Abstract
Ablative doses of stereotactic body radiotherapy (SBRT) may improve pancreatic cancer outcomes but may carry greater potential for gastrointestinal toxicity. Rucosopasem, an investigational selective dismutase mimetic that converts superoxide to hydrogen peroxide, can potentially increase tumor control of SBRT without compromising safety. GRECO-2 is a phase II, multicenter, randomized, double-blind, placebo-controlled trial of rucosopasem in combination with SBRT in locally advanced or borderline resectable pancreatic cancer. Patients will be randomized to rucosopasem 100 mg or placebo via intravenous infusion over 15 min, before each SBRT fraction (5 × 10 Gy). The primary end point is overall survival. Secondary end points include progression-free survival, locoregional control, time to metastasis, surgical resection rate, best overall response, in-field local response and acute and long-term toxicity.
Plain Language Summary
The study design for GRECO-2, a clinical trial to examine treatment of pancreatic cancer with high-dose radiation plus rucosopasem
The use of high doses of radiation delivered directly to tumors (stereotactic body radiation therapy [SBRT]) may improve survival compared with lower doses of radiation in patients with pancreatic cancer, but it may increase side effects. Rucosopasem, an investigational new drug being developed, can potentially improve the ability of SBRT to treat tumors without decreasing safety. In a previous study, median overall survival was improved when patients were treated with SBRT plus avasopasem, a drug that works the same way as rucosopasem. GRECO-2 is a clinical trial of rucosopasem used in combination with SBRT for treatment of localized pancreatic cancer. Patients will be randomly selected to receive either rucosopasem 100 mg or placebo via intravenous infusion over 15 min, before each SBRT treatment. The main result being studied is overall survival. Additional results include amount of time before tumors start to grow, how often patients get tumors surgically removed, best overall response and long-term safety.
Clinical Trial Registration: NCT04698915 (ClinicalTrials.gov)
Pancreatic cancer remains one of the most lethal cancer diagnoses despite advances in treatment. The estimated 5-year survival rate for pancreatic cancer in the USA is only 12%, and it is the third leading cause of cancer-related death in both men and women [Citation1]. Although systemic treatments for pancreatic cancer have improved, patients with borderline resectable or locally advanced pancreatic cancer still have limited treatment options and poor survival rates [Citation2,Citation3]. For those patients ineligible for surgery at presentation, future resection can be limited by treatment toxicity, lack of radiographic response, or disease progression during induction chemotherapy [Citation4,Citation5].
Clinical practice and treatment guidelines for patients with borderline resectable and locally advanced pancreatic cancer vary and include neoadjuvant chemotherapy (NAC) alone, NAC followed by chemoradiation (CRT), or NAC followed by stereotactic body radiation therapy (SBRT) [Citation3,Citation6]. While CRT has been shown to improve local control, the effect on overall survival (OS) remains unclear [Citation7–10]. Recent retrospective analyses have shown that radiation regimens with higher biologically effective dose (BED) may correlate with improvements in OS [Citation11,Citation12]. SBRT utilizes innovations in conformal dose delivery and normal tissue avoidance by more precise and higher dose delivery to tumor areas. Initially used in nonresectable lung cancers, extracranial SBRT use has expanded to multiple tumor types [Citation13,Citation14]. Retrospective studies in patients with pancreatic cancer suggest SBRT may improve clinical outcomes in locally advanced pancreatic cancer, but with an accompanying increase in the risk of toxicity to nearby organs [Citation15–17]. Ablative radiation therapy with doses as high as 10 Gy per fraction have been shown to improve tumor control in patients with medically inoperable or unresectable pancreatic cancer while minimizing toxicity [Citation18–22]. Surgical resection after SBRT (50 Gy in 5 consecutive fractions) appears to be safe, with a low rate of surgical complications and high rates of margin negative resection [Citation23]. The recent Alliance trial conducted by the National Clinical Trials Network randomized patients with borderline resectable pancreatic cancer at diagnosis to either (m)FOLFIRINOX alone or (m)FOLFIRINOX plus hypofractionated radiation therapy (RT) [Citation24]. The study was not powered to answer questions about RT (only 40 patients actually received RT), but patients who did not progress prior to RT and underwent surgery had a similar 18-month OS rate as those who received chemotherapy alone. In addition, patients in the RT arm had a higher CA19-9 and higher event-free OS than patients in the chemotherapy alone arm. Furthermore, the trial utilized a lower BED (up to 40 Gy in five fractions) than what is being tested in more recent studies [Citation24,Citation25]. Therefore, additional clinical trials are needed to clarify the role of SBRT in the borderline resectable pancreatic cancer setting.
