Abstract
Aim:
Describe treatment and dosing patterns of lenvatinib and pembrolizumab combination therapy (lenva+pembro) among endometrial cancer (EC) patients in US clinical practice.
Materials & methods:
Retrospective cohort study among adults with EC initiating lenva+pembro in second line (2L) or third line and later (≥3L) between 17 September 2019 and 30 June 2021.
Results:
110 patients initiated lenva+pembro in 2L and 135 patients in ≥3L. Majority of patients initiated lenva+pembro at label-recommended starting doses/interval. Less than half changed lenvatinib dose over time. At median follow-up of 7.3 and 8.7 months, median (95% CI) duration of therapy was 5.1 (4.7–6.1) and 5.8 (4.2–7.3) months for patients in 2L and ≥3L, respectively.
Conclusion:
Lenva+pembro was mostly initiated at label-recommended dose.
Plain language summary
This study looked at details of lenvatinib and pembrolizumab combination treatment among patients with endometrial cancer (EC) in the USA. Specifically, these patients had received prior chemotherapy or hormone therapy before starting lenvatinib and pembrolizumab. Most patients started lenvatinib and pembrolizumab at the dose recommended by the product label and received the next pembrolizumab injection within the recommended timeframe. Over time, more than half of the patients did not change the dose of lenvatinib, and most patients had the same dose of pembrolizumab. On average, patients were treated with lenvatinib and pembrolizumab for 5–6 months. This study showed that in general, patients were taking lenvatinib and pembrolizumab for treatment of EC as recommended by product labels.
Tweetable abstract
Our retrospective study of lenvatinib and pembrolizumab using IQVIA’s claims database shows that most patients initiated at label-recommended dose, and majority did not have a dose change. At median follow-up of 7.3 and 8.7 months, the median (95% CI) duration of therapy was 5.1 (4.7–6.1) and 5.8 (4.2–7.3) months for patients in 2L and ≥3L, respectively.
Endometrial cancer (EC) is the most common malignancy of the female reproductive system in developed countries [Citation1]. In the USA, there were an estimated 65,950 new cases of EC, with 12,550 predicted deaths due to the disease in 2022 [Citation2]. The prognosis varies across the stages of the cancer, with poor 5-year relative survival reported among women diagnosed with regional stage (71%) and distant stage (20%) disease [Citation3]. A recent multi-center, retrospective, patient chart review study in the USA was conducted among adult women diagnosed with non-microsatellite instability-high (non-MSI-H)/mismatch repair proficient (pMMR) recurrent or advanced EC (aEC) who received at least one prior systemic therapy and progressed [Citation4]. The study reported median overall survival (OS) of 9–10 months, and median real-world progression-free survival (PFS) of 5–6 months in response to second line (2L) chemotherapy or hormonal therapy, further highlighting the burden of aEC, especially among patients with non-MSI-H/pMMR status [Citation4].
Lenvatinib in combination with pembrolizumab received an accelerated approval by the US FDA on 17 September 2019 [Citation5], followed by a full approval on 21 July 2021 [Citation6], for the treatment of patients with aEC that is mismatch repair proficient or not MSI-H (pMMR/non-MSI-H), who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation [Citation5]. Results of the phase III KEYNOTE-775 (KN-775)/Study 309 trial demonstrated clinically meaningful and statistically significant improvement in OS (median 18.3 vs 11.4 months, hazard ratio [HR] = 0.62; p < 0.0001) and PFS (median 7.2 vs 3.8 months, HR = 0.56; p < 0.0001) among patients with aEC treated with lenvatinib and pembrolizumab combination therapy compared with treatment of physician’s choice [Citation7]. Subsequently, the 2022 National Comprehensive Cancer Network (NCCN) guidelines recommended lenvatinib and pembrolizumab combination therapy as the preferred regimen for pMMR/non-MSI-H aEC for 2L treatment [Citation8]. The label-recommended dose of lenvatinib 20 mg/day used in combination with pembrolizumab 200 mg every 3 weeks (or 400 mg every 6 weeks) has been established through multiple clinical trials [Citation9,Citation10].
Since lenvatinib in combination with pembrolizumab is a new therapeutic option, there is limited real-world evidence of utilization and dosing patterns of lenvatinib and pembrolizumab combination therapy among patients with EC treated in the USA. This retrospective, observational cohort study aimed to describe early utilization patterns of lenvatinib and pembrolizumab combination therapy, including dosage and dosing interval, among patients diagnosed with EC who newly initiated lenvatinib and pembrolizumab combination therapy in the 2L setting or later in US clinical practice.
