Abstract
Aim: This study assessed the costs associated with multigene panel tests (MGPTs) in the USA and the impact of coverage on insurance premiums. Materials & methods: We conducted a retrospective claims analysis to estimate total patient costs associated with MGPT use in three solid tumors: advanced non-small-cell lung cancer, advanced melanoma and metastatic colorectal cancer. A decision analytic model was constructed to estimate the premium impact of a 1 million member commercial health plan. Results: In all three tumor types, mean total costs for patients receiving or not receiving MGPTs were not significantly different (p > 0.05). The estimated change in premiums per enrollee per month was estimated to be US$0.040. Conclusion: MGPTs were not associated with higher costs and coverage is expected to have minimal impact on insurance premiums.
Plain language summary
Costs & premiums for tests of biomarkers
We examined whether using tests for many biomarkers at once in three cancer types would lead to higher costs and increase insurance premiums for patients with private insurance. Using real-world data, we found that use of these tests was not linked to higher costs. Furthermore, we estimated that insurance coverage of these tests would result in small changes to insurance premiums.
Advances in the efficacy and number of targeted therapeutics have improved outcomes for patients with cancer [Citation1,Citation2]. For example, in advanced non-small-cell lung cancer (aNSCLC), there are a number of US FDA-approved therapies that target a variety of gene mutations/alterations, including ALK, EGFR, RET, MET, ROS1, NTRK and BRAF [Citation3]. Similarly, there have been advances in diagnostic technology to improve identification of eligible populations who may benefit from targeted therapies. Although gene mutations were identified historically using single-gene tests, such as FISH or immunohistochemistry, the use of multigene panel tests (MGPTs), including next-generation sequencing, that allow for identification of mutations in multiple genes in a single test have become more common [Citation4].
Despite the increase in use of MGPTs, insurance coverage for these tests in the USA has varied. In Medicare, there is a national coverage determination which facilitates access for Medicare beneficiaries [Citation5]; however, similar universal coverage is lacking among commercial insurers and state Medicaid programs. For example, a study performed at a single institution found that only 26% of tests were reimbursed by commercial insurance, despite clinically actionable results found in the majority of denials [Citation6]. Furthermore, an analysis examining alignment of commercial payer policies with clinical guidelines found that 71% of plans were more restrictive than the clinical guidelines [Citation7]. Similarly, a report describing coverage of comprehensive biomarker testing among state Medicaid found that only 39% had coverage for comprehensive biomarker testing [Citation8], which may be a contributor to lower testing rates and poorer outcomes among Medicaid beneficiaries [Citation9].
To address the variable coverage of biomarker testing, some US states have begun to pass laws to improve access to biomarker testing at the state level, while others are considering bills to address access to biomarker testing [Citation10]. One aspect for consideration when assessing the impact of these bills is the potential financial effects on patients which may come in the form of increased premiums to cover the greater access to testing. While some studies have examined the payer budget impact of coverage [Citation11,Citation12], there have been limited data examining the impact of coverage to patient costs in terms of insurance premiums. Therefore, the objective of this study was to assess the total costs associated with the use of MGPTs and the impact of coverage of MGPTs on insurance premiums. Given the use of biomarker testing in aNSCLC, advanced melanoma (aMelanoma) and metastatic colorectal cancer (mCRC) [Citation4], we use these three tumor types as case studies.
Materials & methods
A retrospective cohort analysis was conducted to assess costs associated with the use of MGPTs in three tumor types (aNSCLC, aMelanoma and mCRC). The results were then used as inputs into a decision analytic model to assess the impact of covering MGPTs on insurance premiums.
Claims analysis
This retrospective analysis utilized the IQVIA PharMetrics Plus database, which comprises fully adjudicated pharmacy and medical claims of over 190 million commercially insured patients. Selected patients include those with a lung cancer, melanoma or colorectal diagnosis on at least one inpatient claim or two or more outpatient claims at least 30 days apart from 2015 to 2018. Patients were also required to have 6 months of continuous enrollment prior to their index date (defined below), while also not having any other primary cancer diagnosis during the baseline period, no participation in clinical trials and no Health Maintenance Organization (HMO) insurance.