Rucosopasem is an investigational selective dismutase mimetic that rapidly converts superoxide, which is induced by radiation and damages tissue, to hydrogen peroxide () [Citation26]. Studies have shown that normal cells can tolerate hydrogen peroxide fluxes, converting it to water and oxygen [Citation27,Citation28]. This protects normal cells from oxidative damage, including radiation toxicity. However, tumor cells, including pancreatic cancer cells, appear to be susceptible to the toxic effects of hydrogen peroxide [Citation27–29]. This has been proposed to be due to reduced catalase or other peroxidase levels in cancer cells, resulting in the decreased ability of cancer cells to metabolize hydrogen peroxide [Citation27,Citation28]. In addition, increased production of superoxide has been reported in cancer cells and has been suggested to contribute to uncontrolled mitosis; dismutation of superoxide would, therefore, be expected to impair mitosis in cancer cells [Citation28]. These data suggest that dismutase mimetics have the potential to improve efficacy when used in combination with SBRT, without compromising safety.
Selective dismutase mimetics rapidly and specifically convert superoxide to hydrogen peroxide. Normal cells tolerate hydrogen peroxide fluxes better than cancer cells.
DM: Dismutase mimetic; SOD: Superoxide dismutase.
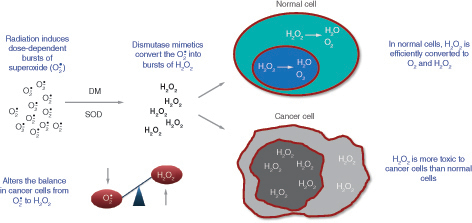
Preclinical and early clinical studies support a synergistic anticancer efficacy effect when a dismutase mimetic is used in combination with SBRT () [Citation29]. In an experimental xenograft mouse model, treatment with a dismutase mimetic augmented the antitumor activity of radiation delivered at doses above approximately 7 Gy per fraction, and this enhancement increased with increasing radiation dose per fraction [Citation29]. The synergy was dependent on the generation of hydrogen peroxide as evidenced by abrogation of the radio-sensitizing effect when catalase was overexpressed, thereby removing hydrogen peroxide. In an exploratory analysis of a phase I/II trial in patients with pancreatic cancer, median overall survival was 17.0 months in patients receiving a dismutase mimetic in combination with SBRT, compared with 13.3 months for placebo with SBRT (HR: 0.48; nominal p = 0.0899; data on file). Local tumor control, time to metastases, and progression-free survival were also improved, with similar rates of adverse events (AEs) in the treatment and control arm.
In an H1299 xenograft model, the selective dismutase mimetic, avasopasem, increased tumor growth delay when given 30 to 60 min before SBRT and daily for 4 days after SBRT. Enhancement of SBRT-mediated tumor growth delay increased with increasing radiation dose per fraction. Average tumor volumes (left column), individual tumor volumes (middle column), and Kaplan–Meier analysis using the IACUC threshold of 1000 mm3 tumor volume as a proxy for survival are shown. Eight to ten animals were included per cohort.
AAAS: American Association for the Advancement of Science; AVA: Avasopasem; Avg.: Average; Ind.: Individual; SBRT: Stereotactic body radiation therapy.
From [Citation29] www.science.org/doi/10.1126/scitranslmed.abb3768. Reprinted with permission from AAAS.
![Figure 2. Synergy of dismutase mimetic and stereotactic body radiation therapy on tumor control. In an H1299 xenograft model, the selective dismutase mimetic, avasopasem, increased tumor growth delay when given 30 to 60 min before SBRT and daily for 4 days after SBRT. Enhancement of SBRT-mediated tumor growth delay increased with increasing radiation dose per fraction. Average tumor volumes (left column), individual tumor volumes (middle column), and Kaplan–Meier analysis using the IACUC threshold of 1000 mm3 tumor volume as a proxy for survival are shown. Eight to ten animals were included per cohort.AAAS: American Association for the Advancement of Science; AVA: Avasopasem; Avg.: Average; Ind.: Individual; SBRT: Stereotactic body radiation therapy.From [Citation29] www.science.org/doi/10.1126/scitranslmed.abb3768. Reprinted with permission from AAAS.](/cms/asset/6693c94b-046b-4907-817d-ecf2aa0af0e3/ifon_a_12367111_f0002.jpg)
Based on these data, the GRECO-2 study was designed to investigate whether rucosopasem can increase the anticancer efficacy of SBRT while protecting normal tissue from superoxide damage.
