Abstract
Aim: To explore treatment selection for relapsed/refractory multiple myeloma (RRMM), which remains complex due to heterogeneity of available treatments and lack of defined standard of care. Patients & methods: The Adelphi Real World MM Disease Specific Programme surveyed physicians in the USA and their patients with MM to collect real-world data on patterns and perceptions of MM treatment across lines of therapy (LOT). Results: Triplets were the most common regimens across each LOT. Physicians reported efficacy-related factors, health insurance coverage, and clinical guidelines as key determinants of treatment choice regardless of LOT. Patients identified better quality of life as the most important treatment benefit. Conclusion: The DSP RW data highlight drivers of RRMM treatment choice from physicians’ and patients’ perspectives and need for a more holistic approach to guidelines and clinical trials that encompasses patient perspectives.
Plain language summary
Multiple myeloma (MM) is an incurable cancer of white blood cells. Treatments tend to become less effective when taken for long periods of time, meaning that patients often receive several different treatments over the course of their disease. Many different drugs and drug combinations are now available for patients with MM. As there is no standardized approach for the treatment of MM, it can be challenging for physicians to choose between the various complex treatment options for their patients, and the key factors that influence physicians’ treatment choices remain unclear. In this study, we used information from a survey of physicians and their patients with MM in the USA to investigate which treatments were used most commonly and how physicians and patients made decisions about their treatment. We found that patients with MM were typically treated with a combination of three drugs which differed between patients. When deciding which treatment to prescribe to their patients, physicians primarily considered factors related to how well a treatment works and, in-turn, prolong a patient’s life, but this was also considered by physicians to be an area for improvement. While patients also favored treatments associated with a longer life, they more commonly favored those associated with a better quality of life. These findings show that quality of life is important to patients receiving treatment for MM and should be taken into account by physicians when choosing a treatment for their patients.
Multiple myeloma (MM) is a hematologic malignancy that involves the proliferation of terminally differentiated plasma cells and can be characterized by renal impairment, bone lesions, hypercalcemia and bone marrow suppression [Citation1–3]. In the USA, the incidence rate from 2014 to 2018 was 7.0 per 100,000, and from 2015 to 2019 the death rate was 3.2 per 100,000 [Citation4]. In 2022, there were an estimated 34,470 new cases of MM and 12,640 MM-related deaths in the USA [Citation4].
Although MM remains incurable, the advent of new anti-myeloma drugs and the refinement of combination approaches using new or existing therapies has improved disease management and outcomes, including those with relapsed or refractory MM (RRMM) [Citation5]. In particular, autologous stem cell transplantation (SCT) has displayed progression-free survival benefit and a significant impact on quality of life (QoL) [Citation6,Citation7]. Current drug treatment options include proteasome inhibitors (PI), immunomodulatory drugs, monoclonal antibodies (mAb), antibody-drug conjugates, chimeric antigen receptor T-cell (CAR-T) therapy, chemotherapy and, more recently, bispecific therapies [Citation8–13]. The growing number of available therapeutic options has led to increasingly complex decision-making for physicians as they aim to optimize treatment choices for patients with MM, especially as disease progresses. Treatment guidelines from the American Society of Clinical Oncology (ASCO), Cancer Care Ontario (CCO) and the European Society for Medical Oncology (ESMO) recommend triplet therapy for patients with RRMM; however, a uniform standard of care has not been defined, and factors including eligibility for SCT and the refractory nature of disease influence treatment recommendations [Citation10,Citation14].
Approximately 40% of MM patients in the real world (RW) do not meet the inclusion criteria for Phase 3 studies on which approvals are based. This represents a significantly underrepresented patient population in Phase 3 trials whose treatment decisions must be based on additional considerations [Citation15]. Therefore, RW information on the treatment and management of disease for patients with RRMM is needed to provide insight into the factors influencing treatment choice by physicians and the benefits of treatment considered most important to patients. Given that alignment of both physician and patient preferences has been associated with improved treatment adherence and outcomes [Citation16], this insight may help to inform treatment decision-making as patients progress through multiple lines of therapy (LOT). The Adelphi Real World (ARW) MM Disease Specific Programme (DSP™) was a point-in-time survey that captured data from physicians and their consulting patients with MM in US clinical practice that can be used to address gaps in the literature, including treatment selection in each LOT, which becomes more complex with later LOTs in RRMM. The RW outcomes of the survey provided an opportunity to improve understanding of current clinical practices in the USA from physician and patient perspectives across LOTs.
The aim of this study was to analyze data from physicians and their consulting patients in the USA to explore current clinical practices in RRMM treatment for patients at various points in their treatment journey. Physician attitudes and perspectives, including reasons for selecting or discontinuing a particular treatment, and areas for improvement of current treatment options were examined, as were patients’ perspectives on their treatments.