Materials & methods
Study design & data sources
We conducted a retrospective cohort study utilizing data from IQVIA’s longitudinal prescription claims database linked to medical claims database. The longitudinal prescription claims database captures information on dispensed prescriptions with 92% coverage of prescriptions from the retail channel, 72% coverage of standard mail service, and 76% coverage of long-term care facilities in the USA. The medical claims database captures over 1 billion pre-adjudicated claims and 3 billion records obtained annually from approximately 800,000 office-based physicians and specialists, with 75% of American Medical Association providers being included. Medical claims from ambulatory and general healthcare sites (as well as outpatient clinics associated with hospitals such as rehabilitation, same day surgery and chemotherapy centers) are also included in the medical claims database. IQVIA’s linked longitudinal prescription claims and medical claims databases were utilized to capture both the prescriptions for lenvatinib (oral medication) as well as procedures for pembrolizumab administration (infusion) and assess the lenvatinib and pembrolizumab combination therapy utilization patterns. All data are de-identified and Health Insurance Portability and Accountability Act (HIPAA)-compliant to protect patient privacy, thus Institutional Review Board approval or waiver was not required for this study.
Patient selection
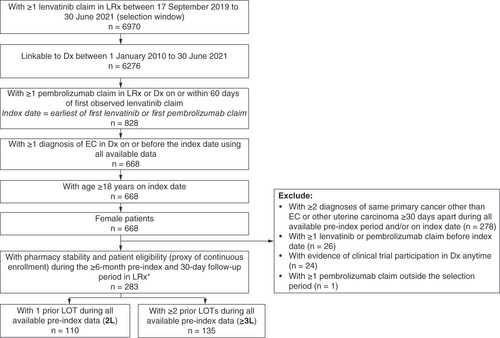
Adult (18 years and older) females diagnosed with EC with ≥1 claim for lenvatinib from 17 September 2019 to 30 June 2021 (selection window) who had ≥1 claim for pembrolizumab on or within 60 days before or after the date of first lenvatinib claim were included into the study. The date of initiating lenvatinib and pembrolizumab combination therapy (index date) was defined as the date of the first lenvatinib claim or the date of the first pembrolizumab claim, whichever came first. Patients were required to have ≥1 diagnosis of EC (International Classification of Diseases, 9th and 10th revision [ICD-9: 182.0; ICD-10: C54.1]) on or before initiating lenvatinib and pembrolizumab combination therapy using all available data (as early as 1 January 2010, if available). Patients were further required to have ≥6 months of baseline and ≥30 days of follow-up pharmacy stability (pharmacies consistently reporting data every month) and patient eligibility (proxy of continuous enrollment). Patients were excluded if they had evidence of other primary cancers (defined as ≥2 diagnoses of the same primary cancer other than EC or other uterine carcinoma on separate days ≥30 days apart) during all available baseline, ≥1 prior lenvatinib or pembrolizumab claim before index date, ≥1 pembrolizumab or lenvatinib claim outside of the selection window, or evidence of clinical trial enrollment in the medical claims database (ICD-9: V70.7; ICD-10: Z00.6) anytime during the study period. Patients who received lenvatinib and pembrolizumab combination therapy in 2L or later settings were included in the study. Patient selection criteria and patient flow are described in . The selected patients were stratified into two mutually exclusive cohorts based on whether they received the index lenvatinib and pembrolizumab combination therapy in the 2L (2L cohort) or third line or later (≥3L cohort) settings, which was determined by the observed number of prior systemic treatment (chemotherapy and hormone therapy) lines received. Therapies were considered as part of the same line of therapy (LOT) if initiated within 30 days. Since the lenvatinib and pembrolizumab combination therapy is approved for patients who have disease progression following prior systemic therapy, patients with no prior observed LOT were not included for analyses.
Study measures
The primary study measures included treatment patterns (number of lenvatinib claims, number of pembrolizumab administrations, duration of lenvatinib and pembrolizumab combination therapy, and reason for end of observable lenvatinib and pembrolizumab combination therapy) and dosing patterns of lenvatinib and pembrolizumab combination therapy (starting dose and/or dosing interval and change in dose and/or dosing interval) during ≥1-month follow-up. The end of follow-up was defined as the end of stable enrollment (last date prior to a 6-month period with no observable records in the longitudinal prescription claims database or medical claims database) or end of study period, whichever came earlier.
