Abstract
Aims: Physicians determine the treatment regimen for metastatic colorectal cancer on a case-by-case bases, according to the individual disease characteristics. We retrospectively compared the baseline characteristics and efficacies of first-line treatment among patients with metastatic colorectal cancer who received intensive therapy involving fluoropyrimidine plus oxaliplatin and/or irinotecan, potentially with molecularly targeted agents as well, versus less intensive fluoropyrimidine and/or bevacizumab therapy. Materials & methods: Data were collected from a medical claims database. The efficacy outcomes were: time to treatment failure, time to first subsequent therapy and overall survival. Results: The less intensive therapy group (n = 633) had higher median age, lower daily activity levels and shorter time to treatment failure, time to first subsequent therapy and overall survival than the intensive therapy group (n = 3829). Combination therapy with molecularly targeted agents and bevacizumab improved treatment efficacy outcomes in the intensive and less intensive groups, respectively. Conclusion: Patient age and daily activity levels were important factors for determining treatment intensity.
Plain Language Summary
In this study we performed a real-world data analysis of treatment for advanced colorectal cancer that had spread to other parts of patients’ bodies, by investigating the medical records of 4462 patients. We wanted to see how well different treatments worked and what kinds of patients received them. We found that the most important factors when choosing between different treatments were the patient’s age and how well they could perform their everyday tasks. We found that using specialized medicines in the intensive treatment group, and a drug called bevacizumab in the less intensive group, resulted in better patient outcomes.
Tweetable abstract
In real-world data on first-line treatment of patients with mCRC, age and daily activity levels were key in choosing intensive or less intensive therapy. Molecularly targeted agents improved outcomes with intensive therapy.
Colorectal cancer (CRC) is the third most common cancer worldwide, with >1.9 million new cases and 915,800 deaths reported in 2020 [Citation1]. Before the 1990s, metastatic CRC (mCRC) had a poor prognosis, with a median overall survival (OS) of approximately 5–8 months without treatment [Citation2–4]. The past 2 decades have seen the evolution of effective new anticancer agents, and combinatory therapies thereof, which have raised the median OS for mCRC to >30 months [Citation5–7].
Based on the results of pivotal phase III studies, doublet (fluoropyrimidine plus oxaliplatin or irinotecan) or triplet (fluoropyrimidine plus oxaliplatin and irinotecan) chemotherapy, with or without molecularly targeted agents, has been established as the standard first-line treatment for mCRC [Citation8–11]. Although these combination therapies are categorized as so-called ‘intensive therapy’ because of their capacity to dramatically improve clinical outcomes, they also fall into the classification of ‘toxic therapy’, having higher incidences and severities of adverse events, especially when used in combination with cytotoxic agents. In several studies, the incidence of grade 3–4 neutropenia and neurotoxicity after combination chemotherapy with fluorouracil, leucovorin and oxaliplatin (FOLFOX) and the incidence of grade 3–4 leukopenia, neutropenia, diarrhea and asthenia after combination chemotherapy with fluorouracil, leucovorin and irinotecan (FOLFIRI) were found to be higher than those after therapy with fluorouracil and leucovorin alone [Citation12,Citation13]. In addition, the incidence of grade 3–4 neutropenia and neurotoxicity was significantly higher after triplet chemotherapy with fluorouracil, leucovorin, oxaliplatin and irinotecan (FOLFOXIRI) than after doublet chemotherapy with FOLFIRI [Citation14].
Because of these issues, not all patients with mCRC (treated with or without molecularly targeted agents) are eligible for doublet or triplet chemotherapy. As such, these therapies should only be used in patients who otherwise have a good medical status (i.e., ‘fit’ patients). Patients for whom these intensive therapies are not indicated (because of advanced age and/or the presence of comorbidities, i.e., ‘vulnerable’ patients) preferentially receive less toxic or ‘less intensive’ therapies. To date, fluoropyrimidine monotherapy has been reported to be as effective and well tolerated in elderly patients as in younger patients [Citation15], and the efficacy of bevacizumab combination therapy has also been confirmed [Citation16–18]. The benefit of anti-EGFR antibody therapy in elderly patients with wild-type KRAS (RAS) has also been reported [Citation19,Citation20]. Therefore, several guidelines also define these therapies as ‘less intensive therapies’ for patients who are not considered candidates for ‘intensive therapy’ [Citation8–11]. In clinical practice, physicians usually decide which treatment regimen to use according to the characteristics of each patient, such as age, performance status score and degree of caregiver support. However, the selection criteria used to differentiate between ‘fit’ and ‘vulnerable’ patients are unclear due to limited data in the literature regarding baseline characteristics that may influence treatment tolerance. Furthermore, limited data exist regarding the clinical outcomes of patients who receive intensive or less intensive therapy in clinical practice. Here we conducted a retrospective analysis based on real-world data from Japan, to determine the baseline characteristics of, and evaluate treatment efficacies in, patients with mCRC who receive intensive or less intensive therapy. Because this study used real-world data, it was difficult to determine whether patients received postoperative adjuvant chemotherapy after curative resection or temporary treatment for mCRC, based solely on the descriptions found in their medical charts; therefore only patients with synchronous metastases and no prior surgeries were included in this study. We hope that the results of this study will help support future decision-making regarding first-line treatment regimens in this patient group.