Methods
Study design
GRECO-2 is a phase II, multicenter, randomized, double-blind, placebo-controlled study (NCT04698915) of rucosopasem with SBRT in patients with borderline resectable or locally advanced pancreatic cancer, following initial chemotherapy with (m)FOLFIRINOX or a gemcitabine-based doublet for at least 6 weeks prior to SBRT (). After initial chemotherapy, patients will be evaluated by computed tomography (CT) and magnetic resonance imaging (MRI) for SBRT eligibility and distant disease metastases. Rigorous SBRT quality assurance has been integrated into the study design, with the first two cases at each center undergoing prospective expert review and each subsequent case undergoing submission into a site for dosimetric review to ensure treatment parameters are met with expert oversight if any issues are detected. Approximately 220 patients will be randomized at approximately 40 sites to receive rucosopasem 100 mg or placebo via intravenous (iv.) infusion over 15 min prior to each SBRT fraction (5 x 10 Gy). A list of countries where data will be collected can be found on clinicaltrials.gov (NCT04698915). At randomization, patients who are eligible for SBRT and do not have distant metastases will be stratified based on disease status, borderline resectable or unresectable, at diagnosis.
1At least 6 weeks of either (m) FOLFIRINOX or gemcitabine-based chemotherapy. There is no upper limit on the number of chemotherapy cycles administered prior to SBRT.
2Patients who remain free of metastases following induction chemotherapy will be eligible for randomization.
3Randomization stratified by disease status at diagnosis (borderline resectable vs locally advanced) as determined by a multidisciplinary tumor review group. Patients may receive additional chemotherapy following randomization and prior to SBRT treatment.
4SBRT should not be initiated until at least 1 week following the end of the last chemotherapy cycle.
5Surgical exploration within 8 weeks following SBRT.
Approximately 160 patients with borderline resectable or locally advanced pancreatic cancer following initial chemotherapy will be randomized to receive rucosopasem 100 mg or placebo via iv. infusion prior to each SBRT fraction. At randomization, patients who are eligible for SBRT and do not have distant metastases will be stratified based on disease status, borderline resectable or unresectable, at diagnosis. Patients judged to be technically and medically resectable after SBRT will be surgically explored. All patients will be followed every 3 months from the completion of SBRT for 2 years and every 6 months thereafter, for a total of up to 3 years.
iv.: Intravenous; PBO: Placebo; QD: Once daily; RUCO: Rucosopasem; SBRT: Stereotactic body radiation therapy.
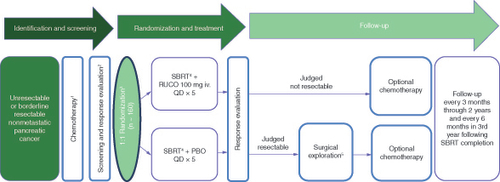
Patients judged to be technically and medically resectable after SBRT will be surgically explored within 8 weeks following SBRT, and the outcomes of margins and pathology will be collected. Following surgery, all patients may receive additional chemotherapy at the discretion of the investigator in accordance with standard of care, institutional practice, and National Comprehensive Cancer Network (NCCN) guidelines. For those not resectable at reevaluation, further management will be per standard of care guidelines in accordance with the patient’s treating physician. All patients will be followed every 3 months from the completion of SBRT for 2 years and every 6 months thereafter, for a total of up to 3 years. Late toxicity will be followed for the first year.
Study population
The study population consists of patients aged 18 or older with newly diagnosed nonmetastatic pancreatic cancer judged by multidisciplinary review to be feasible for (m)FOLFIRINOX or gemcitabine-based doublet and SBRT and either: technically unresectable; potentially resectable but the patient has declined surgery or is considered not a candidate for surgery; or borderline resectable. The participants must not have visible invasion of bulky tumor into the lumen of the bowel or stomach as determined by endoscopy as presence of bulky tumor in the lumen is a contraindication for SBRT [Citation17]. Additional eligibility criteria are summarized in .