Secondary study measures included patient demographics and clinical characteristics that were assessed over the 6-month baseline period. Additionally, evidence of metastasis, prior systemic treatments and procedures (surgery and radiation) for EC since the first diagnosis of the disease observed in all available baseline data (as early as 1 January 2010, if available) were reported. Using data during all available baseline and follow-up periods, systemic therapies for EC indicated in the NCCN guidelines [Citation8] and sequence of treatments up to the fifth observed LOT were identified.
End date of observable lenvatinib and pembrolizumab combination therapy was defined as the earlier of discontinuation date of index lenvatinib and pembrolizumab combination therapy determined by the latest of a treatment gap of >60 days after the end of days’ supply of lenvatinib, a treatment gap of >42 days for pembrolizumab <400 mg (i.e., twice the recommended dosing interval of pembrolizumab 200 mg every 3 weeks), or a treatment gap of >84 days for pembrolizumab ≥400 mg (i.e., twice the recommended dosing interval of pembrolizumab 400 mg every 6 weeks); or (2) one day before switch or augmentation, defined as claim of a new therapeutic agent in replacement of or in addition to the index lenvatinib and pembrolizumab combination therapy. Reasons for end of observable lenvatinib and pembrolizumab combination therapy was defined as discontinuation, switch or augmentation, end of stable enrollment or end of available data.
Statistical analysis
All analyses were descriptive. Baseline measures, lenvatinib and pembrolizumab combination therapy utilization and dosing patterns were described separately for the 2L and ≥3L cohorts. Sankey plot was generated to illustrate the treatment sequences for all patients included in our study. Kaplan–Meier (KM) analysis was conducted to estimate median and 95% CI for duration of lenvatinib and pembrolizumab combination therapy; patients who did not discontinue or switch/augment were censored at their end of follow-up or end of data availability, whichever came first. Time from index lenvatinib claim to first lenvatinib dose decrease was also estimated using KM method; patients were censored if they had no dose change or had dose increase as the first dose change. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., NC, USA) and all plots were created using RStudio [Citation11].
Results
Study population
Our study included 245 patients diagnosed with EC who initiated lenvatinib and pembrolizumab combination therapy in the 2L or later settings (110 patients in the 2L cohort and 135 patients in the ≥3L cohort []). The median age at the initiation of lenvatinib and pembrolizumab combination therapy was 67 years for the 2L cohort and 66 years for the ≥3L cohort (). Patients were from all geographic regions of USA. Nearly all patients were covered by commercial insurance (54.5% of the 2L cohort and 56.3% of the ≥3L cohort) or Medicare (41.8% of the 2L cohort and 40.0% of the ≥3L cohort). Patients had relatively low burden of comorbidities at baseline with mean Charlson Comorbidity Index (CCI; excluding cancer) score of 0.8 for both cohorts. The most commonly reported comorbidities were hypertension (47.3% of the 2L cohort and 37.0% of the ≥3L cohort) and diabetes (27.3 and 25.9%, respectively). Most patients had evidence of metastasis before the initiation of lenvatinib and pembrolizumab combination therapy (71.8% of the 2L cohort and 84.4% of the ≥3L cohort).
Table 1. Baseline demographic and clinical characteristics in patients who initiated lenvatinib and pembrolizumab combination therapy in 2nd line (2L) and 3rd or later lines (≥3L).
Surgery for EC prior to initiation of lenvatinib and pembrolizumab combination therapy was reported in 31.8 and 28.9% of the 2L and ≥3L cohorts, respectively, with the most commonly observed surgery being total hysterectomy (18.2 and 17.8%, respectively). Nearly all patients had evidence of chemotherapy prior to the initiation of lenvatinib and pembrolizumab combination therapy (≥93.6%; ); carboplatin in combination with paclitaxel was the most frequently observed systemic treatment during the baseline (65.5 and 83.7%, respectively).
The most common provider specialty for the first prescription of lenvatinib or administration of pembrolizumab for the index lenvatinib and pembrolizumab combination therapy, whichever observed first, was coded as oncologists specialized in hematology/oncology (45.5% for 2L; 34.1% for ≥3L) followed by gynecologic oncologists (20.9% for 2L; 24.4% for ≥3L) for both cohorts.