Patients & methods
Study design
This retrospective observational study was conducted using electronic medical health records. Data (anonymized to protect personal information) were collected from a medical claims database provided by Medical Data Vision Co., Ltd (MDV; Tokyo, Japan). As of July 2019, MDV had collated data on approximately 28 million patients from 384 hospitals with advanced treatment capabilities, including approximately half of the designated cancer hospitals in Japan. These hospitals employed a diagnostic procedure combination (DPC) / per diem payment system.
The study protocol was approved by the Institutional Review Board of the nonprofit organization MINS (approval No. 200204). The study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000039590). The requirement for informed consent was waived because this study used only anonymized data without any identifying information. The database used in this study is the same one used in a previously reported study of fluoropyrimidine, irinotecan and angiogenesis inhibitors in the secondary treatment of mCRC [Citation21].
Patient population
The inclusion criteria for this study were as follows: CRC diagnosis between April 2008 and September 2019 using DPC disease names (060035: malignant neoplasm of the colon [including the appendix] and 060040: malignant neoplasm of the rectum/anus [from the rectosigmoid to the anus]) or ICD-10 codes (C18: malignant neoplasm of the colon; C19: rectosigmoid malignant neoplasm; and C20: malignant neoplasm of the rectum); no history of surgery for CRC; history of first-line chemotherapy for stage IV CRC; age ≥20 and ≤90 years at the start of first-line chemotherapy; and received a chemotherapy regimen that was recommended as first-line treatment by the Japanese Society for Cancer of the Colon and Rectum guidelines [Citation10] (fluorouracil and l-leucovorin with or without bevacizumab; tegafur/uracil and leucovorin with or without bevacizumab; capecitabine with or without bevacizumab; tegafur/gimeracil/oteracil potassium [S-1] with or without bevacizumab; anti-EGFR antibodies [cetuximab or panitumumab]; FOLFOX; FOLFOX with bevacizumab or anti-EGFR antibodies; FOLFIRI; FOLFIRI with bevacizumab or anti-EGFR antibodies; capecitabine plus oxaliplatin with or without bevacizumab; S-1 plus oxaliplatin with or without bevacizumab; S-1 plus irinotecan with or without bevacizumab; and FOLFOXIRI with or without bevacizumab). Our exclusion criteria were as follows: clinical suspicion of CRC; a colorectal malignancy other than adenocarcinoma, such as carcinoid, neuroendocrine tumor, neuroendocrine carcinoma, sarcoma, stromal tumor or melanoma; cancer of the appendix or anus; or a history of other malignancies.
Data items
Data were collected regarding each patient’s sex, age, activities of daily living (ADL), date of prescription of concomitant drugs (including anticancer drugs), diagnostic codes, hospitalizations, hospital scale and designated cancer hospital.
Efficacy outcomes
The primary efficacy variable of this study was time to treatment failure (TTF). TTF was defined as the time from the start of first-line chemotherapy to the end of treatment or death due to any cause. The end date was defined as the date of the last prescription. Patients with missing data at discontinuation were included up until the date of last confirmed survival. Time to first subsequent therapy (TFST) and OS were secondary efficacy end points. TFST was defined as the time from the start of first-line chemotherapy to the start of the first subsequent therapy or death. Patients with missing data regarding subsequent chemotherapy or death within 90 days after the last prescription date were censored. OS was defined as the time from the start of first-line chemotherapy to death. Progression-free survival and objective response rate were not evaluated for these efficacy analyses because relevant data could not be obtained. The reason for investigating TTF and TFST simultaneously was to confirm the robustness of the study; because a database study has many limitations, it is important to obtain similar tendencies when calculating indices under various assumptions.
Statistical analyses
We first categorized patients who had received first-line chemotherapy into ‘intensive therapy’ and ‘less intensive therapy’ groups, to investigate the differences in baseline characteristics and efficacy outcomes between them. Intensive therapy consisted of doublet (fluoropyrimidine plus oxaliplatin or irinotecan) and triplet (fluoropyrimidine plus oxaliplatin and irinotecan) chemotherapy, with or without molecularly targeted agents (bevacizumab or anti-EGFR antibodies). Less intensive therapy consisted of fluoropyrimidine monotherapy, combination therapy with fluoropyrimidine plus bevacizumab, or anti-EGFR antibody monotherapy. To determine the impact of molecularly targeted agents on intensive therapy, we then compared the efficacy outcomes of doublet or triplet chemotherapy with those of doublet or triplet chemotherapy plus molecularly targeted agents. Finally, to determine the impact of bevacizumab on less intensive therapy, we compared the efficacy outcomes of fluoropyrimidine monotherapy with those of fluoropyrimidine plus bevacizumab combination therapy.