Patients who used MGPTs, including next generation sequencing (Current Procedural Terminology codes: 81445, 81450, 81455) were matched to those who only had any codes for a single-gene test (aNSCLC: 81235, 81275, 81276, 81210; mCRC: 81275, 81276). Owing to their smaller incidence of aMelanoma, patients in the control group were not required to have codes for a single-gene test; however, they were required to have two metastatic codes or systemic treatment post index to limit the analysis to patients with advanced stage cancer. For aNSCLC and aMelanoma, since most use of MGPTs is upfront, the analysis was restricted to previously untreated patients with testing required within 60 days of diagnosis, with the index date defined as their first cancer diagnosis. For mCRC, given that MGPT use varies throughout the patient treatment journey, we constructed lines of therapies to match patients who received or did not receive MGPTs. The MGPT date is the index date for patients who received MGPTs; a pseudo-index date was created for patients who did not receive MGPTs based on the timing of MGPT use relative to lines of therapy for matched patients who received MGPTs. Study design and examples of matching cohorts are shown in Supplementary Figure 1, while additional patient selection and attrition are shown in Supplementary Table 1. Patients were propensity score-matched on age, gender, region, Charlson comorbidity index and baseline total costs, group-matched on post index follow-up time (those with ≤1 year of follow-up were exact-matched; individuals with >1 year of follow-up were group-matched) and exact-matched on receipt of any treatment and surgery (aNSCLC only).
The main outcome was mean post index total costs, including all medical and pharmacy claims for a given patient, adjusted to the 2020 Consumer Prices Index and compared using Wilcoxon rank sum tests. Total costs were estimated with variable follow-up up to 1 year, in order to use as a model input into the decision analytic model to project annual premiums for the following year owing to MGPT coverage. Additionally, the mean cost of MGPTs was estimated.
Decision analytic model
A decision analytic model, representing a hypothetical 1 million member plan, was developed from a US commercial payer perspective using Microsoft Excel to estimate the impact of MGPT coverage on member premiums. The patient population considered included those with aNSCLC, aMelanoma or mCRC, which included both newly diagnosed incident patients as well as patients who had a recurrence after initial diagnosis at an earlier stage of disease. For aMelanoma and mCRC, we also included previously incident and recurrent patients, given that testing with MGPT may be more likely to occur in later treatment lines as well (compared with aNSCLC). Given the focus on the commercially insured population, we estimated the expected number of patients aged <65 years based on published epidemiological data (Supplementary Table 2).
In general, premiums are equal to the sum of two components, the expected costs of services covered (reflecting paid claims, number of members, scope of benefits, cost-sharing requirements) and operational costs/administrative fees (including marketing costs, costs associated with claim processing and other general costs) (Supplementary Figure 2). The medical loss ratio (MLR) is a common metric used to estimate the proportion of premiums used on paid claims and administrative fees and is defined as the ratio of claims payments to total premiums. To estimate the impact of MGPT coverage on premiums, we estimated the difference in premiums in two scenarios: with and without MGPT coverage. To isolate the impact of MGPT coverage on premiums, we focused on the impact of coverage on the paid claims portion of the expected costs of services covered while assuming/holding the other factors (i.e., number of members, cost-sharing requirements, MLR) constant.
We estimated current premiums based on a distribution of coverage types (single, single + 1, family) and annual premiums from the Medical Expenditure Panel Survey. We defined an enrollee as the eligible person who is enrolled in the health plan, while members were defined as the enrollees and their dependents. The number of enrollees paying premiums was determined by coverage type, with family coverage assumed to be four members. Estimates of new premiums were calculated based on the two components described above (expected costs of services covered and operational costs). The expected costs of services covered were determined as the sum of the current paid claims and the additional paid claims associated with MGPT coverage. Current paid claims were estimated by multiplying the MLR (assumed to be 80% in the base case) to the current premiums collected. To estimate the operational costs portion of the new premium with MGPT coverage, we applied the MLR to the expected costs of services covered.
The additional paid claims associated with MGPT coverage were estimated via the retrospective claims analysis described above. In the scenario with MGPT coverage, the average costs for a patient was estimated by taking the weighted average of those who used and did not use MGPTs multiplied by the respective costs for those patients (from the claims analysis). In the scenario without MGPT coverage, the average costs for a patient was estimated in the same way except that the cost of the MGPT was subtracted from the cost associated with MGPT use. The use of MGPTs was based on published literature. In the base case scenario we assumed that coverage of MGPTs would increase utilization marginally (5%) owing to the reimbursement of the tests typically occurring after the test has been ordered. Outcomes of the model included the per member per month (PMPM) budget impact and change in monthly premiums.
