking [Citation16], that may be present prior to the initiation of PD-(L)1 blockade therapy [Citation10,Citation14]. Prior studies of real-world aNSCLC patients have shown that over 80% have a history of smoking [Citation17]. Analysis of claims data has shown that, across diagnoses, 30% of patients use CSs in the 30 days prior to PD-(L)1 blockade initiation [Citation18]. However, little is known about the impact of CSs and other immunosuppressive drugs (ISDs) given before initiation of PD-(L)1 blockade on the incidence of mild-to-moderate AEs and clinical outcomes, as patients with pre-existing conditions requiring ISDs/CSs are usually excluded from clinical trials, and sample size is generally small [Citation19–21].
While not life-threatening, mild-to-moderate AEs can significantly impact patients’ quality of life and their willingness to continue therapy, thereby representing an important avenue for research. To our knowledge, this study is the first to use physician documentation from electronic medical records (EMR) used in routine clinical practice to identify mild-to-moderate AEs in patients receiving PD-(L)1 blockade therapy.
To address this need, we performed a retrospective, observational study using real-world data on patients with aNSCLC receiving PD-(L)1 blockade therapy to describe patient characteristics, treatment patterns and the occurrence of mild and moderate AEs among those who did and did not receive ISDs/CSs within the 180 days prior to initiating PD-(L)1 blockade therapy. We examined the potential effects of baseline ISD/CS use on treatment, progression and survival outcomes. Lastly, we investigated whether the occurrence of mild-to-moderate AEs during PD-(L)1 blockade therapy impacts clinical outcomes.
Patients & methods
Data source
This study was an observational, retrospective evaluation using EMR data from the ConcertAI Patient360 NSCLC dataset, enhanced by additional curation of existing EMR data for select variables. The ConcertAI Patient360 NSCLC dataset is drawn from a range of community and academic oncology practices throughout the USA using data originally collected for clinical use, available to ConcertAI through data-sharing agreements with practices and other data providers. Participating clinical practices are located in both rural and urban settings and are not members of any one group purchasing organization; thus practice patterns reflect the real-world variability of treatment.
Medical records were verified by experienced clinical research nurses, and study data relating to baseline characteristics, treatments, staging, outcomes and other clinical data for each eligible patient were extracted by Structured Query Language query or abstracted by clinical research nurses onto electronic data collection tools and entered into a secure database for analysis.
Study patients
Patients were eligible for inclusion if they were ≥18 years of age at initial diagnosis of advanced/metastatic (stage IIIB–IV) NSCLC and initiated atezolizumab, nivolumab or pembrolizumab as monotherapy or in combination with chemotherapy between 1 January 2015 and 31 October 2019, after a diagnosis of advanced/metastatic disease. The identification period was defined as such to accommodate a potential follow-up time of at least 90 days for patients from the last eligible index date to the end of the study (31 January 2020). Patients were excluded if they received any study drugs (atezolizumab, nivolumab or pembrolizumab) within 6 months prior to the start of first PD-(L)1 blockade therapy after advanced/metastatic diagnosis, received any other immune checkpoint inhibitor (e.g., avelumab, cemiplimab, ipilimumab or durvalumab) within 100 days before initiating PD-(L)1 blockade therapy, had missing derived line-of-therapy data within the Patient360 NSCLC dataset, or initiated PD-(L)1 blockade after three lines of treatment. Stratified random sampling without replacement was used to enhance the identification of eligible patients by line of therapy at PD-(L)1 blockade initiation (balanced to reflect the distribution of treatment line in the eligible Patient360 NSCLC source population) and year of PD-(L)1 blockade initiation (priority given in descending order to consider more recent treatment initiation).
Study design
The study design is summarized in Supplementary Figure 1. The index line of PD-(L)1 blockade therapy was defined as the first PD-(L)1 inhibitor-containing therapy after aNSCLC diagnosis, and the initiation of index PD-(L)1 blockade therapy was set as the index date. AEs and AE management actions were assessed from index date through 100 days after the last dose of PD-(L)1 blockade, initiation of non-PD-(L)1 inhibitor-containing therapy, end of available data, or death (whichever occurred first). Other end points were evaluated from index date to the end of available data or death (whichever occurred first). Study patients were allocated to analysis cohorts based on the existence of no evidence (cohort 1) or positive evidence (cohort 2) of ISD/CS use within 180 days prior to initiation of index PD-(L)1 blockade therapy.