The Kaplan–Meier method was used to estimate the median TTF, TFST and OS with 95% CIs. For the first analysis, univariate Cox regression analysis was performed to estimate the unadjusted hazard ratio (HR). For the second and third analyses, stratified log-rank tests and multivariable Cox regression analyses were performed for time-to-event end points. Age (≥/<70 years), sex (male/female), primary tumor location (right-sided [cecum, ascending colon and transverse colon], left-sided [descending colon, sigmoid colon and rectum], both, or unknown), hospital scale (≥/<500 beds), cancer hospitals designated by the Japanese government (yes/no) and Barthel index (<100/100) were included in the models. The adjusted HR was estimated using a multivariate model with 95% CIs. In the subgroup analysis, we also estimated the medians and adjusted HRs with 95% CIs and calculated p-values for the interaction between the treatment regimen and the subgroup of interest (α-level, 0.1). Categorical variables were compared using Pearson’s χ-squared test, and continuous variables were compared using the Wilcoxon rank-sum test. A two-sided significance level of 5% was used, and no correction for multiple testing was applied. Missing data were excluded from the analyses. All statistical analyses were performed using R software, v. 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
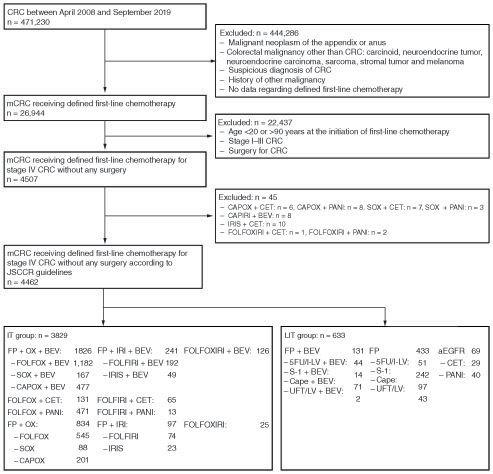
A total of 471,230 patients diagnosed with or suspected of having malignant neoplasms of the colon and rectum (according to the DPC disease names or ICD-10 codes) between 1 April 2008 and 30 September 2019 were identified in the database. After applying the inclusion and exclusion criteria, 4462 patients were included in the analysis, including 3829 who received intensive therapy and 633 who received less intensive therapy (). In the intensive therapy group, 3678 patients (96.1%) received doublet chemotherapy and 2873 patients (75.0%) received molecularly targeted agents (bevacizumab, n = 2193; anti-EGFR antibodies, n = 680). In the less intensive therapy group, fluoropyrimidine monotherapy, combination therapy with fluoropyrimidine plus bevacizumab, and anti-EGFR antibody monotherapy were administered to 433 (68.4%), 131 (20.7%) and 69 patients (10.9%), respectively. Overall, 200 patients (31.6%) received molecularly targeted agents in this group. At the data cutoff date (30 September 2019), the median follow-up times were 17.5 months in the intensive therapy group and 16.2 months in the less intensive therapy group. This study was adjusted for tumor occupancy site at the analysis stage. A subgroup analysis of tumor occupancy site was performed as detailed in . A poorer average prognosis was found for right-sided cases, which is further detailed in the Discussion section.
Figure 1. Patient flow chart.
aEGFR: Anti-epidermal growth factor receptor; BEV: Bevacizumab; Cape: Capecitabine; CAPIRI: Capecitabine and irinotecan; CAPOX: Capecitabine and oxaliplatin; CET: Cetuximab; CRC: Colorectal cancer; FOLFIRI: Fluorouracil, leucovorin and irinotecan; FOLFOX: Fluorouracil, leucovorin and oxaliplatin; FOLFOXIRI: Fluorouracil, leucovorin, oxaliplatin and irinotecan; FP: Fluoropyrimidine; 5-FU: Fluorouracil; IRI: Irinotecan; IRIS: S-1 and irinotecan; IT: Intensive therapy; JSCCR: Japanese Society for Cancer of the Colon and Rectum; LIT: Less intensive therapy; l-LV: l-Leucovorin; LV: Leucovorin; mCRC: Metastatic colorectal cancer; OX: Oxaliplatin; PANI: Panitumumab; S-1: Tegafur/gimeracil/oteracil potassium; SOX: S-1 and oxaliplatin; UFT: Tegafur/uracil.
Baseline characteristics
The proportions of patients with older ages (median: 67 vs 75 years; p < 0.001) and right-sided primary tumors (31.6 vs 37.7%; p = 0.005), those starting first-line chemotherapy between 2008 and 2014 (29.0 vs 33.2%; p = 0.035) and those who had Barthel index scores of <100 (10.4 vs 21.0%; p < 0.001) were significantly lower in the intensive therapy group than in the less intensive therapy group ( & Supplementary Table 1). This study could only evaluate items that were included in the dataset. The study data included ten ADL items obtained at the time of hospitalization, and patient performance status was assessed using items that expressed each patient’s condition. The Barthel index score for each patient was calculated using the ADL data. In the intensive therapy group, younger patients, those with left-sided primary tumors or Barthel index scores of 100 and patients from large hospitals (≥500 beds) or designated cancer hospitals frequently received combination chemotherapy with molecularly targeted agents (Supplementary Table 2). The proportions of patients aged 70 years or older and those with Barthel index scores of <100 were both significantly higher in the less intensive treatment group than in the intensive treatment group (Supplementary Table 3). Among the patients who received less intensive therapy, the proportion of patients who were older and had Barthel index scores of <100 was significantly higher in patients who received combination therapy with fluoropyrimidine plus bevacizumab than in those who received fluoropyrimidine monotherapy (Supplementary Table 3).
Table 1. Patient characteristics.