Table 1. Inclusion and exclusion criteria.
Study interventions
Patients will receive at least 6 weeks of chemotherapy consisting of FOLFIRINOX, mFOLFIRINOX, or a gemcitabine-based doublet regimen (e.g., gemcitabine combined with nab-paclitaxel, cisplatin, capecitabine) prior to SBRT. There is no limit on the number of pre-SBRT chemotherapy cycles that a patient may receive as long as there is no evidence of metastatic spread during treatment with the initial chemotherapy regimen. Patients may change chemotherapy regimens for issues of toxicity or intolerance as long as they remain on a FOLFIRINOX, mFOLFIRINOX or a gemcitabine-based doublet regimen. Patients who commence chemotherapy with single-agent gemcitabine, but who are then able to tolerate either a gemcitabine-based doublet, FOLFIRINOX or mFOLFIRINOX may also be included in the trial if they fulfill the requirements for at least 6 weeks of pre-SBRT chemotherapy with the regimens outlined above.
Patients will be randomized 1:1 to receive either rucosopasem 100 mg or placebo via iv. over 15 min prior to each fraction of SBRT. To mitigate the risk of radiosensitizing due to fluorouracil and gemcitabine, patients will have at least 1 week of rest between the final chemotherapy cycle and the start of SBRT.
SBRT will be administered as five fractions of 10 Gy applied to the residual gross tumor volume (GTV) with a 3 mm margin to the clinical target volume (CTV) that also includes areas of suspected perineural and vascular invasion to at least 30 Gy, while normal tissue constraints overrule GTV coverage criteria. Fractions will be given sequentially daily within 180 min from the end of rucosopasem or placebo infusion. All five fractions must be given within a maximum of 10 calendar days, with a minimum of 18 h and a maximum of 72 h (96 h in instances where a holiday or machine breakdown interferes with administration) between fractions.
All patients, investigators and study personnel involved in the conducting of the study will be blinded to treatment assignment. Randomization codes are generated and assigned using a Randomization and Trial Supply Management System. Electronic access to the codes will be granted in cases where the treatment assignment is needed by the safety team, risk management, or drug supply oversight.
Planned study period
The duration of the study is expected to be up to approximately 41 months including the 28-day screening period, chemotherapy, SBRT, surgery where possible, and additional chemotherapy and follow-up for survival, progression, and long-term safety. If after the 90-day AE follow-up period there is distant disease progression, patients will be followed every 3 months through 2 years post-SBRT and then every 6 months through 3 years post-SBRT to assess survival status.
Study objectives & end points
The primary objective of this trial is to determine the effect of adding rucosopasem compared with placebo to SBRT on OS in patients with unresectable or borderline resectable nonmetastatic pancreatic cancer. Secondary end points include progression-free survival (PFS), locoregional control (LRC), and time to distant metastases (TDM) as well as best overall response and in-field local response. The proportion of patients in whom R0 or R1 surgical resection is achieved, pathological response in resected specimens, and acute and late toxicities observed after SBRT are additional secondary end points. Exploratory end points include PRO-CTCAE, CA19-9 normalization, and pharmacokinetics.
Study assessments
CT imaging will be used to assess secondary efficacy end points based on RECIST 1:1 and will be centrally reviewed retrospectively. MRI and CT will be used to determine SBRT eligibility and post-SBRT technical resectability. The determination will be validated by the local multidisciplinary review group.
CA19-9 values will be collected at diagnosis, screening, 4 weeks after SBRT, and every 3 months for 1 year follow-up or until documented progression. CA19-9 normalization, defined as reaching the value of 37 U/ml or below from screening across all follow-up time points, will be categorized into three categories: normalization throughout 6 months, decrease of >30% but no normalization and increase over 90 U/ml before 6 months or no significant decrease (<30%). Distribution across the categories will be compared across the groups and related to resection rates and survival.
Blood samples for pharmacokinetic (PK) analysis will be obtained on the first day of SBRT; 1) prior, 2) immediately after and 3) 24 h after the study drug infusion (but before the second infusion). PK blood samples will also be collected in the same way on day 4 of SBRT. Vital signs, including blood pressure and an electrocardiogram, will be taken at the same timepoints.