End points
The primary end points were incidence and management actions of mild/moderate AEs. For this study, a mild or moderate AE was defined as an event that did not result in hospitalization, emergency department visit or death, was not indicated by the provider to be a grade 4 or 5 AE and was not documented by the provider as ‘severe’. The specific AEs that were evaluated were categorized as follows: dermatological (rash, pruritus, vitiligo), gastrointestinal (nausea, vomiting, diarrhea, colitis), endocrine (evidence of new or worsening hypothyroidism, hyperthyroidism, hypophysitis), hepatic (hepatitis), respiratory (pneumonitis), rheumatic (inflammatory arthritis) and general (fatigue). The following management actions for the first AE were monitored: early anticancer agent discontinuation based on provider documentation of discontinuation and planned number of treatment cycles, anticancer agent treatment hold based on provider documentation, dose reduction of anticancer agent, administration of CSs and administration of antiemetics.
Duration of therapy (DOT), time-to-next-treatment (TTNT), OS and PFS by cohort and by occurrence of mild/moderate AEs by 3 months were also examined. DOT was defined as the time from index date to the end date of the last PD-(L)1 inhibitor-containing therapy in the continuous PD-(L)1 blockade treatment segment. A switch between PD-(L)1 inhibitors was not considered a discontinuation of PD-(L)1 blockade therapy. TTNT was defined as the time from the index date to the start of the next anticancer regimen not containing a PD-(L)1 inhibitor. PFS was defined as the time from index date to the earlier of disease progression or death, and OS was defined as the time from index date to death. For patients without a record of the terminal event of interest, analysis of DOT, TTNT, OS and PFS was censored at the last date known alive. Secondary end points were baseline demographic and clinical characteristics and treatment patterns of PD-(L)1 blockade therapy from aNSCLC diagnosis through three lines of therapy, end of available data or death (whichever occurred first).
Statistical analysis
Among the aNSCLC patients who fulfilled the eligibility criteria (n = 1017), 400 patients were randomly selected to address the study objectives while also effectively curating additional variables of interest (e.g., mild/moderate AEs and management actions, confirmation of ISD/CS administration) from the database. As scarce data are available on which to base sample size calculations, no formal assessment of statistical power was conducted. The distribution of race and gender for the 400-patient study sample was compared with that of the entire eligible population to verify that the study population was representative of the source population of eligible patients within the ConcertAI Patient360 NSCLC dataset.
Descriptive statistics were generated for all continuous and categorical study variables for the overall sample as well as for each cohort. Missing data were reported as unknown or not documented, and no missing data imputation was performed.
Distributions of study variables between cohorts were compared using t-tests for continuous variables when the Shapiro–Wilk test failed to reveal evidence of non-normality or when the sample size for each group was at least 30; otherwise, the nonparametric equivalent Wilcoxon rank-sum test was used. For categorical variables, χ-square tests were used when up to 20% of cells for comparison had expected frequencies less than five; otherwise, Fisher’s exact test was used.
Kaplan–Meier analyses were performed for time-to-event/survival end points (DOT, TTNT, PFS and OS), stratified by cohort. For each Kaplan–Meier analysis, the log-rank test was conducted to evaluate differences between cohorts. Kaplan–Meier methods were also used to generate survival probability and 95% CIs at 12 and 24 months for OS and PFS. For OS, an associated Cox regression analysis was conducted. The Cox regression examined the difference in OS between cohorts, generally following methods previously described by Hosmer et al. [Citation22]. The full multivariate Cox proportional hazards model included baseline exposure to ISDs/CSs, baseline patient characteristics (demographic and clinical), line of therapy of PD-(L)1 blockade initiation, and index treatment regimen. A correlation matrix was generated to test for collinearity of the covariates considered for the model, and univariate Cox proportional hazards were generated to determine which covariates would be considered for inclusion in the full model. Covariates that were determined to be statistically significantly associated with OS in the univariate analyses at α = 0.10 and that were not highly correlated with other considered covariates (|r| <0.6) were included in the full model. Finally, covariates were chosen via stepwise selection into the final Cox model using α = 0.10 as threshold. If multiple covariates were highly correlated (|r| <0.6) with others that met the criteria for inclusion in the final model, only one was included. Age at index date, gender and race were included in the final model regardless of statistical significance. Assumptions of proportional hazards were assessed by visual evaluation of the Kaplan–Meier plot.