Sensitivity analyses were conducted to assess the robustness of the model results. Total costs with and without MGPTs and the cost of MGPTs were varied by ±20% in one-way sensitivity analyses. Given the uncertainty of the assumptions around MGPT utilization, we ran two-way sensitivity analyses on this in combination with the differences in patient costs for those using and not using MGPTs. Additionally, MLR values were varied to reflect differences in plan types. An MLR of 80% was assumed to represent individual and small employer plans, while an MLR of 85% was assumed to represent large self-funded plans and an MLR equal to 97% was assumed to represent large fully insured plans [Citation13].
Results
Costs for MGPT tested patients versus non-MGPT tested patients
In total, 1056 patients were included in the analysis, with 273 aNSCLC, 56 aMelanoma and 95 mCRC MGPT tested patients matched to non-MGPT tested patients. After matching, baseline characteristics were similar between MGPT tested and non-MGPT tested patients within each tumor type ().
Table 1. Baseline characteristics.
In all three tumor types, the mean total costs for MGPT tested patients were not significantly different from those for non-MGPT tested patients (p > 0.05) (). Additionally, total medical and pharmacy costs were not significantly different between cohorts (p > 0.05). While mean cancer treatment related costs were not significantly different for aNSCLC (mean [standard deviation; SD] costs for MGPT tested vs non-MGPT tested: $60,747 [$93,706] vs $55,305 [$97,944]; p > 0.05) and mCRC (mean [SD] costs for MGPT tested vs non-MGPT tested: $58,168 [$67,689] vs $65 353 [$80,865]; p > 0.05), cancer treatment related costs were significantly higher for MGPT tested patients with aMelanoma than for non-MGPT tested patients (mean [SD]: $136 409 [$144,188] vs $109,369 [$173,391]; p = 0.04). MGPT related costs were approximately $1000 across the tumor types (mean [SD]: aNSCLC: $1076 [$2126]; aMelanoma: $1243 [$2292]; mCRC: $891 [$2007]).
Table 2. Total costs for patients with multigene panel test claims versus without multigene panel test claims.
Impact of MGPT coverage on insurance premiums
In a 1 million member commercial plan, we estimated a total of 436 patients with aNSCLC (n = 142), aMelanoma (n = 59) or mCRC (n = 235) (). Among these patients, 188 were estimated to receive MGPTs in the scenario without MGPT coverage and 198 were expected to receive MGPTs in the scenario with MGPT coverage. The estimated 1 year budget impact of covering MGPTs across the three tumor types was $195,171, or $0.016 PMPM. The change in premiums per enrollee per month was estimated to be $0.040 for individual and small employer plans (MLR = 80%), $0.037 for large self-funded plans (MLR = 85%) and $0.033 for large fully insured plans (MLR = 97%).
Table 3. Base case estimates for impact on insurance premiums for a hypothetical 1 million member commercial health plan.
Model sensitivity analyses
One-way sensitivity analyses showed variation between $0.00 and $0.08, with the impact on premiums being most sensitive to the total costs for patients with aNSCLC for whom MGPTs were used, followed by the total costs for patients with aNSCLC who did not have MGPT testing and lastly the total costs for patients with mCRC who did not have MGPT testing (). The cost of MGPTs resulted in changes of premiums between $0.036 and $0.034.
aMelanoma: Advanced melanoma; aNSCLC: Advanced non-small-cell lung cancer; mCRC: Metastatic colorectal cancer; MGPT: Multigene panel test.
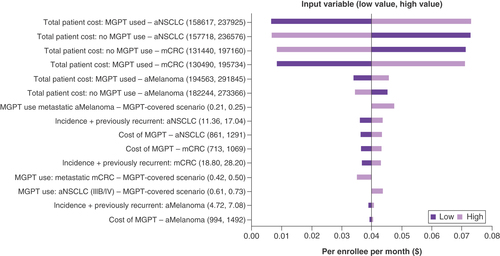
Two-way sensitivity analyses examining the relationship between changes in MGPT utilization (with coverage) and differences in total costs between patients using and not using MGPTs showed that the results were robust across the ranges of values tested (). Changes in premiums ranged from $0.018 to $0.026 in aNSCLC, from $0.003 to $0.018 in aMelanoma and from $0.008 to $0.017 in mCRC.
Table 4. Two-way sensitivity analysis: assessment of varying multigene panel test utilization and costs simultaneously on the change in insurance premiums.