After 20 patients were randomized, an independent Data Monitoring Committee (DMC) was convened to review safety data through the week 4 visit after SBRT and recommended that the study proceed without modification.
Safety monitoring & toxicity management
AEs and serious AEs (SAEs) will be monitored and categorized using common terminology for AEs (CTCAE) version 5.0 for 90 days after SBRT completion. After 90 days, patients will be monitored for specific late toxicities (i.e., gastrointestinal toxicities, cardiovascular toxicities, and persisting hematological, neurological, kidney, or liver dysfunctions) for 1 year or until distant disease progression. In cases of grade 2 or greater hypotension occurring within 1 hour of rucosopasem, or placebo infusion or grade 3 or greater AEs judged by the investigator to be possibly attributable to rucosopasem infusion, the infusion time of rucosopasem or placebo will be extended by 15 min. Patients will be permitted three infusion modifications before they must discontinue treatment with study drug, but will continue SBRT at the discretion of the investigator.
Sample size
The sample size calculation is based on the primary end point of OS. Median OS in the control group is expected to be approximately 11 months from randomization, based on historical rates [Citation7,Citation11,Citation30], with 120 deaths needed to provide 90% power to detect a reduction in the OS hazard ratio to 0.55 using a two-sided type I error rate of 0.05 and accounting for a possible interim analysis after 84 events.
Statistical considerations
The primary efficacy analysis will be the comparison of OS between the two treatment groups using a stratified log-rank test at an overall two-sided 5% level of significance. The OS distribution will be estimated using the Kaplan–Meier method and the hazard ratio for OS will be estimated along with its 95% CI using a stratified Cox model.
Assuming that the OS is significant at the two-sided 5% significance level, PFS may be formally tested at a two-sided 5% significance level. Other secondary end points and exploratory end points will be summarized and compared descriptively. Safety including both acute (for 90 days) and late (until distant progression or 1 year) toxicities will be summarized descriptively.
Ethics
The study was approved by the ethics committee at every participating institution and was conducted according to the recommendations of Good Clinical Practice, the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines. All patients provided written informed consent to participate in the study.
Conclusion
While treatment of pancreatic cancer has advanced, survival rates remain low, highlighting the need for additional treatment approaches to improve local control and overall survival outcomes. Higher radiation dose delivery is being evaluated as a potential strategy to improve surgical resection rates and overall outcomes in patients with locally advanced or borderline resectable pancreatic cancer. The GRECO-2 trial will evaluate whether the addition of rucosopasem can increase anticancer efficacy of SBRT without an increase in toxicity, potentially leading to the future integration of dismutase mimetic therapy into the current paradigm of chemotherapy and radiation therapy.
Background & rationale
Pancreatic cancer remains one of the most lethal cancer diagnoses despite advances in treatment.
Stereotactic body radiotherapy (SBRT) may improve clinical outcomes, but may carry a greater potential for gastrointestinal toxicity when delivered at ablative doses.
Rucosopasem is an investigational selective dismutase mimetic that converts superoxide to hydrogen peroxide.
Dismutase mimetics have the potential to improve anticancer efficacy without compromising safety.
Preclinical and early clinical studies support a synergistic anticancer efficacy effect when a dismutase mimetic is used in combination with SBRT.
These data support the hypothesis that rucosopasem may increase the anticancer efficacy of SBRT while protecting normal tissue from superoxide damage.
The GRECO-2 trial
This is a phase II, multicenter, randomized, double-blind, placebo-controlled study (NCT04698915) of rucosopasem with SBRT in patients with borderline resectable or locally advanced pancreatic cancer, following initial chemotherapy with (m)FOLFIRINOX or a gemcitabine doublet for at least 6 weeks prior to SBRT.
The study population consists of patients aged 18 or older with newly diagnosed nonmetastatic pancreatic cancer judged by a multidisciplinary review group to be feasible for (m)FOLFIRINOX or gemcitabine doublet and SBRT and either: technically unresectable; potentially resectable but the patient has declined surgery or is considered not a candidate for surgery; or borderline resectable. The participants must not have visible invasion of bulky tumor into the lumen of the bowel or stomach as determined by endoscopy as presence of bulky tumor in the lumen is a contraindication for SBRT.