Efficacy
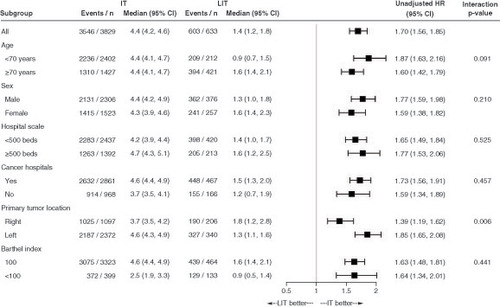
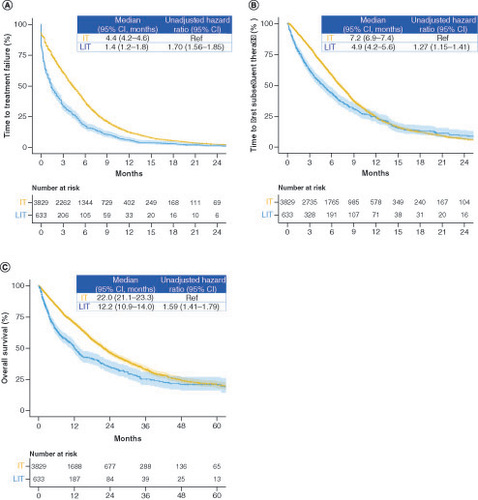
The median TTF (4.4 vs 1.4 months; HR: 1.70; 95% CI: 1.56–1.85), TFST (7.2 vs 4.9 months; HR: 1.27; 95% CI: 1.15–1.41) and OS (22.0 vs 12.2 months; HR: 1.59; 95% CI: 1.41–1.79) were longer in the intensive therapy group than in the less intensive therapy group (). In the TTF subgroup analysis, the primary efficacy outcome was worse in all subgroups in the less intensive therapy group than in the intensive therapy group. A statistically significant difference was observed in the interaction test between age and primary tumor location (). Subgroup analyses of TFST and OS showed similar trends to that of TTF (Supplementary Figure 1).
Figure 3. Time to treatment failure, time to first subsequent therapy and overall survival in the intensive and less intensive therapy groups.
Kaplan–Meier curves of (A) time to treatment failure, (B) time to first subsequent therapy and (C) overall survival.
IT: Intensive therapy; LIT: Less intensive therapy.
In the intensive therapy group, combination therapy with molecularly targeted agents significantly improved the median TTF (2.1 vs 5.3 months; HR: 0.50; 95% CI: 0.46–0.54; stratified log-rank p < 0.001), TFST (5.2 vs 7.9 months; HR: 0.62; 95% CI: 0.57–0.68; stratified log-rank p < 0.001) and OS (16.1 vs 23.6 months; HR: 0.74; 95% CI: 0.66–0.83; stratified log-rank p < 0.001) compared with combination therapy without molecularly targeted agents (). Among patients treated with combination therapy involving molecularly targeted agents, there was no significant difference in the median TTF (5.3 vs 4.9 months; HR: 1.00; 95% CI: 0.91–1.09; stratified log-rank p = 0.884) and TFST (8.0 vs 7.8 months; HR: 0.96; 95% CI: 0.86–1.06; stratified log-rank p = 0.471) between patients who received bevacizumab versus anti-EGFR antibodies. However, OS was significantly longer in patients who received combination chemotherapy with bevacizumab than in those who received combination chemotherapy with anti-EGFR antibodies (median OS: 24.2 vs 22.0 months; HR: 0.80; 95% CI: 0.70–0.92; stratified log-rank p = 0.006). The subgroup analysis of TTF showed a better trend in patients treated with molecularly targeted agents than in those treated without molecularly targeted agents across all subgroups (). There were statistically significant differences in the interaction effects of age, hospital scale and cancer hospital on TTF. The subgroup analyses of TFST and OS showed similar trends to that of TTF (Supplementary Figure 2).
Figure 4. Time to treatment failure, time to first subsequent therapy and overall survival in the groups receiving treatment with and without molecularly targeted agents.
Kaplan-Meier curves of (A) time to treatment failure, (B) time to first subsequent therapy and (C) overall survival.
MTA: Molecularly targeted agent; w/: With; w/o: Without.
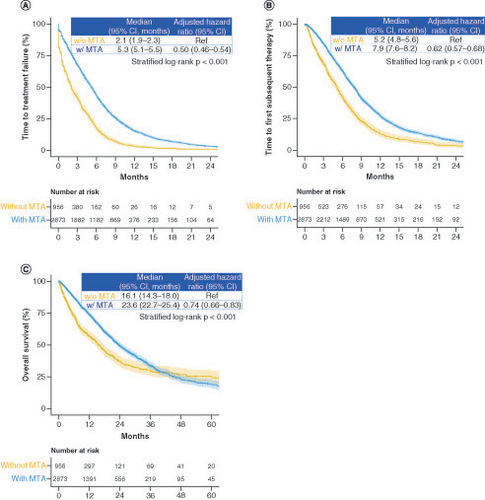
Figure 5. Subgroup analysis of time to treatment failure between the groups treated.
(A) With and without a molecularly targeted agent and (B) with and without bevacizumab.
BEV: Bevacizumab; HR: Hazard ratio; MTA: Molecularly targeted agent.
