Abstract
Despite recent treatment advances, the prognosis for patients with locally recurrent inoperable or metastatic triple-negative breast cancer (TNBC) remains poor. The antibody–drug conjugate datopotamab deruxtecan (Dato-DXd) is composed of a humanized anti-TROP2 IgG1 monoclonal antibody linked to a topoisomerase I inhibitor payload via a stable, cleavable linker. The phase III TROPION-Breast02 trial in patients previously untreated for locally recurrent inoperable or metastatic TNBC, who are not candidates for PD-1/PD-L1 inhibitors is evaluating efficacy and safety of Dato-DXd versus investigator’s choice of chemotherapy (ICC). Approximately 600 patients will be randomized 1:1 to Dato-DXd 6 mg/kg iv. every 3 weeks or ICC (paclitaxel, nab-paclitaxel, carboplatin, capecitabine or eribulin mesylate). Dual primary end points are progression-free survival by blinded independent central review and overall survival.
Plain language summary
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that is hard to treat. Tumors lack receptors for estrogen and progesterone, which means that standard endocrine therapy is ineffective, and it does not express HER2, so HER2 therapies are also not appropriate. However, the majority of TNBC tumors do possess a cell surface protein called TROP2 which provides a way of directing treatment inside tumor cells that is more selective than traditional chemotherapy.
Datopotamab deruxtecan (Dato-DXd) is a drug that consists of two parts: datopotamab (an antibody) and DXd (the cancer-cell killing toxic component), which are joined via a stable linker. Datopotamab binds to the TROP2 protein found on TNBC tumors and is taken into the cell. The linker is then broken and releases DXd, which kills the tumor cell. By binding to cancer cells before releasing the payload, treatment is directed to the tumor, minimizing side effects in the rest of the body.
The TROPION-Breast02 study aims to discover whether Dato-DXd is more effective than standard-of-care chemotherapy, allowing patients with TNBC to live longer without their breast cancer getting worse. This study is also looking at how Dato-DXd may affect patients’ overall functioning and quality of life. TROPION-Breast02 will recruit approximately 600 patients who:
Have cancer that has spread from the original site (metastatic), or cancer that returned to the same site (locally recurrent) that cannot be surgically removed
Have not received any prior treatment for this stage of cancer
Cannot receive an alternative type of anticancer treatment called PD-(L)1 inhibitors
Had any length of time between their last treatment with the aim of cure and return of their disease
Eligible patients will be randomly assigned to a treatment group in equal numbers to either Dato-DXd or an appropriate chemotherapy (one of five available options, chosen by the treating doctor). Each patient will generally continue to receive their designated treatments if the tumor is controlled by the drug, there are no unacceptable side effects, or the patient chooses to stop treatment.
Clinical Trial Registration: NCT05374512 (ClinicalTrials.gov)
Of the 2.26 million cases of breast cancer diagnosed worldwide each year [Citation1], approximately 15–20% of patients will have triple-negative breast cancer (TNBC) [Citation2,Citation3] – a subtype that is characterized by a lack of estrogen receptors (ER) and progesterone receptors (PgR), as well as the absence of human epidermal growth factor 2 (HER2) overexpression/amplification [Citation2,Citation4,Citation5]. The lack of these actionable treatment targets and an aggressive nature, where many patients experience early local recurrence following therapy, a high propensity for metastasis, and poor 5-year survival [Citation2–4] make TNBC an area of high unmet medical need. Despite recent advances in breast cancer therapy, the prognosis for patients diagnosed with locally recurrent inoperable or metastatic TNBC remains poor [Citation2,Citation4,Citation5].
While single-agent chemotherapy continues to play an important role in the treatment of metastatic TNBC, additional treatment options have recently become available for patients with programmed cell death-ligand 1 (PD-L1)-positive tumors or germline BRCA mutations [Citation6,Citation7]. In patients with metastatic TNBC and PD-L1-positive tumors (around 40% of patients with metastatic disease [Citation8]) anti-PD-1/PD-L1 agents in combination with chemotherapy are now recommended as first-line treatment based on recent clinical trial data. Results from the phase III, placebo-controlled IMpassion130 trial (NCT02425891) indicated that the addition of atezolizumab to nab-paclitaxel increased the median progression-free survival (PFS) and resulted in a clinically meaningful, but non-statistically significant improvement in median overall survival (OS) (both primary end points) versus placebo and nab-paclitaxel in treatment-naive patients with PD-L1-positive (≥1% immune cells, per VENTANA SP142 PD-L1 assay), locally advanced unresectable or metastatic TNBC (PFS: hazard ratio [HR]: 0.62; 95% CI: 0.49–0.78; p < 0.001; OS: HR: 0.67; 95% CI: 0.53–0.86) [Citation9,Citation10]. Notably, these patients had a treatment-free interval of at least 12 months from curative intent therapy, or de novo metastatic disease. The KEYNOTE-355 (NCT02819518) trial provided additional data on the use of immunotherapy in combination with chemotherapy [Citation11]. This phase III trial showed that pembrolizumab plus investigator’s choice of chemotherapy (ICC; i.e., nab-paclitaxel, paclitaxel, or gemcitabine-carboplatin) significantly improved both PFS and OS in previously untreated patients with PD-L1-positive (combined positive score ≥10, per PD-L1 immunohistochemistry 22C3 assay) locally recurrent inoperable or metastatic TNBC versus placebo plus ICC (PFS HR: 0.66; 95% CI: 0.50–0.88; OS HR: 0.73; 95% CI: 0.55–0.95; p = 0.0185), with a manageable safety profile.