The primary objective of this trial is to determine the effect of adding rucosopasem compared with placebo to SBRT on overall survival in patients with unresectable or borderline resectable nonmetastatic pancreatic cancer.
Secondary end points include progression-free survival, locoregional control, and time to distant metastases as well as best overall response and in-field local response. The proportion of patients in whom R0 or R1 surgical resection is achieved, pathological response in resected specimens and acute and late toxicities observed after SBRT are additional secondary end points.
Conclusion
The GRECO-2 trial will evaluate whether the addition of rucosopasem can increase anticancer efficacy of SBRT without an increase in toxicity, potentially leading to the future integration of dismutase mimetic therapy into the current paradigm of chemotherapy and radiation therapy.
Author contributions
All authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
Acknowledgments
The authors thank all the patients and their families and the investigators and staff at the clinical sites for their participation in this study.
Financial disclosure
Funding for this study (NCT04698915 available from www.clinicaltrials.gov) was provided by Galera Therapeutics, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
SE Hoffe reports employment with MyCareGorithm, has patents, royalties or other intellectual property from UpToDate, has other relationships with Beyond the White Coat, holds stock and other ownership interests from Rittenhouse Book Distributors, received honoraria from UpToDate; received research funding from Galera Therapeutics, Inc., and has uncompensated relationships with Galera Therapeutics, Inc. and ViewRay. TA Aguilera reports research funding from Apexigen and Galera Therapeutics, Inc., patents, royalties and other intellectual property from UCSD/ Avelas Biosciences and Stanford/AKSO Biosciences, honoraria from Apexigen and stock ownership with AKSO Biopharmaceutical and Avelas Biosciences. PJ Parikh reports speakers’ fees, honoraria and institutional research funding from ViewRay, research funding from Galera Therapeutics, Inc., and stock ownership with Nuvaira. MM Ghaly reports institutional research funding from Galera Therapeutics, Inc. JM Herman reports research funding from 1440 for the Canopy Cancer Collective, travel and accommodations from Varian Medical Systems, honoraria from Boston Scientific, honoraria, stock, and other ownership interests from HistoSonics, and honoraria with MyCareGorithm. JM Caster reports institutional research funding from GSK and Galera Therapeutics, Inc. DW Kim reports research funding from Bold Therapeutics. James Costello reports honoraria from ViewRay and institutional research funding from Galera Therapeutics, Inc. MP Malafa reports research funding from Galera Therapeutics, Inc. Elizabeth C. Moser reports employment from Deka Biosciences, Inc, and advisory roles with Galera Therapeutics, Inc. EP Kennedy reports employment, leadership, and stock and other ownership interests from Galera Therapeutics, Inc. K Terry reports employment and stock with Galera Therapeutics, Inc. M Kurman reports consulting or advisory roles with Galera Therapeutics, Inc. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Medical writing and editorial support (BG Richardson, assembling tables and figures, collating author comments, copyediting, fact checking and referencing) and graphic services were provided by AOIC, LLC and were funded by Galera Therapeutics, Inc.
Ethical conduct of research
The study was approved by the ethics committee at every participating institution and was conducted according to the recommendations of Good Clinical Practice, the Declaration of Helsinki, and the Council for International Organizations of Medical Sciences International Ethical Guidelines. All patients provided written informed consent to participate in the study.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Data sharing statement
As this is a Clinical Trial Protocol, there is no data to share at this time.
References
- American Cancer Society . Cancer Facts & Figures 2023 (2023). Available at: www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf (Accessed: 19 July 2023 ).
- Murphy JE, Wo JY, Ryan DPet al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase II Clinical Trial. JAMA Oncol. 4(7), 963–969 (2018).
- Palta M, Godfrey D, Goodman KAet al. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 9(5), 322–332 (2019).
- Lambert A, Schwarz L, Borbath Iet al. An update on treatment options for pancreatic adenocarcinoma. Ther. Adv. Med. Oncol. 11, 1758835919875568 (2019).
- Maggino L, Malleo G, Marchegiani Get al. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surg. 154(10), 932–942 (2019).
- National Comprehensive Cancer Network . Pancreatic Adenocarcinoma. Version 1.2022. (Accessed 3 October 2022 ).