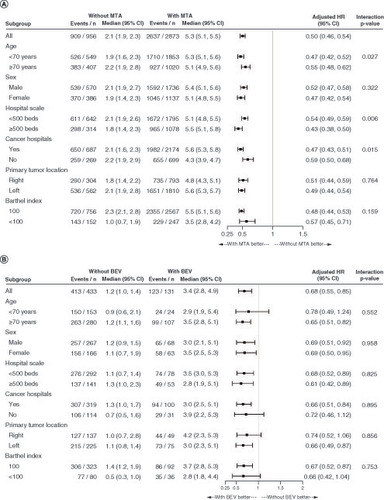
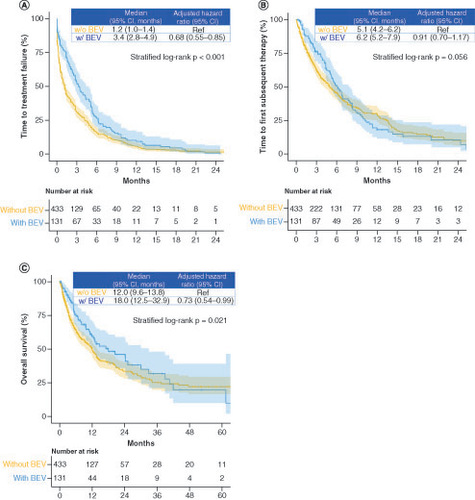
In the less intensive therapy group, combination therapy with fluoropyrimidine plus bevacizumab significantly improved the median TTF (1.2 vs 3.4 months; HR: 0.68; 95% CI: 0.55–0.85; stratified log-rank p < 0.001) and OS (12.0 vs 18.0 months; HR: 0.73; 95% CI: 0.54–0.99; stratified log-rank p = 0.021) but not the TFST (5.1 vs 6.2 months; HR: 0.91; 95% CI: 0.70–1.17; stratified log-rank p = 0.056), compared with fluoropyrimidine monotherapy (). In the subgroup analysis of TTF, patients treated with combination therapy (fluoropyrimidine plus bevacizumab) tended to perform better than those treated with fluoropyrimidine monotherapy, and there was no significant difference in the interaction test (). However, our subgroup analysis of OS did not reveal a beneficial trend for combination therapy with bevacizumab among younger patients (<70 years) and patients with Barthel index scores of <100 (Supplementary Figure 3).
Discussion
There are various treatment options for mCRC, and the choice of which to use is typically determined based on the characteristics of each tumor, patient and treatment [Citation8]. Although doublet or triplet chemotherapy is a standard first-line treatment for mCRC, it cannot be used in all patients due to its toxicity profile. In clinical practice, the true indications for intensive therapy are unclear and are usually left to the judgment of each physician. This study was not interventional or observational but was an analysis that used claims data. In general, it is desirable for patients with right-sided tumors to receive more intensive treatment; however, some patients choose to receive less intensive treatments based on their backgrounds or the judgments of their attending physicians. Because the patients in this study began treatment between April 2008 and September 2019, we assumed that many patients were selected for treatment regardless of primary tumor location. In this study, a large-scale analysis of individual patient data collected from a medical database revealed that older age and lower ADL were important factors for choosing less intensive therapy. Abundant data from clinical trials of less intensive therapies in older patients or in patients with poor Eastern Cooperative Oncology Group performance status (ECOG PS) scores may have also influenced this result [Citation16–19,Citation21].
In this study, patients with right-sided primary tumors received less intensive therapy more frequently than those with left-sided tumors. Although primary tumor location has been reported to be a prognostic factor in mCRC and a predictor of response to anti-EGFR antibodies [Citation22], it is unclear whether it influences the decision to choose between intensive and less intensive therapies. Shida et al. reported that compared with patients with left-sided tumors, those with right-sided tumors were older and had poorer ECOG PS scores, higher prevalences of peritoneal metastases and higher frequencies of poorly differentiated or mucinous histological tumor types [Citation23]. These differences in baseline characteristics between patients with right-sided tumors may have affected our results.
The chemotherapy data in the MDV database consisted solely of prescriptions, and information on tumor response or reasons for treatment termination (e.g., disease progression or adverse events) was unavailable. Therefore it was not possible to assess progression-free survival or response rates. In addition, if patients were transferred to other hospitals, data on their survival and subsequent treatment could not be tracked. Therefore we used TTF as the primary efficacy outcome and TFST and OS as secondary efficacy outcomes. Because this study was an analysis based on medical record entries reflecting daily clinical practice, it was difficult to determine the exact reasons for delays between the end of primary treatment and the beginning of secondary treatment, but possible reasons include recovery time from adverse events during the previous treatment, timing of clinical decision due to timing of imaging and patient intent.
All efficacy outcomes in our study were better in the intensive therapy group than in the less intensive therapy group; however, no statistical tests or adjustments were performed because of the large treatment selection bias, especially regarding patient characteristics. In some cases, less intensive therapy is the only option, due to a patient’s medical condition. In other cases, a sequential treatment approach starting with less intensive therapy may be an option when the tumor is asymptomatic, non-life-threatening, non-bulky or slow-progressing. Kwakman et al. reported that primary tumor location and synchronous metastatic disease were the main predictors of improved survival with combination or sequential chemotherapy [Citation24]. In the subgroup analysis regarding tumor location, our results supported those of their study in that prognosis for the right side was significantly worse than that of the left side, regardless of whether patients received molecularly targeted agents, and with regard to the fact that patients who received combination therapy with molecularly targeted agents and bevacizumab had better prognoses. We suggest exercising caution when considering tumor location as the primary factor in decision-making for first-line treatment regimens. However, the subgroup analysis in this study – which included only synchronous metastases without prior surgery – did not identify any patients for whom less intensive therapy was more appropriate than intensive therapy.