Patients & methods
Study population
This study drew data from the ARW MM DSP RW survey of physicians and their consulting patients with MM in the USA between August 2020 and July 2021. The DSP methodology has been described and validated previously [Citation17–19].
A geographically representative sample of physicians across the USA who were actively involved in prescribing decisions for patients with RRMM, were recruited to participate in the survey by local fieldwork agents. Physicians were required to have seen a minimum of six patients with RRMM per month, although to ensure physicians with lower caseloads were considered, a degree of flexibility with these screening criteria was applied.
The patient population was generated by convenience sampling, whereby the next eight eligible patients with MM seen by the physician after the study start date were invited to participate in the study. These eight patients comprised a quota of two patients per LOT (from first line [1L] to fourth line and later [4L+]). Eligible patients were aged at least 18 years old, had a confirmed diagnosis of MM, and were receiving active drug treatment at the time of data collection. Patients participating in a clinical trial for MM and those no longer receiving an active systemic drug treatment for their MM (e.g., those receiving palliative care only) were excluded.
Data sources
Data were collected from four ARW MM DSP sources that included subjective and objective elements: (I) an attitudinal physician survey on physician perceptions and treatment decisions; (II) a physician workload survey completed by the physician for each day over a 5-day period to record the number of patients they consult with; (III) detailed physician-completed patient record forms (including patient history and treatment) for each of their next eight patients and (IV) a patient self-completion questionnaire voluntarily completed by a subset of patients whose information was recorded in the patient record form immediately after consultation and independent from the physician.
Outcomes
This study assessed physician and patient characteristics, treatment patterns and physician and patient perspectives on MM treatment, including physicians’ reasons for treatment selection. The physician surveys were used to assess physician characteristics as well as their perspectives on MM treatment. The physician workload surveys were used to assess the number of patients managed by physicians. The patient record forms were used to assess patient characteristics and treatment patterns (including factors influencing their choice of treatment, level of satisfaction with treatment choice, areas for improvement, and reasons for treatment cessation, all provided as a list of pre-defined responses that physicians were asked to select all that applied). As retrospective data were captured, data from the same patients may be included in the results for multiple LOTs. The patient self-completion questionnaires were used to assess patients’ perspectives on the most important benefits of treatment, other than a cure. The choice of most important treatment benefit was an open text question where patients were permitted to provide more than one response.
For patients beyond 1L, retrospective data on treatment patterns for their prior LOTs were captured; for example, for patients in 4L, data were collected on their treatments received in third line (3L), second line (2L) and 1L.
Data analysis
Analyses focused on subgroups of interest that were stratified by physician characteristics (practice setting: academic vs community-based) as determined by the surveys, and also patient characteristics (LOT: 1L vs 2L vs 3L vs 4L; SCT status: SCT vs no SCT) as determined by the physician-completed patient-report forms. Patients in 4L and beyond (4L+) were combined when assessing treatment patterns by LOT.
Descriptive statistics (median, interquartile range [IQR] and frequency) were calculated. Categorical variables are presented as frequency and percentage, ordinal variables are reported as frequency and percentage and/or median (IQR), and continuous variables are presented as the mean (standard deviation [SD]) or median (IQR) and range. Responses were analyzed as observed, with no imputation of missing data or aggregation across questions.
Study ethics
This research was submitted to the Western Institutional Review Board, study protocol number AG8847. Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines, and as such it did not require ethics committee approval. Patients provided informed consent to take part in the survey, and data were collected and pseudo-anonymized such that patients and physicians could not be identified directly. Each survey was performed in full accordance with relevant legislation at the time of data collection.
Results
Physician & patient characteristics
A total of 63 physicians (61 hemato-oncologists and two hematologists) responsible for managing patients with MM participated in the survey. The physicians that completed at least one patient record (n = 49) completed a mean (SD) of 7.7 (2.97) forms per physician. Of the participating physicians, 28 (44%) were in academic practices and 35 (56%) were in community-based practices. At data collection, physicians were managing a median (IQR) of 52 (40.0–83.8) patients with MM and 20 (32%) participated in the Oncology Care Model () [Citation20].
Table 1. Physician demographics at time of data collection.
Physicians completed patient record forms for a total of 377 patients (Supplementary Table 1). The majority of patients were male (67%) and of white race (64%), with a mean (SD) age of 68.3 (8.14) years. Overall, at the time of data collection, 61% of patients had an Eastern Cooperative Oncology Group (ECOG) score of 1, 36% had an International Staging System (ISS) disease stage of II and 45% had an ISS disease stage of III, and for the 83% of patients with a known date of MM diagnosis, the median (IQR) time since MM diagnosis was 20.8 (6.5–41.3) months. In total, 195 patients (52%) received a biomarker test for cytogenetic risk, with 61% identified as having no risk and 39% identified as having cytogenetic risk (defined by the presence of del[17p], t[4;14], t[14;16], del[17/17p], gain 1q genetic abnormalities [Citation21]). At the time of data collection, 118 patients (31%) were treated in 1L, 90 (24%) in 2L, 85 (23%) in 3L, and 84 (22%) in 4L+.