Real-world utilization patterns of lenvatinib & pembrolizumab combination therapy
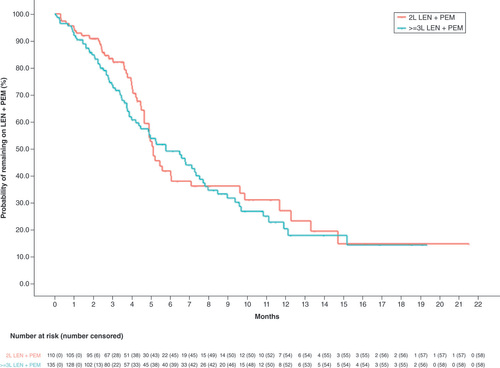
Over the median length of follow-up of 7.3 (interquartile range 3.0–12.6) months and 8.7 (3.9–12.4) months in the 2L and ≥3L cohorts, respectively, over half of patients had at least three claims for lenvatinib (59.1% of 2L and 54.8% of ≥3L cohorts); for pembrolizumab, the majority of patients had at least five pembrolizumab administrations (50.0% of 2L and 51.1% of ≥3L cohorts; ). The median (95% CI) duration of lenvatinib and pembrolizumab combination therapy was 5.1 (4.7–6.1) months for patients in the 2L cohort and 5.8 (4.2–7.3) months for patients in the ≥3L cohort ( & ). Reasons for the end of observable lenvatinib and pembrolizumab combination therapy in the 2L and ≥3L cohort, respectively, were discontinuation (35.5 and 35.6%), end of available data (34.5 and 27.4%), end of stable enrollment (18.2 and 15.6%) and switch or augmentation (11.8 and 21.5%).
Table 2. Treatment and dosing patterns of lenvatinib and pembrolizumab combination therapy in patients who initiated the combination therapy in 2nd line (2L) and 3rd or later lines (≥3L).
LEN: Lenvatinib, PEM: Pembrolizumab.
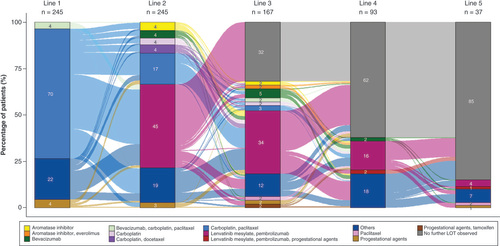
Sequences of EC-directed systemic therapies observed for patients included in our study are illustrated in . The columns denote line of therapy; each column is comprised of patients (%) receiving each treatment regimen during that line. The flows connecting the columns denote the therapy sequences. Of the 245 patients treated with lenvatinib and pembrolizumab combination therapy in the 2L or later settings in our study, 70% of patients had carboplatin and paclitaxel combination therapy in 1L. About 45, 34, 16 and 4% of the overall cohort had levatinib and pembrolizumab (denoted in magenta color) as 2L, 3L, 4L and 5L, respectively. The majority of patients who discontinued lenvatinib and pembrolizumab combination therapy did not have evidence of subsequent therapy; a small proportion of patients received additional treatments, varying in treatment regimens.
n in the column headers indicate the number of patients with treatments received during the LOT.
‘Others’ category in the legend includes all other treatment regimens for EC not specified; each regimen was used in <1% of patients.
EC: Endometrial cancer; LOT: Line of therapy.
Real-world dosing patterns of lenvatinib & pembrolizumab combination therapy
Among the 245 patients who initiated lenvatinib and pembrolizumab combination therapy for EC in 2L or later settings, the majority of the 2L cohort (70.9%) and the ≥3L cohort (63.7%) initiated lenvatinib on label-recommended 20 mg daily dose for EC () [Citation9]. During the index lenvatinib and pembrolizumab combination therapy, 48.2% of the 2L cohort and 47.4% of the ≥3L cohort remained on the daily dose of 20 mg lenvatinib.