Kaplan–Meier landmark analyses were conducted for all survival end points to assess the effect of occurrence of an AE by 3 months following the index date. The methods described by Anderson et al. were used [Citation23]. The landmark analyses were stratified by occurrence of at least one mild/moderate AE by 3 months following the initiation of index therapy and excluded patients who were censored or experienced a terminal event prior to 3 months. The log-rank test was conducted for each Kaplan–Meier analysis to evaluate differences between strata.
All analyses were performed using R v. 4.0.2, and survival analyses were conducted using the R survival package v. 3.1.
Results
Patient demographics
From a total of 1017 eligible patients, a stratified random sample of 400 patients was generated and allocated to cohort 1 (n = 131) or cohort 2 (n = 269) (). The mean (± standard deviation [SD]) patient age at aNSCLC diagnosis was 67.1 ± 10.0 years and was older in cohort 1 (69.4 ± 10.1 years) than in cohort 2 (66.1 ± 9.8 years) (). The majority of patients were male (51.3%) and White (71.0%), and a larger proportion of patients in cohort 2 were non-Hispanic than in cohort 1 (90.0 vs 78.6%). Nearly one-third (32.5%) of patients were current smokers at the time of diagnosis, and patients in cohort 2 were more likely to be current smokers than patients in cohort 1 (36.1 vs 25.2%) ().
aNSCLC: Advanced or metastatic non-small-cell lung cancer; CS: Corticosteroid; ISD: Immunosuppressive drug; LOT: Line of therapy; NSCLC: Non-small-cell lung cancer.
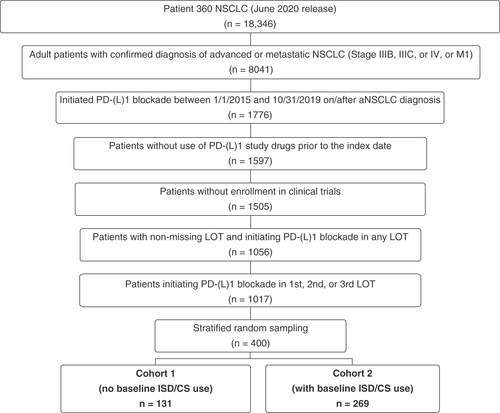
Table 1. Patient characteristics at advanced/metastatic non-small-cell lung cancer diagnosis.
Disease characteristics
Most patients had stage IV NSCLC at initial diagnosis (61.8%), and the most common histological type overall was adenocarcinoma (65.0%) followed by squamous cell carcinoma (22.0%) (). A total of 73.5% of patients had metastatic disease (78.6% in cohort 1; 71.0% in cohort 2) at aNSCLC diagnosis, and brain and bone were the most common sites of metastasis overall (35.0 and 32.8%, respectively). A larger proportion of patients in cohort 2 had brain metastasis than in cohort 1 (42.0 vs 20.6%), while the proportion of patients with malignant pleural effusion was greater in cohort 1 (14.5 vs 6.7%) than in cohort 2. Overall, 49.3% of patients had an Eastern Cooperative Oncology Group performance score of 0 (14.5%) or 1 (34.8%) at aNSCLC diagnosis, while the score was missing for 29.3% of patients.
Table 2. Disease characteristics at advanced/metastatic non-small-cell lung cancer diagnosis.
Index PD-(L)1 blockade treatment
The time from aNSCLC diagnosis to initiation of PD-(L)1 blockade therapy ranged from 0 to 1222 days (median 37 days for cohort 1 and 69 days for cohort 2; difference not significant) (). Most patients (75.0%) initiated index therapy in combination with chemotherapy, and 19.3% had pembrolizumab monotherapy. The distribution of index treatments differed significantly between groups (p = 0.001), with the PD-(L)1 blockade + chemotherapy combination less common in cohort 1 (61.1%) than in cohort 2 (81.8%), and pembrolizumab monotherapy more common in cohort 1 than in cohort 2 (30.5 vs 13.8%). PD-(L)1 blockade initiation was most likely to occur as first-line therapy overall (57.3%), and more likely initiated in first line for patients in cohort 1 than cohort 2 (71.0 vs 50.6%; p = 0.001). Accordingly, compared with those in cohort 1, patients in cohort 2 were more likely to initiate PD-(L)1 blockade therapy during second-line (39.8 vs 24.4%) and third-line (9.7 vs 4.6%) regimens.