Discussion
Using real-world medical and pharmacy claims data, we found the total costs up to 1 year for patients with aNSCLC, aMelanoma or mCRC who had claims for MGPTs to not be significantly different compared with patients without claims for MGPTs. Using the claims analysis results as inputs into a model to estimate the impact of MGPT coverage for the three tumor types in a 1 million member commercial plan found the expected impact to payers’ budgets and patients’ premiums to be minimally cost additive. The limited impact to patients and payers is driven by both the similarity in costs for patients with claims for MGPTs and no claims for MGPTs, as well as the relatively few number of patients with the three tumor types. To our knowledge, this is the first study to assess the real-world total costs associated with patients with MGPT claims versus no MGPT claims and to estimate the impact on patient premiums.
Prior research has shown MGPTs to have a range of costs. For example, Dalal et al. found the average cost of an MGPT to be $2860 [Citation14], while Desai et al. found the average cost per test varied from $1269 to $2058 [Citation15]. The cost of an MGPT in our study was lower than the costs found in previous studies, with the average cost ranging from $891 to $1243 depending on the tumor type. While it is not clear why the costs were less in the present analysis, one possibility is that the composition of payers was different between the studies. Nonetheless, our study builds upon the previous work on MGPT costs by looking at an expanded view of the economic impact by examining the total real-world costs associated with patients with and without claims for MGPTs, for which the cost of the MGPT appears to be a small fraction of the total patient costs.
Previous studies examining the budget impact of MGPTs have mostly been limited to aNSCLC and have found MGPTs to be associated with cost savings or to have a minimal impact on budget. Pennel et al. examined the budget impact of MGPT versus different testing strategies and found MGPTs to be cost saving (although downstream costs such as treatment were excluded) [Citation11], while Yu et al. found the use of MGPTs versus single-gene testing to have a small budget impact (5 year PMPM: $0.007) [Citation12]. Our analysis found similar results in aNSCLC (1 year PMPM: $0.008) while also finding that coverage across the three tumor types would still result in a limited budget impact. Given our use of real-world patient cost data to estimate the costs associated with MGPTs rather than relying on assumptions, the consistency of our results with prior findings strengthens the evidence of the limited cost impact of MGPTs.
Generally, changes in health plan spending are a proxy for insurance premiums; however, there are limited data on the impact of coverage of MGPTs in oncology. In our study, we found that the estimated impact on insurance premiums would be minimal – approximately $0.04 – across different plan types (individual/small employer, large self-funded, large fully insured). Furthermore, given that many employers contribute to their employees’ insurance coverage, the portion by plan enrollees would be even smaller (approximately $0.01 per enrollee per month). The findings here are consistent with a recent white paper which found that the impact of broadening coverage of biomarker testing (not specific to MGPTs) across oncology on insurance premium ranges from $0.14 to $0.51 PMPM [Citation13]. Given that many US states have passed or are considering passing legislation to improve access to biomarker testing at the state level [Citation10], evidence of the limited impact of MGPT coverage on insurance premiums may be an important consideration for policymakers who are concerned about the potential impact of legislation on patient costs.
Limitations
There are several limitations of this analysis to consider. First, there is some uncertainty in the cost estimates comparing patients receiving and not receiving MGPTs. This may be due to smaller sample sizes or potential misclassification of MGPTs owing to challenges in identifying MGPTs in claims data. For example, MGPTs may be partially reimbursed using stacked individual biomarker codes, which may result in some patients receiving MGPTs being included in the non-MGPT cohort. While the misclassification may bias our cost estimates in the claims analysis to be more similar, and the smaller sample sizes may lead to larger uncertainty around the estimates, sensitivity analyses in the model showed results to be robust when varying the cost estimates by 20%. Second, our analysis had a limited time horizon of 1 year and it is unknown how expanding this may affect the results. For example, delayed progression due to more targeted treatments identified via MGPT may not occur until after 1 year, thus potentially leading to greater cost savings in the MGPT cohort. Further research and more complex modeling would be required to estimate the longer-term impact on insurance premiums. Third, owing to the limitations of administrative claims data, other outcomes, such as patient quality of life or clinical outcomes (such as treatment response), could not be measured in this analysis, but could add further context to cost results in this study. For example, additional information on treatment response could provide insight into the reasons for the similarity in costs between the MGPT and non-MGPT cohorts. Finally, the results of this analysis may not be generalizable to all commercial plans or payers. While our claims analysis includes only a subset of payers in the USA, there are multiple payers included in the database. Additional research leveraging other data sources with different payers may be warranted to confirm the generalizability of the findings.