Treatment patterns by LOT
Data on current or retrospective treatments were available for all 377 patients in 1L, for 259 patients in 2L, for 169 patients in 3L and for 84 patients in 4L+. Patients typically received combination therapies across all LOTs, with triplet regimens the most commonly administered (1L: 76%, 2L: 78%, 3L: 63%, 4L+: 39%; Supplementary Figure 1). The most common triplet regimens were bortezomib/lenalidomide/dexamethasone (VRd, 51%) in 1L; carfilzomib/lenalidomide/dexamethasone (KRd, 17%) or daratumumab/lenalidomide/dexamethasone (DRd, 17%) in 2L; KRd (13%) in 3L; and daratumumab/pomalidomide/dexamethasone (DPd, 6%), elotuzumab/pomalidomide/dexamethasone (EPd, 6%), or isatuximab/pomalidomide/dexamethasone (IsPd, 6%) in 4L+ (). Doublet regimens accounted for 17% of treatments in 1L, 14% in 2L, 25% in 3L and 38% in 4L+, while monotherapies accounted for 2% in 1L, 6% in 2L, 8% in 3L and 18% in 4L+ (Supplementary Figures 1 & 2).
Patients may be included in multiple LOTs as retrospective data were captured.
1L: First line; 2L: Second line; 3L: Third line; 4L+: Fourth line and beyond; B: Bendamustine; C: Cyclophosphamide (including high-dose [cytotoxin]); D: Daratumumab; d: Dexamethasone; Do: Doxorubicin; E: Elotuzumab; Is: Isatuximab; Ix: Ixazomib; K: Carfilzomib; LOT: Line of therapy; P: Pomalidomide; R: Lenalidomide; S: Selinexor; T: Thalidomide; V: Bortezomib.
![Figure 1. Treatment patterns by line of therapy among patients receiving triplet regimens for multiple myeloma.Patients may be included in multiple LOTs as retrospective data were captured.1L: First line; 2L: Second line; 3L: Third line; 4L+: Fourth line and beyond; B: Bendamustine; C: Cyclophosphamide (including high-dose [cytotoxin]); D: Daratumumab; d: Dexamethasone; Do: Doxorubicin; E: Elotuzumab; Is: Isatuximab; Ix: Ixazomib; K: Carfilzomib; LOT: Line of therapy; P: Pomalidomide; R: Lenalidomide; S: Selinexor; T: Thalidomide; V: Bortezomib.](/cms/asset/6c487a32-9040-49ac-9a74-b6480c4e1943/ifon_a_12333824_f0001.jpg)
Across 1L, 2L and 3L, PI in combination with an immunomodulatory drug was the most common treatment class used (1L: 61%, 2L: 28%, 3L: 26%, 4L: 8%), while in 4L+ PI was more commonly used alone (19%). Overall, retreatment with the same class of drug occurred in 48% of patients treated with a PI, in 46% of those treated with an immunomodulatory drug and in 4% of those treated with an anti-CD38 antibody.
Physician-reported treatment attributes that generally influence treatment selection for each LOT
When considering the top five most important treatment attributes that generally influence treatment selection, ‘prolonged overall survival’ (OS; 1L: 70%, 2L: 70%, 3L: 56%, 4L: 59%), ‘durable response’ (1L: 60%, 2L: 49%, 3L: 44%, 4L: 41%), ‘high median progression-free survival’ ‘(PFS; 1L: 48%, 2L: 52%, 3L: 51%, 4L: 51%)’, ‘achieves a deep response’ (1L: 46%, 2L: 35%, 3L: 40%, 4L: 30%) and ‘evidence-based efficacy overall’ (1L: 40%, 2L: 35%, 3L: 33%, 4L: 32%) were most frequently indicated by physicians across all LOTs and independent of practice setting (academic or community-based). ‘Long-term safety’ was in the top ten most frequently indicated treatment attributes that influenced 1L and 2L therapy selection, but not 3L or 4L selection. On the other hand, ‘improved patient QoL’ was not one of the top ten most frequently indicated for 1L-3L treatment selection but was considered by 24% of physicians when selecting 4L treatment options ().
For stratification, physician practice setting was assigned to each patient record form based on the survey response of the treating physician. Data are presented as the relative frequency (%) of observed responses for each influential factor of drug treatment choice.
1L: First line; 2L: Second line; 3L: Third line; 4L: Fourth line; CR: Complete response; ORR: Overall response rate; OS: Overall survival; PFS: Progression-free survival; QoL: Quality of life; VGPR: Very good partial response.