Table 3. PD-(L)1 blockade therapy treatment patterns.
Incidence of AEs & management actions
Mild or moderate AEs were reported for 257 patients (64.3%) during index treatment, with similar frequencies in cohort 1 (61.8%) and cohort 2 (65.4%). The most common type of AE overall and in both cohorts was gastrointestinal AEs (56.8%). The most common specific AEs were fatigue (55.3%), nausea (28.0%) and diarrhea (15.8%) (). Pruritus was more common in cohort 1 (12.2%) than in cohort 2 (6.3%). Distributions of occurrence of other AEs were similar across cohorts.
Table 4. Incidence of mild/moderate adverse events of interestTable Footnote† during index PD-(L)1 blockade therapy by category and severity.
Of the patients who experienced mild/moderate AEs (n = 257), 58.4% received at least one management action for their first AE, and these occurred at similar frequencies in cohort 1 (59.3%) and cohort 2 (58.0%) (). Of the patients for whom management actions were performed, the most common were ISD/CS administration (50.0%) followed by treatment hold (48.7%) and antiemetic administration (37.3%). Frequencies of specific management actions were similar between cohorts, although treatment hold (54.2%) followed by ISD/CS administration (52.1%) were the most common actions taken in cohort 1, while ISD/CS administration (49.0%) followed by treatment hold (46.1%) were the most common in cohort 2 ().
Table 5. Management actions performed for the first occurrence of mild/moderate adverse events of interestTable Footnote† after initiation of index PD-(L)1 blockade therapy.
Clinical effectiveness outcomes
No significant differences were observed in clinical effectiveness outcomes in any of the landmark analyses by AE status at or before 3 months (Supplementary Figure 2).
Overall, patients in cohort 1 had significantly better outcomes than patients in cohort 2. The median DOT in cohort 1 was longer than in cohort 2 (9.6 vs 5.9 months; p = 0.012) (). Similarly, TTNT was significantly longer in cohort 1 than in cohort 2 (median: 8.8 vs 5.0 months; p = 0.012) (). Patients in cohort 1 had longer PFS compared with patients in cohort 2 (median: 8.6 vs 4.5 months; p = 0.031) (). Overall, 12-month PFS probability was 36% and was higher in cohort 1 (45%; 95% CI: 37–55) than in cohort 2 (31%; 95% CI: 25–38). 24-month survival was similar between cohorts (27%; 95% CI: 18–40 in cohort 1 vs 23%; 95% CI: 17–31 in cohort 2; data not shown).
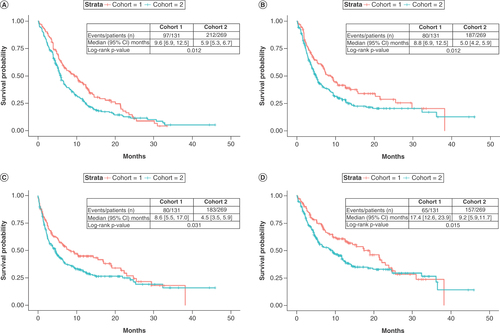
The mean follow-up for analysis for the study population was 9.5 ± 8.7 months, with a median of 6.6 months and an interquartile range of 2.9–13.2 months. Follow-up was longer in cohort 1 (mean ± SD: 11.0 ± 8.6 months; median: 9.4; IQR: 3.7–15.5) than in cohort 2 (mean ± SD: 8.8 ± 8.7 months; median: 5.5; IQR: 2.5–11.8) (). Median OS was significantly longer for cohort 1 than cohort 2 (17.4 months; 95% CI: 12.6–23.9 vs 9.2 months; 95% CI: 5.9–11.7; p = 0.015) (). Similar to PFS, the 12-month OS probability was higher in cohort 1 than in cohort 2 (61%; 95% CI: 52–70 vs 42%; 95% CI: 36–49, respectively; data not shown), and 24-month survival probability was similar between cohorts (34%; 95% CI: 24–49 in cohort 1 vs 33%; 95% CI: 27–41 in cohort 2; data not shown).