Among patients who had at least two lenvatinib claims (76 patients in 2L, and 93 patients in ≥3L), change in index lenvatinib dosing was observed in approximately one third of each cohort (38.2% of the 2L cohort and 34.4% of the ≥3L cohort with at least 2 lenvatinib claims). Among the patients reporting a dose change, majority reported a dose decrease from starting dose, and most of them had a single dose decrease during the index treatment. The mean time from the first lenvatinib claim in the index combination therapy to first lenvatinib dose decrease was 4.5 months for the 2L cohort and 5.3 months for the ≥3L cohort ().
Of patients with two or more pembrolizumab claims (95 patients in 2L, and 109 patients in ≥3L), the majority initiated pembrolizumab on label-recommended starting dose and dosing interval for EC [Citation12]; 85.3% of the 2L cohort and 93.6% of the ≥3L cohort received 200 mg of pembrolizumab every 3 weeks, and 2.1% of the 2L cohort and 1.8% of the ≥3L cohort received 400 mg of pembrolizumab every 6 weeks.
Among patients with at least three pembrolizumab claims (82 patients for 2L; 92 patients for ≥3L), change in pembrolizumab dose and dosing interval were rarely observed (4.9% of the 2L cohort and 7.6% of the ≥3L cohort). Most of these changes were from 200 mg every 3 weeks to 400 mg every 6 weeks (both label-recommended doses [Citation12]).
Discussion
Our retrospective cohort study utilized a combination of longitudinal prescription claims and medical claims to identify 245 women diagnosed with EC who initiated lenvatinib in combination with pembrolizumab in the 2L setting or later in US real world clinical practice. Our study found that the majority of patients initiated lenvatinib on the label recommended starting dose of 20 mg daily, with most of these patients remaining on 20 mg daily during the index lenvatinib and pembrolizumab combination therapy. These results are consistent with results from a multicenter retrospective study in South Korea by Kim et al. [Citation13], where 83% initiated at the 20 mg starting dose, but differ from the findings from a single-center retrospective study in the USA by How et al. [Citation14], in which the majority of patients (77.1%) initiated lenvatinib at a dose lower than 20 mg daily. The difference we observed between our study and the study by How et al. may be potentially due to our study using a nationally representative claims database compared with data from a single institution, which may have led to differences in practice patterns. Additionally, for the majority of patients in our study, we did not observe lenvatinib dose reduction during the index lenvatinib and pembrolizumab combination therapy. The study by How et al. found that over half of patients (57.1%) did not have lenvatinib dose reduction [Citation14]. However, a little over half (56.2%) of patients in Kim et al. experienced lenvatinib dose reduction [Citation13].
The duration of follow-up in our study (median 7.3–8.7 months) was comparable to that observed in other retrospective studies (median 6.9–7.0 months) but shorter than the median follow-up of 12.2 months observed in the KN-775/Study 309 trial [Citation13–15]. The limited follow-up duration due to end of available data and end of stable enrollment in our study may have underestimated the median duration of the index lenvatinib and pembrolizumab combination therapy (5.1–5.8 months), which was shorter than the median duration of 7.7 months observed in the KN-775/Study 309 trial [Citation15]; the differences in patient population, use in later lines, and care setting in real-world from clinical trials (where patients are monitored more closely) may also play a role. In addition, the limited follow-up duration could potentially impact the treatment sequences we observed (), as it is likely that we were not able to capture information on if subsequent therapies were used post the index lenvatinib and pembrolizumab combination therapy in most patients. The prior treatments captured in the treatment sequences we observed () only applies to our patient population who initiated lenvatinib and pembrolizumab combination therapy in 2L and later settings, not the general EC population. A recent claims-based study reported that among patients with EC initiating systemic therapy, 33% received 2L and 13.2% received 3L, indicating high attrition as patients’ disease progress to the next LOTs [Citation16].
The median age of patients in our study was consistent with that of advanced endometrial cancer patients from the phase III KN-775/Study 309 trial (64 years old) [Citation15]. Treatments for EC prior to initiating lenvatinib and pembrolizumab combination therapy observed in our study were generally consistent with the NCCN guidelines and previously published studies [Citation7,Citation8]. As our study population consisted of patients who initiated lenvatinib and pembrolizumab combination therapy in 2L and later settings, nearly all patients in our study (>90%) had received at least one chemotherapy prior to lenvatinib and pembrolizumab combination therapy initiation. Of these patients, carboplatin in combination with paclitaxel was the most common chemotherapy regimen, albeit observed in only 70% of the cohort; lower than expected given this is considered standard of care in first line [Citation4,Citation8,Citation17].