- Hammel P, Huguet F, van Laethem JLet al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 315(17), 1844–1853 (2016).
- Jang JY, Han Y, Lee Het al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase II/3 Trial. Ann. Surg. 268(2), 215–222 (2018).
- Versteijne E, Suker M, Groothuis Ket al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 38(16), 1763–1773 (2020).
- Versteijne E, van Dam JL, Suker Met al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 40(11), 1220–1230 (2022).
- Krishnan S, Chadha AS, Suh Yet al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 94(4), 755–765 (2016).
- Rudra S, Jiang N, Rosenberg SAet al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 8(5), 2123–2132 (2019).
- Iyengar P, Kavanagh BD, Wardak Zet al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J. Clin. Oncol. 32(34), 3824–3830 (2014).
- Timmerman R, Paulus R, Galvin Jet al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303(11), 1070–1076 (2010).
- Zhong J, Patel K, Switchenko Jet al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer 123(18), 3486–3493 (2017).
- Murphy JD, Christman-Skieller C, Kim J, Dieterich S, Chang DT, Koong AC. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 78(5), 1420–1426 (2010).
- Chuong MD, Bryant J, Mittauer KEet al. Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided Radiation Therapy With On-Table Adaptive Replanning and Elective Nodal Irradiation for Inoperable Pancreas Cancer. Pract. Radiat. Oncol. 11(2), 134–147 (2021).
- Taniguchi C, Frakes J, Aguilera Tet al. Stereotactic Body Radiation Therapy with or without a selective dismutase mimetic in pancreatic adenocarcinoma: an adaptive, randomised, double-blind, placebo-controlled phase 1b/2 trial. Lancet Oncol. 24(12), 1387–1398 (2023).
- Hassanzadeh C, Rudra S, Bommireddy Aet al. Ablative Five-Fraction Stereotactic Body Radiation Therapy for Inoperable Pancreatic Cancer Using Online MR-Guided Adaptation. Adv. Radiat. Oncol. 6(1), 100506 (2021).
- Koay EJ, Hanania AN, Hall WAet al. Dose-Escalated Radiation Therapy for Pancreatic Cancer: A Simultaneous Integrated Boost Approach. Pract. Radiat. Oncol. 10(6), e495–e507 (2020).
- Reyngold M, O’Reilly EM, Varghese AMet al. Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol. 7(5), 735–738 (2021).
- Parikh PJ, Lee P, Low DAet al. A Multi-Institutional Phase II Trial of Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided On-Table Adaptive Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 19, S0360-3016(23)00499-6 (2023) ( Online ahead of print).
- Bryant JM, Palm RF, Liveringhouse Cet al. Surgical and Pathologic Outcomes of Pancreatic Adenocarcinoma (PA) After Preoperative Ablative Stereotactic Magnetic Resonance Image Guided Adaptive Radiation Therapy (A-SMART). Adv. Radiat. Oncol. 7(6), 101045 (2022).
- Katz MHG, Shi Q, Meyers Jet al. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase II Randomized Clinical Trial. JAMA Oncol. 8(9), 1263–1270 (2022).
- Hall WA, Dawson LA, Hong TSet al. Value of Neoadjuvant Radiation Therapy in the Management of Pancreatic Adenocarcinoma. J. Clin. Oncol. 39(34), 3773–3777 (2021).
- Riley DP, Schall OF. Structure-Activity Studies and the Design of Synthetic Superoxide Dismutase (SOD) Mimetics as Therapeutics. Template Effects and Molecular Organization. Advances in Inorganic Chemistry (2006).233–263
- Doskey CM, Buranasudja V, Wagner BAet al. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox. Biol. 10, 274–284 (2016).
- El-Mahdy MA, Alzarie YA, Hemann C, Badary OA, Nofal S, Zweier JL. The novel SOD mimetic GC4419 increases cancer cell killing with sensitization to ionizing radiation while protecting normal cells. Free Radic. Biol. Med. 160, 630–642 (2020).
- Sishc BJ, Ding L, Nam TKet al. Avasopasem manganese synergizes with hypofractionated radiation to ablate tumors through the generation of hydrogen peroxide. Sci. Transl. Med. 13(593), eabb3768 (2021).
- Mellon EA, Hoffe SE, Springett GMet al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta. Oncol. 54(7), 979–985 (2015).