The adjusted multivariate regression analysis revealed that baseline exposure to ISD/CS (cohort 2) was associated with a 38% increase in mortality rate compared with no baseline exposure to ISD/CS (cohort 1) (hazard ratio [HR]: 1.38; 95% CI: 1.02–1.86; p = 0.038). The highest-magnitude predictors of increased mortality were impaired performance status (HR: 1.67; 95% CI: 1.19–2.34 vs unimpaired), presence of brain metastasis (HR: 1.55; 95% CI: 1.16–2.06 vs no brain metastasis) and presence of comorbid diabetes at aNSCLC diagnosis (HR: 1.50; 95% CI: 1.04–2.15 vs no comorbid diabetes) (). Male gender (vs female; HR: 1.31; 95% CI: 1.00–1.72) and White race (vs non-White; HR: 1.37; 95% CI: 1.01–1.86) were associated with moderate significant increases in mortality rate compared with their counterparts. Incidence of mild/moderate AEs was not included in the final model due to lack of statistical significance. Line of therapy of PD-(L)1 inhibitor initiation, PD-(L)1 inhibitor monotherapy versus PD-(L)1 inhibitor + chemotherapy combination regimen, BMI, stage at initial diagnosis, histology, smoking status, other measured sites of distant metastasis and other measured comorbid conditions were included in the stepwise selection process but did not meet the significance threshold to be included in the final adjusted model.
Table 6. Factors associated with overall survival from PD-(L)1 blockade treatment initiation.Table Footnote†
Discussion
This real-world, retrospective, observational study aimed to describe the incidence of mild and moderate AEs among patients receiving PD-(L)1 blockade for aNSCLC by CS or other ISD, as well as the impact of baseline use of ISDs/CSs and AE incidence on clinical effectiveness outcomes.
In the present study of a stratified random sample of aNSCLC patients receiving PD-(L)1 blockade in the ConcertAI Patient360 NSCLC dataset, approximately two-thirds of patients had received ISDs/CSs in the 180 days prior to initiating PD-(L)1 blockade therapy (cohort 2). The proportion of baseline ISD/CS use observed in this study is higher than previously reported [Citation13,Citation18]; this may be due to the extended timeframe for ISD/CS use identification (e.g., 180 days vs 30 days prior to PD-(L)1 blockade initiation). Additionally, COPD was the most commonly reported comorbid condition at initiation of PD-(L)1 blockade therapy; as previously mentioned, COPD is often treated using CS. The proportion of patients with comorbid COPD and smoking history in this study is similar to that reported in other real-world studies of aNSCLC patients [Citation17]. Patients with baseline ISD/CS use were more likely to have initiated PD-(L)1 blockade therapy in later lines of therapy, which may explain the shorter available follow-up for this group.
A majority (64.3%) of patients in this study experienced mild-to-moderate AEs. Distributions were generally similar between cohorts 1 and 2 in terms of overall AE incidence, type and management. The occurrence of mild-to-moderate AEs in our study is lower than those previously reported in interventional clinical trials [Citation5–7]. This is expected as, unlike interventional clinical trials, in medical records the documentation of AEs does not follow an operationalized definition, and potential side effects are generally only documented when the treating physician judges them to be clinically relevant or requiring intervention. This likely results in underascertainment of AEs in this study compared with clinical trials. While minor differences were observed in baseline characteristics including age, race/ethnicity and smoking history, these factors were not significant confounders of the association between baseline CS/ISD use and OS, and thus were not included in the final multivariate model.
Consistent with the previously described safety profile of these agents [Citation9], we found that mild-to-moderate AEs most commonly affected the gastrointestinal and dermatological organ systems. Similarly, our finding that management actions were performed for approximately 60% of patients who experienced any mild/moderate AEs (most frequently, ISD/CS administration and treatment hold) generally aligns with treatment guidelines that recommend no intervention for grade 1 AEs and ISD/CS administration and/or treatment hold for grade 2 and 3 AEs [Citation9,Citation24,Citation25].