In the intensive therapy group, 75% of patients received molecularly targeted agents, and combination therapy with molecularly targeted agents significantly improved all efficacy outcomes. These trends were maintained in most subgroups. The efficacy of combination therapy with molecularly targeted agents has been shown in previous reports [Citation25–28]. There may be several reasons for the poorer long-term survival data observed in patients treated with bevacizumab compared with conventional therapy. First, our analyses were conducted using data from routine clinical practice, collected from a medical claims database. Second, all tumors were stage IV at diagnosis and were unresectable. Due to the data cutoff date (30 September 2019), the median follow-up period was as short as 17.5 months in the intensive therapy group and 16.2 months in the less intensive therapy group. In particular, there were very few ‘not at risk’ data after 36 months, as shown in ; therefore it is difficult to determine the reliability and validity of these data. In this study, we compared combination therapy with bevacizumab or anti-EGFR antibodies, and our results revealed that bevacizumab was significantly more effective than anti-EGFR antibodies in terms of improving OS. Because of the inconsistency of results reported in previous phase III studies, it is debatable whether bevacizumab or anti-EGFR antibodies should be used in combination with doublet chemotherapy as first-line treatments for (K)RAS wild-type mCRC [Citation29–31]. However, the results of a pooled analysis suggest that RAS status is a strong predictor of the efficacy of anti-EGFR antibodies. As such, anti-EGFR antibodies are preferable in patients with wild-type RAS and left-sided tumors [Citation22]. We were unable to compare the efficacies of bevacizumab and anti-EGFR antibodies in this regard because the RAS status of the patients was unknown. Furthermore, some cases included in this study were managed before some predictors of efficacy – such as RAS testing and primary tumor location – were introduced into clinical practice.
In the less intensive therapy group, a small number of patients were treated with anti-EGFR antibody monotherapy (n = 69); therefore we only investigated the impact of bevacizumab on efficacy outcomes. Combination therapy with fluoropyrimidine plus bevacizumab significantly prolonged TTF and OS, but not TFST. TFST may not have been prolonged due to the small number of events. Most patients treated with less intensive therapy may have been in poor medical condition to begin with, which may have made it difficult for them to receive second-line treatments. The rate of second-line treatment in this study was relatively low (32.1%) and was comparable to those reported in previous clinical trials of older or frail patients (21–65%) [Citation16–19]. In our subgroup analysis of TTF, bevacizumab combination therapy resulted in a better trend in all subgroups. On the other hand, in our subgroup analysis of OS, bevacizumab combination therapy did not show a better trend in patients aged <70 years or in those with Barthel index scores of <100. Therefore, bevacizumab combination therapy may not be useful for younger patients who are not candidates for intensive care, or who have inadequate ADL. However, due to the small number of patients in this analysis, a definitive conclusion could not be drawn.
Limitations
Our study had some limitations that should be acknowledged. First, the database contained limited information regarding patient baseline characteristics. No data were available regarding ECOG PS and RAS/BRAF mutation status, which are well-known predictive and prognostic factors of the efficacy of chemotherapy and survival in mCRC [Citation10]. Second, the accurate dose intensity could not be calculated for each regimen – especially for patients who received oral fluoropyrimidines such as capecitabine, S-1, and tegafur/uracil + leucovorin – because the actual number of dosing days was unknown. Third, in the efficacy analysis, the progression-free survival and response rates could not be evaluated. It was also difficult to determine whether the prescribed treatment in the database was adjuvant chemotherapy for curatively resected CRC or first-line chemotherapy for mCRC. For this reason, only patients with synchronous metastases and no prior surgeries were included in this study. Therefore the median OS was shorter in the intensive and less intensive therapy groups than has been reported previously [Citation5–7,Citation16–19]. Fourth, a safety analysis could not be performed because the MDV database did not contain information regarding adverse events according to the Common Terminology Criteria for Adverse Events or Medical Dictionary for Regulatory Activities [Citation32,Citation33 ]. Thus adverse events could only be inferred from the medical procedures and disease names that were recorded. Information regarding adverse events that did not require medical procedures was unavailable, and it was not possible to determine whether the medical procedures that were performed were associated with chemotherapy or not. Therefore, based on our dataset, we could not conclude whether intensive therapy confers more benefits in terms of safety and patient-reported outcomes. However, our findings did reveal differences in baseline characteristics between the intensive and less intensive therapy groups. In addition, our analysis of real-world data revealed the impact of molecularly targeted agents in the intensive therapy group, and of bevacizumab in the less intensive therapy group. Therefore we believe that our findings are clinically valuable.
Conclusion
As this study used a retrospective cohort design, we could not draw definitive conclusions. However, our analysis of real-world data revealed that patient age and ADL were important factors in the decision of whether to select intensive or less intensive therapy for the treatment of mCRC. Furthermore, molecularly targeted agents in the intensive therapy group, and bevacizumab in the less intensive therapy group, seemed to improve treatment efficacy outcomes.