Conclusion
Patients with aNSCLC, aMelanoma or mCRC and claims for MGPTs did not have significantly different total costs than patients without claims for MGPTs. Coverage of MGPTs by commercial insurers was estimated to result in minimal budget impact and changes in insurance premiums.
Total costs associated with the use of multigene panel tests (MGPTs) were not significantly different from those associated with patients who did not use MGPTs.
The cost of MGPTs was a small fraction of the total patient costs.
The estimated budget impact on payers for coverage of MGPTs was expected to be minimal.
The impact of coverage on insurance premiums was expected to be minimal across different plan types.
The expected minimal impact on insurance premiums may address policymaker concerns about the potential impact of legislation improving access to biomarker testing on patient costs.
Author contributions
Concept and design: D Sheinson, TM To, W Wong. Acquisition, analysis or interpretation: Y Liu, D Sheinson, TM To, W Wong, Y Liu. Drafting or revising manuscript: Y Liu, D Sheinson, TM To, W Wong. Final approval of manuscript: Y Liu, D Sheinson, TM To, W Wong.
Financial & competing interests disclosure
This study was funded by Genentech, Inc. D Sheinson, TM To and W Wong are employees of Genentech, Inc. and have Roche Stock. Y Liu is an employee of Genesis Research, which received funding from Genentech, Inc. in connection with this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by J Carthy and R Hornby of Oxford PharmaGenesis (Oxford, UK), and was funded by Genentech, Inc.
Supplemental Figure 1
Download MS Word (208.2 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2023-0094
Additional information
Funding
References
- Li M , GoldmanDP, ChenAJ. Spending on targeted therapies reduced mortality in patients with advanced-stage breast cancer. Health Aff. (Millwood)40(5), 763–771 (2021).
- Howlader N , ForjazG, MooradianMJet al. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med.383(7), 640–649 (2020).
- American Cancer Society . Targeted drug therapy for non-small-cell lung cancer (2023). www.cancer.org/cancer/lung-cancer/treating-non-small-cell/targeted-therapies.html
- Sheinson DM , WongWB, FloresC, OgaleS, GrossCP. Association between Medicare’s national coverage determination and utilization of next-generation sequencing. JCO Oncol. Pract.17(11), e1774–e1784 (2021).
- Centers for Medicare & Medicaid Services . Medicare coverage database (2023). www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=372
- Hsiao SJ , SireciAN, PendrickDet al. Clinical utilization, utility, and reimbursement for expanded genomic panel testing in adult oncology. JCO Precis. Oncol.4, 1038–1048 (2020).
- Wong WB , AninaD, LinCW, AdamsDV. Alignment of health plan coverage policies for somatic multigene panel testing with clinical guidelines in select solid tumors. Per. Med.19(3), 171–180 (2022).
- Lungevity . State Medicaid coverage policy and impact on lung cancer outcomes (2023). www.lungevity.org/sites/default/files/state-scorecards/LUNGevity-scorecard-030920.pdf
- Gross CP , MeyerCS, OgaleS, KentM, WongWB. Associations between Medicaid insurance, biomarker testing, and outcomes in patients with advanced NSCLC. J. Natl Compr. Canc. Netw.20(5), 479–487.e472 (2022).
- Sadigh G , GoecknerHG, KazerooniEAet al. State legislative trends related to biomarker testing. Cancer128(15), 2865–2870 (2022).
- Pennell NA , MutebiA, ZhouZYet al. Economic impact of next-generation sequencing versus single-gene testing to detect genomic alterations in metastatic non-small-cell lung cancer using a decision analytic model. JCO Precis. Oncol.3, 1–9 (2019).
- Yu TM , MorrisonC, GoldEJ, TradonskyA, ArnoldRJG. Budget impact of next-generation sequencing for molecular assessment of advanced non-small cell lung cancer. Value Health21(11), 1278–1285 (2018).
- Milliman, Inc. The landscape of biomarker testing coverage in the United States (2023). www.milliman.com/en/insight/the-landscape-of-biomarker-testing-coverage-in-the-us
- Dalal AA , GuerinA, MutebiA, CulverKW. Economic analysis of BRAF gene mutation testing in real world practice using claims data: costs of single gene versus panel tests in patients with lung cancer. J. Med. Econ.21(7), 649–655 (2018).
- Desai K , HookerG, GilbertK, CropperC, MetcalfR, KachrooS. Real-world trends in costs of next generation sequencing (NGS) testing in U.S. setting. J. Clin. Oncol.39(Suppl. 15), e18824–e18824 (2021).