Contrary to previous literature suggesting that AEs can indicate better response to treatment and lead to better clinical outcomes [Citation15,Citation26], in this study the occurrence of mild or moderate AEs by 3 months had no observed effect on subsequent outcomes for any clinical effectiveness measures assessed. This may be because the majority of patients who experienced mild/moderate AEs subsequently had at least one management action performed, including treatment changes and ISD/CS administration. These management actions may mitigate the effect of mild/moderate AEs on PD-(L)1 blockade efficacy and survival outcomes. Mediation analysis of AE management on outcomes was not assessed in the present study.
Unadjusted analysis of clinical effectiveness outcomes revealed that patients with evidence of baseline ISD/CS use showed significantly shorter duration of PD-(L)1 blockade treatment, time to next non-PD-(L)1 blockade treatment, PFS and OS than those without evidence of baseline use. It is possible that the poorer outcomes associated with baseline ISD/CS use may be due to confounding related to underlying differences between the two groups, as patients with baseline ISD/CS use were more likely to smoke and have brain metastasis at aNSCLC diagnosis. As previously noted, brain metastases are often managed by administration of CSs, which could explain the higher proportion of brain metastasis seen in cohort 2. It is also possible that there were other unmeasured adverse health conditions requiring treatment or management with ISDs/CSs that may have an adverse effect on clinical outcomes, though there were no significant differences observed in the distribution of measured comorbid conditions. However, adjusted analysis including presence of comorbid conditions still showed a significant reduction in OS among patients who received baseline ISDs/CSs.
Limitations of this study reflect those of all retrospective, observational designs in that they can detect associations but not determine causality. Additionally, data are based on medical records collected for clinical practice and not research purposes. As mentioned above, this may lead to an underestimation of the occurrence of AEs. Similarly, lack of evidence of ISD/CS use does not necessarily imply lack of use, and some patients who used ISDs/CSs may not have this information documented in their medical record. However, we believe that this occurs rarely and randomly across the sample and as such would bias results toward the null. While the AEs evaluated in this study resemble the profile of AEs associated with immune checkpoint inhibitor treatment, documentation confirming that the AEs were immune-related or non-immune-related was not available. Therefore, it is possible that AEs reported in this study, although contemporaneous with immune checkpoint inhibitor treatment, were not considered to be immune-related. The dose and indication of CS/ISD use were not collected, so we are unable to determine whether the CS/ISD dose is causally related to the mild/moderate AEs, which may impact the interpretation of outcomes. Furthermore, the data used in this study were obtained primarily in the community oncology setting among a selected cohort of patients, which may not fully represent the wider population of patients with aNSCLC treated with PD-(L)1 blockade. Finally, atezolizumab and nivolumab were underrepresented in this analysis, and this study observed a lower proportion of current or former smokers than past studies [Citation17], which may also affect the generalizability of the results.
Strengths of this study include a robust and detailed data source that permitted a large population-based analysis to provide a real-world view of patient characteristics, treatment patterns and outcomes for patients treated with PD-(L)1 blockade for aNSCLC. While most real-world studies are limited to the structured fields of EMRs, the ConcertAI Patient360 NSCLC dataset also includes manually curated unstructured data in physicians’ notes, images and other uploaded files, thereby allowing us to leverage richer and deeper information, especially information on mild-to-moderate AEs, which may only be found in the unstructured notes.
Future studies may wish to evaluate the sequence and timing of treatment administration; the onset of mild/moderate AEs; the type of AE (immune-related vs non-immune-related AE); and the indications for, timing, and dosing of ISDs/CSs as predictors or modifiers of clinical effectiveness of PD-(L)1 blockade therapy. While this study includes the largest sample size investigating ISD/CS use prior to PD-(L)1 blockade therapy identified by these authors, repeating this analysis with a larger patient population is needed to confirm the conclusions drawn.
Conclusion
This study is the first to investigate the real-world impact of baseline ISD/CS use on the incidence of mild or moderate AEs and clinical outcomes of PD-(L)1 blockade therapy and shows that, while common in real-world settings, ISD/CS use immediately prior to initiating PD-(L)1 blockade therapy for aNSCLC can have adverse effects on clinical outcomes. In addition, the occurrence of mild or moderate AEs may not be a predictor of PD-(L)1 blockade effectiveness.