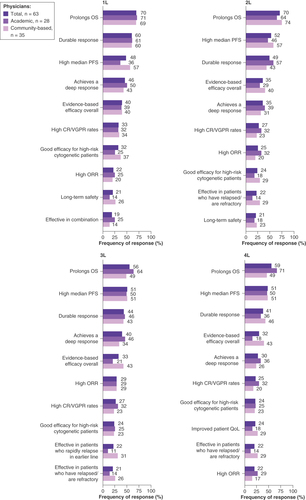
A numerically higher proportion of physicians in community-based settings versus those in academic settings reported ‘effective in patients who have relapsed/are refractory’ as an important factor in 2L+ treatment selection (2L: 29 vs 14%, 3L: 26 vs 14%, 4L: 29 vs 14%, respectively), as well as ‘improved patient quality of life’ in 4L treatment (29 vs 18%).
Physician-reported reasons for selecting a particular treatment for each patient in each LOT
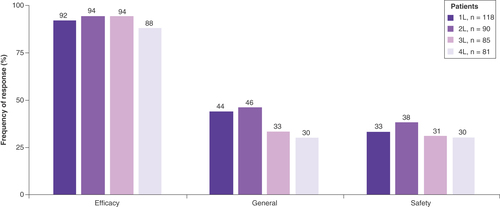
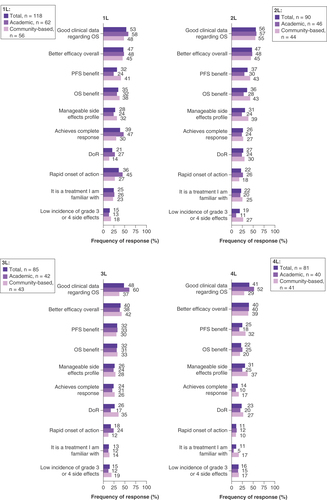
When considering the factors that influenced the selection of their patients’ current MM treatment, almost all physicians stated factors in the efficacy-related category for each LOT (1L: 92%, 2L: 94%, 3L: 94%, 4L: 88%), while fewer physicians reported consideration of factors in the safety-related category (1L: 33%, 2L: 38%, 3L: 31%, 4L: 35%; ). The specific treatment attributes considered most important in driving this treatment selection were ‘good clinical data regarding OS’ (1L: 53%, 2L: 56%, 3L: 48%, 4L: 41%) and ‘better efficacy overall’ (1L: 47%, 2L: 47%, 3L: 40%, 4L: 40%) across all LOTs (). The influence of factors attributed to efficacy on physicians’ choice for MM treatment at each LOT was observed regardless of whether their patient had received SCT (Supplementary Figure 3).
Data are presented as the relative frequency (%) of a reported response for each category of influential factors: efficacy (rapid onset of action, better efficacy overall, good clinical data regarding OS, duration of response, achieves complete response, achieves partial response, achieves stable disease, progression-free survival benefit, OS benefit, impacts MRD, depth of response), safety (manageable side effects profile, low incidence of Grade 3 or 4 side effects, safe for long-term use, has few contraindications). General it is a treatment I am familiar with, maintains or improves patient’s performance status, maintains or improves patient’s QoL, high compliance rate, convenient dosing regimen, mode of administration, standard of care, recommended by national guidelines, in accordance with clinical guidelines, included in hospital formulary, easy to secure reimbursed access, positive cost:benefit ratio, cost (in the absence of consideration of benefit).
1L: First line; 2L: Second line; 3L: Third line; 4L: Fourth line; MRD: Minimal residual disease; OS: Overall survival; QoL: Quality of life.
For stratification, physician practice setting was assigned to each patient record form based on the survey response of the treating physician. Data are presented as the relative frequency (%) of observed responses for each influential factor of current treatment choice.
1L: First line; 2L: Second line; 3L: Third line; 4L: Fourth line; DoR: Duration of response; OS: Overall survival; PFS: Progression-free survival.
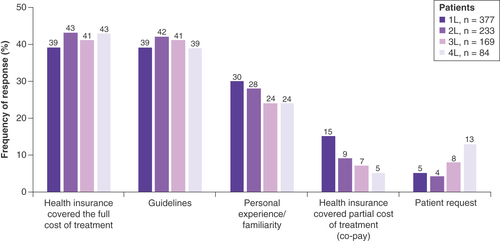
When considering all the treatments selected for their patients throughout their MM treatment history, the most common physician-reported reasons were ‘health insurance covering full cost of treatment’ and ‘guidelines’ for all LOT (). Compared with community-based physicians, a numerically greater proportion of academic physicians selected treatments based on ‘guidelines’ (1L: 43 vs 35%, 2L: 53 vs 31%, 3L: 51 vs 33%, 4L: 51 vs 35%; Supplementary Table 2). For patients who had received SCT, the most common reason for physician choosing treatment was ‘guidelines’ (1L: 59%, 2L: 57%, 3L: 60%, 4L: 67%), whereas in patients who have not received SCT the most common reason was ‘whether health insurance covered the full cost of treatment’ (1L: 41%, 2L: 45%, 3L: 43%, 4L: 44%; Supplementary Table 2).
n Indicates the number of patients receiving LOT at the time of data collection. Data are presented as the relative frequency (%) of an observed response for each reason of treatment choice.