Our study leveraged a large, nationally representative administrative claims database covering broad prescribers and practice types to capture early utilization patterns of lenvatinib and pembrolizumab combination therapy. The study design employed up to 10 years of data before initiation of lenvatinib and pembrolizumab combination therapy, where available, in order to capture the LOT of index lenvatinib and pembrolizumab combination therapy and treatment history to the best possible extent. However, our study had a few limitations. Due to the recent approval of lenvatinib and pembrolizumab combination therapy and lag in data availability of claims databases in general, the sample size was relatively small and available follow-up was limited. We minimized patient attrition by requiring relatively short baseline (6 months) and follow-up (30 days) and utilized variable follow-up to retain as many patients as possible to accurately reflect the real-world treatment patterns; however, this may have led to underestimation of both the number of observable LOTs prior to initiation of lenvatinib and pembrolizumab combination therapy and the duration of lenvatinib and pembrolizumab combination therapy in some patients. Future studies with longer follow-up and a larger sample size would be valuable. The longitudinal prescription and medical claims databases leveraged in this study lack inpatient data; to that end, prior surgeries conducted in the inpatient setting would not be captured, likely contributing to the lower than expected frequency of prior surgeries among the study population.
Other study limitations are inherent to the administrative nature of data, including potential misclassification of diagnoses and lack of clinical data to help understand patient cancer-specific characteristics (e.g., histology subtypes), confirm LOT, and note reasons for treatment discontinuation (e.g., adverse events). For example, information on patient clinical characteristics such as disease stage were not available, therefore, our study was not able to confirm recurrent and advanced disease (except observable metastasis diagnoses) to determine if the treatment was prescribed as per label. Biomarker information such as MSI or MMR were also not available, so the MSI/MMR status of our study cohort could not be confirmed. Future studies with expanded data including more clinical information such as MSI/MMR test results, disease stage and clinical outcomes are warranted. Lastly, the study findings are based on the US population and may not be generalizable to patient populations outside USA.
Conclusion
Our retrospective cohort study using a nationally representative claims database is one of the first studies that provide early evidence on real-world utilization and dosing patterns of lenvatinib and pembrolizumab combination therapy among patients diagnosed with EC in clinical practice in USA, when used in the 2L or later settings. Lenvatinib and pembrolizumab combination therapy was mostly initiated at the label recommended starting dose and dosing interval in US clinical practice, and the majority of patients did not have any dose change both for lenvatinib and pembrolizumab. Future research with longer follow-up on clinical outcomes observed in real world clinical practice in this patient population is warranted.
Lenvatinib in combination with pembrolizumab is a new therapeutic option for endometrial cancer (EC). Given its recent approval, there is limited real-world evidence of utilization and dosing patterns of lenvatinib and pembrolizumab combination therapy among patients with EC treated in the USA.
This retrospective cohort study utilizing data from IQVIA’s longitudinal prescription claims database linked to medical claims database included adult females with EC initiating lenvatinib and pembrolizumab combination therapy in second line (2L) or later settings between 17 September 2019 and 30 June 2021.
The majority of 2L (70.9%) and third line and later (≥3L) cohorts (63.7%) initiated lenvatinib at the label-recommended 20 mg daily starting dose and change in lenvatinib dose was observed in <40% of the patients. The majority of patients (>87%) initiated pembrolizumab on label-recommended dose and interval.
Over median follow-up duration of 7.3 (2L)–8.7 (≥3L) months since treatment initiation, 35.6% of patients discontinued the combination therapy; the majority of patients remained on combination therapy at data cut-off.
Author contributions
K Wada: conceptualization, investigation, methodology, project administration, validation, writing – original draft, writing – review & editing. J Zhang: conceptualization, investigation, methodology, supervision, writing – review & editing. I Lee: conceptualization, investigation, methodology, project administration, validation, writing – original draft, writing – review & editing. Y Wang: data curation, formal analysis, software, validation, writing – review & editing. A Near: conceptualization, investigation, methodology, supervision, writing – review & editing. VS Prabhu: conceptualization, funding acquisition, investigation, methodology, supervision, writing – review & editing.
Ethical conduct of research
All data are de-identified and Health Insurance Portability and Accountability Act (HIPAA)-compliant to protect patient privacy, thus Institutional Review Board approval or waiver was not required for this study.