Summary points
Doublet or triplet chemotherapy with or without molecularly targeted agents has been widely accepted as intensive first-line treatment for patients with metastatic colorectal cancer (mCRC) who are in otherwise good medical condition.
Less intensive treatments, such as fluoropyrimidine monotherapy with or without bevacizumab, are administered to patients who are in poorer clinical condition. However, the selection criteria for and clinical outcomes of each treatment option remain unclear.
Large-scale real-world data revealed that patient age and activities of daily living were important factors for selecting therapy intensity.
Molecularly targeted agents in the intensive therapy group, and bevacizumab in the less intensive therapy group, improved treatment efficacy and outcomes.
There are various treatment options for mCRC, and the choice of which to use is typically determined based on the characteristics of the tumor, patient and treatment. Age and activities of daily living should be considered important factors for the selection of treatment intensity in clinical practice.
Based on the results of pivotal phase III studies, intensive therapy with or without molecularly targeted agents has been established as the standard first-line treatment for mCRC.
Our study showed that these results could also be adapted to real-world patients whose characteristics do not fully match the enrollment criteria of clinical studies.
Limited data are available regarding the clinical outcomes of patients who received less intensive therapy. Less intensive bevacizumab combination therapy may be optimal for patients for whom intensive therapy is not indicated.
Author contributions
K Yamazaki: conceptualization, investigation, methodology, project administration, resources, supervision, visualization and writing (review and editing). S Yuki: conceptualization, visualization and writing (review and editing). E Oki: conceptualization, visualization and writing (review and editing). F Sano: conceptualization, investigation, methodology, resources, visualization and writing (review and editing). M Makishima: conceptualization, investigation, methodology, resources, visualization and writing (review and editing). K Aoki: conceptualization, investigation, methodology, resources, visualization and writing (review and editing). T Hamano: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, visualization and writing (review and editing). K Yamamoto: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, visualization. All authors have read and approved the final version of the manuscript.
Acknowledgments
The authors would like to thank E Nemoto and M Moro for their excellent assistance, and would like to acknowledge Editage (https://www.editage.com/) for English-language editing.
Writing disclosure
Writing assistance (English-language editing) was provided by Editage and supported by the 22nd Century Cutting-Edge Medical IT Organization.
Ethical conduct of research
The study protocol was approved by the Institutional Review Board of the nonprofit organization MINS (approval No. 200204). The study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000039590). The requirement for informed consent was waived because this study used only anonymized data without any identifying information.
Table S1. Patient characteristics and Barthel indexscores
Download MS Word (29.5 KB)Table S2. Patient characteristics in the with- and without-molecularly-targeted agent (MTA)subgroups.
Download MS Word (32.3 KB)Table S3. Patient characteristics in the IT (intensive therapy) and LIT(less-intensive therapy) subgroups.
Download MS Word (32.2 KB)Supplementary Material
Download TIFF Image (852.2 KB)Supplementary Material
Download TIFF Image (912.9 KB)Supplementary Material
Download TIFF Image (873.4 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2022-1284
Financial & competing interests disclosure
This work was conducted by the 22nd Century Cutting-Edge Medical IT Organization and supported by Chugai Pharmaceutical Co., Ltd. K Yamazaki reports personal fees from Chugai Pharmaceutical Co., Ltd; Daiichi Sankyo; Yakult Honsha Co., Ltd; Takeda Pharmaceutical Co., Ltd; Bayer Yakuhin, Ltd; Merck Serono; Taiho Pharmaceutical Co., Ltd; Eli Lilly K.K.; Sanofi K.K.; Ono Pharmaceutical Co., Ltd; MSD K.K.; and Bristol-Myers Squibb Co., Ltd, outside of the submitted work. S Yuki reports personal fees from Takeda Pharma; Ono Pharma; Sanofi K.K.; Bayer; Eli Lilly; Bristol-Myers Squibb; Merck Biopharma Co., Ltd; Chugai Pharma; Taiho Pharma; Yakult Honsha; and MSD, outside of the submitted work. E Oki reports personal fees from Taiho Pharma, Bayer, Chugai Pharma, Takeda Pharma, Ono Pharma, Merck Biopharma, MSD and Yakult Honsha, outside of the submitted work. F Sano, M Makishima and K Aoki were Chugai employees during the time when the study was conducted. T Hamano reports personal fees from the 22nd Century Cutting-Edge Medical IT Organization (22CEMIT), during the time when the study was conducted and personal fees from Chugai Pharma, outside of the submitted work. T Yamanaka reports grants and personal fees from Takeda, Chugai, Boehringer Ingelheim, Taiho, Daiichi-Sankyo and Bayer; grants from Ono, Merck Serono, Astellas and Eli Lilly; and personal fees from Pfizer, Sysmex, Huya Biosciences and Gilead Sciences, outside of the submitted work. K Yamamoto reports grants and personal fees from Chugai Pharmaceutical, personal fees from Otsuka Pharmaceutical, personal fees from CMIC holdings, J-Pharma, Craif, Johokiko, Triceps, Kanagawa Prefectural Hospital Organization and Kanagawa Medical Practitioners Association; and grants from Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, Takeda Pharmaceutical, Bayer Yakuhin, Daiichi-Sankyo, Astellas and Kyowa Kirin, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Data sharing statement
Data generated and/or analyzed during this study will not be shared due to concerns about data protection regulations in our country.