1L: First line; 2L: Second line; 3L: Third line; 4L: Fourth line; LOT: Line of therapy.
Physician-reported level of treatment satisfaction, areas for improvement in patients’ current MM treatment, & reasons for treatment cessation
Across all LOTs, approximately half of the physicians were ‘satisfied’ with their treatment choice. Around a third were ‘very satisfied’ by their 1L and 2L treatment choices (33 and 39%, respectively), which dropped to 18 and 20% at 3L and 4L, respectively. Levels of ‘moderate satisfaction’ rose from 9% at 1L to 25% at 3L and 4L. Very few physicians reported being ‘dissatisfied’ with their treatment choice (3L: 2.4%, 4L: 2.5%; A). Reasons given by the two physicians who reported treatment dissatisfaction at 3L and 4L were ‘negative impact on patient QoL’ (3L: n = 1/2, 4L: n = 2/2), ‘poor efficacy’ (3L: n = 1/2, 4L: n = 1/2), and ‘poor tolerability’ (3L: n = 1/2, 4L: n = 1/2).
Data are presented as the relative frequency (%) of an observed response for each level of satisfaction (A) and areas for improvement (B).
1L: First line; 2L: Second line; 3L: Third line; 4L: Fourth line; OS: Overall survival; PFS: Progression-free survival.
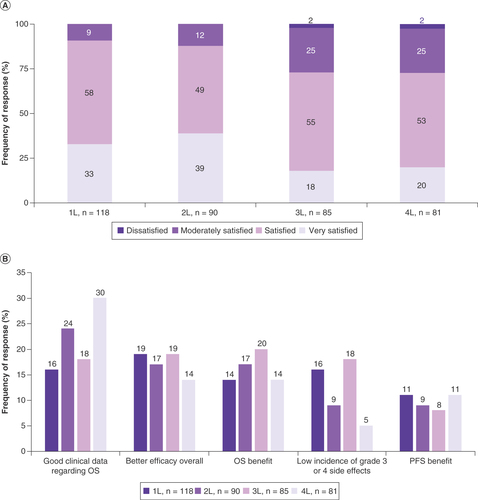
When considering areas for improvement in the treatment of MM, physicians predominantly highlighted efficacy-related factors as the most important across all LOTs (1L: 59%, 2L: 64%, 3L: 73%, 4L: 63%). The specific factors most commonly identified as areas for improvement were ‘better efficacy overall’ for patients treated in 1L (19%), ‘good clinical data regarding OS’ for patients in 2L (24%) and in 4L (30%) and ‘OS benefit’ for patients in 3L (20%; B). Physicians also predominantly highlighted efficacy-related factors as the most important area for improvement in treatment regardless of whether SCT was received (Supplementary Figure 4). Finally, disease progression/relapse was the most frequently reported reason for treatment cessation in each LOT (1L: 52%, 2L: 63%, 3L: 63%, 4L: 100%; Supplementary Figure 5A) and regardless of whether SCT was received (Supplementary Figure 5B).
Patient-reported perspectives on most important treatment benefits
Of the 377 patients with completed patient record forms, 132 (35%) had completed the voluntary self-completion questionnaire. Of these, 35 patients (27%) with similar demographics to the overall study population (Supplementary Table 3) responded to the question about the most important benefit of MM treatment (other than a cure for their MM).
The benefits reported as most important by >10% of all patient respondents were ‘better QoL’ (20%), ‘live longer’ (14%), ‘peace of mind/sense of security’ (11%) and ‘reduce fear/anxiety/nervousness’ (11%; ). The most important benefits varied between patients on different LOTs, although there were relatively few patient respondents in each LOT: ‘better QoL’ (19%) and ‘live longer’ (19%) were the most important benefits for patients treated in 1L (n = 16); ‘make me feel well/better’ (25%) and ‘reduce fear/anxiety/nervousness’ (25%) were the most important benefits for patients in 2L (n = 8); ‘better QoL’ (50%) was the most important benefit for patients in 3L (n = 6) and ‘live longer’ (40%) was the most important benefit for patients in 4L (n = 5).
Table 2. Patient perspectives on the most important benefit of treatment (other than a cure for multiple myeloma)Table Footnote† at line of therapy at time of data collection.