Previous Presentations
Part of this research was presented at the National Comprehensive Cancer Network (NCCN) 2022 Annual Conference [Citation18].
Financial disclosure
This study was co-funded by Merck Sharp & Dohme (MSD) LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and Eisai Inc., Nutley, NJ, USA. I Lee, Y Wang and A Near are employees of IQVIA Inc., which received funding from MSD to conduct this study. K Wada was an employee of IQVIA Inc. at the time of study execution. J Zhang was an employee of Eisai, Inc., Nutley, NJ, USA, at the time of study execution. VS Prabhu is an employee of MSD and owns shares of Merck & Co., Inc., Rahway, NJ, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
- Ferlay J , SoerjomataramI, DikshitRet al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer136(5), E359–E386 (2015).
- American Cancer Society . Cancer Facts & Figures 2022. www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html (Accessed 15December2022).
- American Cancer Society . Survival Rates for Endometrial Cancer. www.cancer.org/cancer/endometrial-cancer/detection-diagnosis-staging/survival-rates (Accessed 29April2022).
- Kelkar SS , PrabhuVS, ZhangJet al. Treatment patterns and real-world clinical outcomes in patients with advanced endometrial cancer that are non-microsatellite instability high (non-MSI-high) or mismatch repair proficient (pMMR) in the United States. Gynecologic Oncology Reports42, DOI: 10.1016/j.gore.2022.101026 (2022).
- U.S. Food and Drug Administration (FDA) . NDA 206947/S-011 Accelerated Approval. www.accessdata.fda.gov/drugsatfda_docs/appletter/2019/206947Orig1s011ltr.pdf (Accessed 14April2022).
- U.S. Food and Drug Administration (FDA) . NDA 206947/S-020 Supplement approval/fulfillment of postmarketing requirement. www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/206947Orig1s020ltr.pdf (Accessed 14April2022).
- Makker V , ColomboN, HerráezACet al. A multicenter, open-label, randomized, Phase III study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab versus treatment of physician’s choice in patients with advanced endometrial cancer. Gynecol. Oncol.162, S4 (2021).
- National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Uterine Neoplasms. www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (Accessed 20June2022).
- U.S. Food and Drug Administration (FDA) . Prescribing information for lenvatinib. www.accessdata.fda.gov/drugsatfda_docs/label/2022/206947s021lbl.pdf (Accessed 20June2022).
- Motzer RJ , TaylorMH, EvansTRJet al. Lenvatinib dose, efficacy, and safety in the treatment of multiple malignancies. Expert Review of Anticancer Therapy22(4), 383–400 (2022).
- R Studio . RStudio: Integrated Development for R. www.rstudio.com/
- U.S. Food and Drug Administration (FDA) . Prescribing information for pembrolizumab. www.accessdata.fda.gov/drugsatfda_docs/label/2022/125514s131lbl.pdf (Accessed 22June2022).
- Kim J , NohJJ, LeeTKet al. Real-world experience of pembrolizumab and lenvatinib in recurrent endometrial cancer: a multicenter study in Korea. Gynecol. Oncol.165(2), 369–375 (2022).
- How JA , PatelS, FellmanBet al. Toxicity and efficacy of the combination of pembrolizumab with recommended or reduced starting doses of lenvatinib for treatment of recurrent endometrial cancer. Gynecol. Oncol.162(1), 24–31 (2021).
- Makker V , ColomboN, CasadoHerráez Aet al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med.386(5), 437–448 (2022).
- Kebede N , ShahR, ShahA, CormanS, NwankwoC. Treatment patterns and economic burden among cervical and endometrial cancer patients newly initiating systemic therapy. Future Oncology18(8), 953–964 (2022).
- Kelkar SS , PrabhuVS, CormanSet al. Treatment patterns and real-world clinical outcomes in patients with advanced endometrial cancer who are microsatellite instability (MSI)-high or are mismatch repair deficient (dMMR) in the United States. Gynecol. Oncol.169, 154–163 (2023).
- Wada K , PrabhuV, WangY, ZhangJ. HSR22-177: Real-World Utilization of Lenvatinib+Pembrolizumab Combination Therapy for the Treatment of Advanced Endometrial Cancer in the United States: ENdometrial cancer DOsing in the real-World (ENDOW) Study. J. Nat. Compreh. Cancer Netw.20(3.5), HSR22–177 (2022).