For patients treated with PD-(L)1 blockade for advanced/metastatic non-small-cell lung cancer (NSCLC), the occurrence of mild/moderate adverse events (AEs) and the effect of baseline corticosteroid (CS) use on clinical outcomes in real-world settings is largely unknown.
This retrospective cohort study aimed to describe the effect of baseline immunosuppressive drugs (ISDs)/CSs and the incidence of mild or moderate AEs on the clinical effectiveness of PD-(L)1 blockade.
The ConcertAI Patient360 NSCLC dataset was used to analyze the medical histories of adult patients with stage IIIB–IV NSCLC who initiated treatment with atezolizumab, nivolumab or pembrolizumab after a diagnosis of advanced/metastatic NSCLC in the USA between 1 January 2015 and 31 October 2019.
Duration of PD-(L)1 blockade treatment, time-to-next-treatment, progression-free survival and overall survival were compared between patients without evidence of ISD/CS use within 180 days before PD-(L)1 blockade initiation (cohort 1) and patients with ISD/CS use during that timeframe (cohort 2), and by occurrence of any mild/moderate AEs.
Eligible patients (n = 400) were randomly selected and then allocated to cohort 1 (n = 131) or cohort 2 (n = 269).
A majority of patients (64.3%) developed one or more mild/moderate AE during PD-(L)1 blockade therapy; the most common AEs were fatigue (55.3%), nausea (28.0%) and diarrhea (15.8%).
Duration of PD-(L)1 blockade treatment (p = 0.012), time to next treatment (p = 0.012), progression-free survival (p = 0.031) and overall survival (p = 0.015) were significantly reduced for patients in cohort 2 compared with those in cohort 1. Occurrence of mild/moderate AEs did not affect any clinical outcomes.
Patients in cohort 1 and cohort 2 had similar profiles of AEs and management actions performed; however, patients in cohort 2 showed significant reductions in clinical outcomes compared with those in cohort 1, revealing an association between prior ISD/CS use and a poorer prognosis.
Author contributions
Y Zhang, C Doran, K Le, B Dreyfus, L Lal, J Penrod and S Meadows Shropshire were responsible for study conception and/or design; Y Zhang, C Doran, K Le, B Dreyfus, N Kola, J Penrod, B Sylvester and S Meadows Shropshire were responsible for data interpretation; Y Zhang, C Doran, K Le and L Lal were responsible for data analysis; and C Doran, K Le and N Kola were responsible for drafting the manuscript. All authors were involved in critically reviewing and revising the manuscript and approved the final draft.
Financial & competing interests disclosure
Bristol Myers Squibb sponsored this study and provided financial support for the conduct of the research and preparation of the article. C Doran and L Lal are employees of ConcertAI and report research funding to their institution from Bristol Myers Squibb. Y Zhang, K Le, B Dreyfus, N Kola, B Sylvester, J Penrod and S Meadows Shropshire are employed by Bristol Myers Squibb and hold stock or stock options. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors thank N Connors (NCC Medical & Scientific Communications, Inc.) for professional assistance with manuscript preparation. This was funded by Bristol Myers Squibb.
Ethical conduct of research
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This research was reviewed and approved by the Institutional Review Board of IntegReview, Austin, TX, USA. This research study was conducted retrospectively from data obtained for clinical purposes. An IRB waiver of consent was granted from IntegReview.
Supplemental Figure 1. Study Schema
Download MS Word (84.2 KB)Supplemental Figure 2. Kaplan-Meier plots comparing patients with vs. without an AE by 3 monthsfor (A) duration of therapy, (B) time to next treatment, (C) progression-free survival; and (D) overall survival from the index date.
Download MS Word (371.9 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2022-1117
Additional information
Funding
References
- Siegel RL , MillerKD, FuchsHE, JemalA. Cancer statistics, 2021.CA Cancer J. Clin.71(1), 7–33 (2021).
- US Food and Drug Administration . Oncology (cancer)/hematologic malignancies approval notifications (2022). www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications (Accessed 7April2022).
- National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology: non-small cell lung cancer (2022). www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (Accessed 15September2022).