Discussion
This study used RW data from physicians and their patients in the USA to examine current clinical practices in RRMM treatment decision-making at various points of a patient’s treatment journey. The study describes physician attitudes and perspectives on the treatment attributes that influence treatment selection or treatment cessation, identifies areas for improvement in current treatments and explores patient perspectives of the most important treatment benefits.
In line with guidelines for the treatment of RRMM [Citation10,Citation14], patients in any LOT predominantly received triplet regimens that included a PI in combination with an immunomodulatory drug. In particular, VRd was used for over half of all patients at 1L; however, there was considerable variation in the specific combinations used, with most individual triplet regimens accounting for less than 10% of patients in any LOT. Anti-CD38 mAb-based regimens were also used across all LOTs, with daratumumab-based regimens (DRd and DPd) among the most common in 2L, 3L and 4L+.
Despite the myriad of combination therapies available to patients with MM, clinical efficacy outcomes generally decline as patients relapse and require successive LOTs [Citation22]. A US database study of 650 patients with RRMM who had received 3 or more prior LOTs reported that 72% of patients were double-class refractory and 34% were triple-class refractory [Citation23]. These patients showed high rates of retreatment with agents to which they had already been exposed or become refractory to and poorer clinical outcomes at later LOTs [Citation23]. In the present study, rates of retreatment were over 45% for both PI and immunomodulatory drugs. Together, this evidence highlights the challenge of treatment selection and the need for a uniform standard of care for patients with RRMM, given that patients with prior exposure to immunomodulatory drugs, PI, and anti-CD38 antibodies in tandem have a particularly poor prognosis [Citation24].
Previous research has suggested that physicians in clinical practice place primary importance on patient survival, with less consideration of minimizing treatment-related side effects in patients with RRMM [Citation25]. This may reflect the priorities of regulatory authorities whereby treatment approval is predominantly based on available clinical trial efficacy data, such as OS and PFS, with less weight given to evidence regarding QoL benefits [Citation26]. Similarly, in this study, efficacy-related factors, including OS, PFS and duration and depth of treatment response, were included in physicians’ top five most important drivers of treatment selection in general, with fewer physicians considering safety-related factors in their top five. Additionally, efficacy-related factors such as good clinical data regarding OS, better efficacy overall and PFS benefit were key drivers in the selection of their patients’ current treatment at the time of data collection, highlighting the commitment of physicians to prolonging patients’ lives. Interestingly, however, when considering the factors that historically influenced the selection of their patients’ current and previous treatments, health insurance coverage and clinical guidelines were reported as primary reasons for their particular treatment choice, which was largely independent of the LOT. Notably, ‘clinical guidelines’ more commonly cited as the main reason for selecting treatment for patients who had SCT, whereas health insurance coverage drove the treatment decision for patients without SCT, indicating a discordance in physician’s approach to treatment selection depending on patient characteristics. Disease progression/relapse was the most frequently reported reason for treatment cessation regardless of LOT or SCT status. Conversely, physician-reported areas for improvement in their patients’ current treatment varied by LOT, with better efficacy overall most frequently reported for 1L therapy, good clinical data regarding OS most frequently reported for both 2L and 4L therapy and OS benefit most frequently reported for 3L.
In contrast to physicians’ perspectives, patients most frequently considered improvement of health-related QoL (HRQoL) as the most important treatment benefit ahead of improved survival. This is consistent with a recent multinational qualitative study in which patients with MM placed importance on prolonged life expectancy and reduction of disease burden, while equally expressing concern for the impact of treatment burden as it related to pain and general function [Citation27], and HRQoL. On the other hand, in this study, improved HRQoL was not as strongly emphasized by physicians and was only considered in the top five most important treatment attributes by 24% of physicians, and only when it came to selection of 4L therapy. These findings underline the importance of considering factors that are important to patients in treatment selection, as effective alignment between physician and patient preferences is associated with improved treatment adherence and outcomes [Citation16].
The DSP data are subject to limitations which should be considered when interpreting this analysis. The quality of data depended to a large extent on the accurate reporting of information by physicians and patients, which may be subject to recall bias; however, the likelihood of recall bias was reduced by the data being collected at the time of each patient’s appointment and the ability of physicians to access patient medical records for data extraction. As is common with patient surveys in general, self-completion was voluntary. In addition, patients were not required to respond to all questions. Some items therefore had low numbers of responses, especially at individual LOTs, which may have made the study vulnerable to responder bias. In addition, due to the point-in-time nature of the survey, it was not possible to explore changes in physicians’ or patients’ perspectives over time that may result from physician experience, changes in symptom severity at different stages of treatment or disease state, or indeed as patients age [Citation15]. Although the patient population analyzed was relatively small and may not be representative of the broader MM population, the systematic approach to recruitment used in the DSP (including the next prespecified number of presenting patients meeting the criteria) reduced selection bias otherwise observed in retrospective chart audits. Despite the limitations of the data source, RW data play an important role in highlighting areas of interest that are not addressed in clinical trials, and the present study provides valuable insight into drivers of treatment choice, together with patient perspectives on treatment, across different LOTs. An advantage of the DSP is that the data were collected from a variety of sources and therefore provide an overview of how both patient and physician preferences impact MM treatment patterns, which could help to identify opportunities for the development of new treatments.