References
- International Agency for Research on Cancer . GLOBOCAN 2020: estimated cancer incidence, mortality, and prevalence worldwide in 2018. Cancer fact sheets (2020). https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- Nordic Gastrointestinal Tumor Adjuvant Therapy Group . Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. J. Clin. Oncol.10(6), 904–911 (1992).
- Scheithauer W , RosenH, KornekGV, SebestaC, DepischD. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ306(6880), 752–755 (1993).
- Simmonds PC . Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ321(7260), 531–535 (2000).
- Yamada Y , DendaT, GamohMet al. S-1 and irinotecan plus bevacizumab versus mFOLFOX6 or CapeOX plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer (TRICOLORE): a randomized, open-label, phase III, noninferiority trial. Ann. Oncol.29(3), 624–631 (2018).
- Yamazaki K , NagaseM, TamagawaHet al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann. Oncol.27(8), 1539–1546 (2016).
- Yamada Y , TakahariD, MatsumotoHet al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol.14(13), 1278–1286 (2013).
- Van Cutsem E , CervantesA, AdamRet al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol.27(8), 1386–1422 (2016).
- Yoshino T , ArnoldD, TaniguchiHet al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO–ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol.29(1), 44–70 (2018).
- Hashiguchi Y , MuroK, SaitoYet al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol.25(1), 1–42 (2020).
- National Comprehensive Cancer Network . NCCN guidelines for patients: colon cancer (Version1.2022) (2022). www.nccn.org/patients/guidelines/colon/index.html
- de Gramont A , FigerA, SeymourMet al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol.18(16), 2938–2947 (2000).
- Douillard J , CunninghamD, RothADet al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet355(9209), 1041–1047 (2000).
- Falcone A , RicciS, BrunettiIet al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J. Clin. Oncol.25(13), 1670–1676 (2007).
- Folprech G , CunninghamD, RossPet al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann. Oncol.15(9), 1330–1338 (2004).
- Cunningham D , LangI, MarcuelloEet al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomized phase 3 trial. Lancet Oncol.14(11), 1077–1085 (2013).
- Nishina T , MoriwakiT, ShimadaMet al. Uracil–tegafur and oral leucovorin combined with bevacizumab in elderly patients (aged ≥75 years) with metastatic colorectal cancer: a multicenter, phase II trial (Joint Study of Bevacizumab, Oral Leucovorin, and Uracil-Tegafur in Elderly Patients [J-BLUE] Study). Clin. Colorectal Cancer15(3), 236–242 (2016).
- Moriwaki T , SakaiY, IshidaHet al. Phase II study of S-1 on alternate days plus bevacizumab in patients aged ≥75 years with metastatic colorectal cancer (J-SAVER). Int. J. Clin. Oncol.24(10), 1214–1222 (2019).
- Sastre J , MassutiB, PulidoGet al. First-line single-agent panitumumab in frail elderly patients with wild-type KRAS metastatic colorectal cancer and poor prognostic factors: a phase II study of the Spanish Cooperative Group for the Treatment of Digestive Tumours. Eur. J. Cancer51(11), 1371–1380 (2015).
- Pietrantonio F , CremoliniC, AprileGet al. Single-agent panitumumab in frail elderly patients with advanced RAS and BRAF wild-type colorectal cancer: challenging drug label to light up new hope. Oncologist20(11), 1261–1265 (2015).
- Yamazaki K , YukiS, OkiEet al. Real-world evidence on second-line treatment of metastatic colorectal cancer using fluoropyrimidine, irinotecan, and angiogenesis inhibitor. Clin. Colorectal Cancer20(3), e173–e184 (2021).
- Arnold D , LuezaB, DouillardJYet al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol.28(8), 1713–1729 (2017).
- Shida D , TanabeT, BokuNet al. Prognostic value of primary tumor sidedness for unresectable stage IV colorectal cancer: a retrospective study. Ann. Surg. Oncol.26(5), 1358–1365 (2019).
- Kwakman JJM , van KruijsdijkRCM, EliasSGet al. Choosing the right strategy based on individualized treatment effect predictions: combination versus sequential chemotherapy in patients with metastatic colorectal cancer. Acta Oncol.58(3), 326–333 (2019).
- Saltz LB , ClarkeS, Díaz-RubioEet al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol.26(12), 2013–2019 (2008).
- Van Cutsem E , KöhneCH, HitreEet al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med.360(14), 1408–1417 (2009).
- Bokemeyer C , BondarenkoI, MakhsonAet al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol.27(5), 663–671 (2009).
- Douillard JY , SienaS, CassidyJet al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol.28(31), 4697–4705 (2010).
- Heinemann V , von WeikersthalLF, DeckerTet al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomized, open-label, phase 3 trial. Lancet Oncol.15(10), 1065–1075 (2014).
- Stintzing S , ModestDP, RossiusLet al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomized open-label phase 3 trial. Lancet Oncol.17(10), 1426–1434 (2016).
- Venook AP , NiedzwieckiD, LenzHJet al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA317(23), 2392–2401 (2017).
- Common terminology criteria for adverse events or medical dictionary for regulatory activities (2017). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- Medical dictionary for regulatory activities (2022). https://admin.meddra.org/sites/default/files/guidance/file/intguide_25_1_English.pdf