- Mencoboni M , CeppiM, BruzzoneMet al. Effectiveness and safety of immune checkpoint inhibitors for patients with advanced non small-cell lung cancer in real-world: review and meta-analysis. Cancers (Basel) 13(6), 1388 (2021).
- Bristol-Myers Squibb . Study of nivolumab (BMS-936558) in patients with advanced or metastatic squamous cell nonsmall-cell lung cancer who have received at least 2 prior systemic regimens (2021). ( NLM Identifier: NCT01721759). https://clinicaltrials.gov/ct2/show/NCT01721759 (Accessed 23May2022).
- Hoffman-La Roche . A randomized phase 2 study of atezolizumab (an engineered anti-PDL1 antibody) compared with docetaxel in participants with locally advanced or metastatic non-small cell lung cancer who have failed platinum therapy – ‘POPLAR’ (2019). ( NLM Identifier: NCT01903993). http://clinicaltrials.gov/show/NCT01903993 (Accessed 8April2022).
- Merck Sharp & Dohme Corp . Study of two doses of pembrolizumab (MK-3475) versus docetaxel in previously treated participants with non-small cell lung cancer (MK-3475-010/KEYNOTE-010) (2021). ( NLM Identifier: NCT01905657). https://clinicaltrials.gov/ct2/show/NCT01905657 (Accessed 23May2022).
- Wang P-F , ChenY, SongS-Yet al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 8, 730 (2017).
- Puzanov I , DiabA, AbdallahKet al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 5(1), 95 (2017).
- Arbour KC , MezquitaL, LongNet al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J. Clin. Oncol. 36(28), 2872–2878 (2018).
- Fuca G , GalliG, PoggiMet al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 4(1), e000457 (2019).
- Petrelli F , SignorelliD, GhidiniMet al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers (Basel) 12(3), 546 (2020).
- Bernal GM , MezquitaL, AuclinEet al. Baseline corticosteroids (CS) could be associated with absence of benefit to immune checkpoint inhibitors (ICI) in advanced non-small cell lung cancer (NSCLC) patients. Ann. Oncol. 28(Suppl. 5), v472 (2017).
- Scott SC , PennellNA. Early use of systematic corticosteroids in patients with advanced NSCLC treated with nivolumab.J. Thorac. Oncol.13(11), 1771–1775 (2018).
- Zhou X , YaoZ, YangHet al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 18(1), 87 (2020).
- Centers for Disease Control and Prevention . Chronic obstructive pulmonary disease among adults – United States, 2011.MMWR Morb. Mortal. Wkly Rep.61(46), 938–943 (2012).
- Simeone JC , NordstromBL, PatelK, KleinAB. Treatment patterns and overall survival in metastatic non-small-cell lung cancer in a real-world, US setting.Future Oncol.15(30), 3491–3502 (2019).
- George S , BellEJ, ZhengYet al. The impact of adverse events on health care resource utilization, costs, and mortality among patients treated with immune checkpoint inhibitors. Oncologist 26(7), e1205–e1215 (2021).
- Connell CM , RabyS, BehIet al. Cancer immunotherapy trial registrations increase exponentially but chronic immunosuppressive glucocorticoid therapy may compromise outcomes. Ann. Oncol. 28(7), 1678–1679 (2017).
- Della Corte CM , MorgilloF. Early use of steroids affects immune cells and impairs immunotherapy efficacy.ESMO Open4(1), e000477 (2019).
- Skribek M , RounisK, AfsharSet al. Effect of corticosteroids on the outcome of patients with advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Eur. J. Cancer 145, 245–254 (2021).
- Hosmer DW , LemeshowS, MayS. Applied Survival Analysis: Regression Modeling of Time to Event Data (2nd Edition). Wiley Interscience, NJ, USA (2008). https://onlinelibrary.wiley.com/doi/book/10.1002/9780470258019
- Anderson JR , CainKC, GelberRD. Analysis of survival by tumor response.J. Clin. Oncol.1(11), 710–719 (1983).
- Brahmer JR , LacchettiC, SchneiderBJet al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 36(17), 1714–1768 (2018).
- National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology (NCCN Guidelines®): management of immunotherapy-related toxicities. Version 3.2021 (2021). https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- Maillet D , CorbauxP, StelmesJJet al. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur. J. Cancer 132, 61–70 (2020).