Conclusion
These RW findings highlight drivers of treatment choice among physicians treating patients with MM, patient perspectives, areas for improvement in current treatment, and the unmet need for new treatment strategies and a uniform standard of care for patients with RRMM. Physicians considered clinical efficacy outcomes to be key factors influencing their treatment choice for their patients with MM, while patients reported improved QoL as the most important benefit. Despite the recent advances in MM therapy, efficacy was identified as a key area for improvement by physicians. Our findings indicate that guidelines strongly influence physicians’ prescribing decisions, while regulatory bodies give more weight to efficacy factors than QoL. We recommend the incorporation of a more holistic, comprehensive approach to guidelines and clinical trials that also encompasses patient perspectives. Together, these findings may enhance physicians’ understanding of what matters to patients and encourage them to place more consideration on QoL-related outcomes in clinical practice to improve disease outcomes and patient treatment satisfaction.
Treatment selection in multiple myeloma (MM) is a complex process due to the range of available anti-myeloma treatments and the various combination approaches, in the absence of a uniform standard of care.
Patients with relapsed or refractory MM (RRMM) may undergo multiple lines of therapy (LOT), with triplet regimens being the most common in each line.
‘Prolonged overall survival (OS)’ is the treatment attribute that generally influences physicians’ treatment selection in each LOT.
Efficacy-related factors such as ‘good clinical data regarding OS’ and ‘better efficacy overall’ were the most common factors that influenced physicians’ selection of their patients’ treatment in each LOT.
Over 90% of physicians were ‘moderately’ to ‘very satisfied’ with their patients’ current treatment in each LOT.
‘Better efficacy overall’, ‘good clinical data regarding OS’ and ‘OS benefit’ were identified by physicians as areas for improvement in their patients’ current treatments.
Other than a cure, treatment benefits most important to patients were ‘better quality of life’ and to ‘live longer’.
This study highlights drivers of RRMM treatment choice from physicians’ and patients’ perspectives and the need for a more holistic approach to guidelines and clinical trials that encompasses patient perspectives.
This study may help to inform treatment decision-making and improve treatment adherence by aligning both physician and patient preferences.
Author contributions
All authors contributed to the conception or design of the study, the analysis and interpretation of data and the writing and approval of this manuscript. A Ribbands, A Bailey, E Luke and A Lambert also contributed to the acquisition of data.
Financial & competing interests disclosure
A Ribbands, A Bailey, E Luke and A Lambert are employees of ARW, and N Boytsov and B Gorsh are employees of GSK. Data collection was undertaken by ARW as part of an independent survey, entitled the MM DSP. GSK did not influence the original survey through either contribution to the design of questionnaires or data collection. The analysis described here, funded by GSK (study 209997) used data from the ARW MM DSP. The DSP is a wholly owned ARW product. Publication of survey results was not contingent on the subscriber’s approval or censorship of the manuscript. GSK subscribed to the MM DSP survey. The authors would also like to thank Phoebe Salmon of Adelphi Real World for statistical analytics support; the analysis was funded by GSK (study 209997). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance was provided by P Wisniewski and M Condon of Fishawack Indicia Ltd, part of Fishawack Health, and funded by GSK.
Ethical conduct of research
Using a checkbox, patients provided informed consent to take part in the survey. Data were collected in such a way that patients and physicians could not be identified directly.
Physician and patient data were pseudo-anonymized. A code was assigned when data were collected. Upon receipt by ARW, data were pseudo-anonymized again to mitigate against tracing them back to the individual. Data were aggregated before being shared with the subscriber and/or for publication.
This research was submitted to the Western Institutional Review Board, study protocol number AG8847. Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines [Citation24], and as such it did not require ethics committee approval. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996 [Citation25] and Health Information Technology for Economic and Clinical Health Act legislation [Citation26]. Complete descriptions of the DSP methodology and their generalizability have been published [Citation14–16].
Data sharing statement
All data, i.e., methodology, materials, data and data analysis, that support the findings of this survey are the intellectual property of ARW. All requests for access should be addressed directly to Amanda Ribbands at [email protected].
Supplementary Figure 1. Treatment regimens received by LOT
Download MS Word (381.8 KB)Acknowledgments
The ARW MM DSP is a multi-sponsored program.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2023-0020
Additional information
Funding
References
- Kumar SK , RajkumarV, KyleRAet al. Multiple myeloma. Nat. Rev. Dis. Primers3, 17046 (2017).
- Cowan AJ , AllenC, BaracAet al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol.4(9), 1221–1227 (2018).
- van de Donk N , PawlynC, YongKL. Multiple myeloma. Lancet397(10272), 410–427 (2021).
- American Cancer Society . Cancer Statistics Center: myeloma. ( Eds). (2022).
- Mikhael J . Treatment Options for Triple-class Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk20(1), 1–7 (2020).
- Dhakal B , SzaboA, ChhabraSet al. Autologous Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Novel Agent Induction: A Systematic Review and Meta-analysis. JAMA Oncol.4(3), 343–350 (2018).
- Chakraborty R , HamiltonBK, HashmiSK, KumarSK, MajhailNS. Health-Related Quality of Life after Autologous Stem Cell Transplantation for Multiple Myeloma. Biol. Blood and Marrow Transplant.24(8), 1546–1553 (2018).
- Gengenbach L , GrazianiG, ReinhardtHet al. Choosing the Right Therapy for Patients with Relapsed/Refractory Multiple Myeloma (RRMM) in Consideration of Patient-, Disease- and Treatment-Related Factors. Cancers (Basel)13(17), (2021).
- Gengenbach L , ReinhardtH, IhorstGet al. Navigating the changing multiple myeloma treatment landscape: clinical practice patterns of MM patients treated in- and outside German DSMM study group trials. Leuk. Lymphoma59(11), 2692–2699 (2018).
- Mikhael J , IsmailaN, CheungMCet al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J. Clin. Oncol.37(14), 1228–1263 (2019).
- Dima D , JiangD, SinghDJet al. Multiple Myeloma Therapy: Emerging Trends and Challenges. Cancers14(17), 4082 (2022).
- Pfizer . Pfizer’s Elranatamab Granted FDA Breakthrough Therapy Designation for Relapsed or Refractory Multiple Myeloma (2022). Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-elranatamab-granted-fda-breakthrough-therapy
- FDA . FDA approves teclistamab-cqyv for relapsed or refractory multiple myeloma (2022) Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-teclistamab-cqyv-relapsed-or-refractory-multiple-myeloma
- Dimopoulos MA , MoreauP, TerposEet al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.32(3), 309–322 (2021).
- Terpos E , MikhaelJ, HajekRet al. Management of patients with multiple myeloma beyond the clinical-trial setting: understanding the balance between efficacy, safety and tolerability, and quality of life. Blood Cancer Journal11(2), 40 (2021).
- Fifer SJ , HoKA, LybrandS, AxfordLJ, RoachS. Alignment of preferences in the treatment of multiple myeloma – a discrete choice experiment of patient, carer, physician, and nurse preferences. BMC Cancer20(1), 546 (2020).
- Anderson P , BenfordM, HarrisN, KaravaliM, PiercyJ. Real-world physician and patient behaviour across countries: Disease-Specific Programmes – a means to understand. Curr. Med. Res. Opin.24(11), 3063–3072 (2008).
- Babineaux SM , CurtisB, HolbrookT, MilliganG, PiercyJ. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open6(8), e010352 (2016).
- Higgins V , PiercyJ, RoughleyAet al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr. Obes.9, 371–380 (2016).
- Centers for Medicare and Medicaid Services . Oncology Care Model. Available from: https://innovation.cms.gov/innovation-models/oncology-care [Accessed November 2022]. ( Eds). (2022).
- Sonneveld P , Avet-LoiseauH, LonialSet al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood127(24), 2955–2962 (2016).
- Yong K , DelforgeM, DriessenCet al. Multiple myeloma: patient outcomes in real-world practice. Br. J. Haematol.175(2), 252–264 (2016).
- Wang PF , YeeCW, GorshBet al. Treatment patterns and overall survival of patients with double-class and triple-class refractory multiple myeloma: a US electronic health record database study. Leuk. Lymphoma ( In press).
- Gandhi UH , CornellRF, LakshmanAet al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia33(9), 2266–2275 (2019).
- Batchelder L , PhilpottS, DivinoVet al. Physician treatment preferences for relapsed/refractory multiple myeloma: a discrete choice experiment. Future Oncol.18(25), 2843–2856 (2022).
- Kazandjian D , LandgrenO. A look backward and forward in the regulatory and treatment history of multiple myeloma: approval of novel-novel agents, new drug development, and longer patient survival. Semin. Oncol.43(6), 682–689 (2016).
- Janssens R , LangT, VallejoAet al. Patient Preferences for Multiple Myeloma Treatments: A Multinational Qualitative Study. Front Med. (Lausanne)8, 686165 (2021).