Additionally, bevacizumab in combination with chemotherapy is approved as a first-line treatment for metastatic TNBC in some countries. However, this regimen is not approved globally (including in the USA), as clinical data have shown that the addition of bevacizumab resulted in an increase in median PFS, but did not result in a statistically significant improvement in overall survival [Citation12–14].
In patients with germline BRCA mutations, who represent ∼20% of patients with TNBC [Citation15,Citation16], poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors are now an alternate treatment option to chemotherapy [Citation7] based on results of the OlympiAD (NCT02000622) [Citation17] and EMBRACA (NCT01945775) [Citation18] trials. The phase III OlympiAD trial reported that the PARP inhibitor, olaparib, significantly increased median PFS versus non-platinum ICC (7.0 months vs 4.2 months; HR: 0.58; 95% CI: 0.43–0.80; p < 0.001) [Citation17]. Similarly, the phase III EMBRACA trial showed that the PARP inhibitor, talazoparib, significantly increased median PFS compared with ICC (8.6 months vs 5.6 months; HR: 0.54; 95% CI: 0.41–0.71; p < 0.001) [Citation18]. In both studies, no significant differences were observed in OS.
However, for the majority of patients, first-line treatment remains chemotherapy [Citation7]. As chemotherapy is associated with relatively short PFS and low treatment response rates [Citation9,Citation11,Citation19–22], there remains an urgent unmet need for new TNBC treatment options.
Antibody drug conjugates (ADC) have rapidly emerged as a new modality for the delivery of cytotoxic therapy in advanced breast cancer. These agents deliver a cytotoxic payload linked to a tumor-directed antibody. One potential candidate for tumor-directed therapy is trophoblast cell surface antigen 2 (TROP2). TROP2 is a transmembrane protein that has been shown to modulate the MAPK and PI3K/AKT intracellular signalling pathways and is associated with proliferation, migration and invasion of cancerous cells. As TROP2 is often highly expressed on tumors [Citation23,Citation24], including TNBC [Citation25], it represents an attractive potential antigen target for anti-TROP2 monoclonal antibodies (mAb). Indeed, recent data have demonstrated a role for TROP2-directed agents in pre-treated TNBC. The anti-TROP2 ADC sacituzumab govitecan, which has a topoisomerase I inhibitor (SN38) payload and is dosed on days 1 and 8 of a 21-day cycle, was found to increase PFS and OS versus single-agent chemotherapy in a phase III trial of patients with relapsed or refractory metastatic TNBC who had received prior treatment with a taxane (PFS: 5.6 vs 1.7 months [HR: 0.41; 95% CI: 0.32–0.52; p < 0.001]; OS: 12.1 vs 6.7 months [HR: 0.48; 95% CI: 0.38–0.59; p < 0.001]) [Citation26].
Datopotamab deruxtecan (Dato-DXd) is an investigational TROP2-directed ADC that consists of a humanized anti-TROP2 IgG1 monoclonal antibody conjugated to a potent topoisomerase I inhibitor via a serum stable tetrapeptide-based tumor-selective cleavable linker (A) [Citation23,Citation27–29]. Consistent with the stable linker, Dato-DXd exhibits an extended half-life (t1/2) of approximately 45.12 ± 13.92 h following intravenous (iv.) administration [Citation23]. This extended t1/2 prolongs the exposure of the intact ADC to the tumor, which may increase anti-tumor effect and durability of response. The extended t1/2 also allows for dosing once every 3 weeks (on day 1 of a 21-day cycle). Upon binding of the antibody to tumor-surface expressed TROP2, the entire molecule is internalized and the linker is enzymatically cleaved to release DXd (B) [Citation23,Citation30]. In contrast to the intact ADC, free DXd exhibits a short t1/2in vivo [Citation27] resulting in low systemic exposure to the payload and a safety profile that is differentiated from standard-of-care chemotherapy. DXd, an exatecan derivative, is more potent than irinotecan, the active metabolite of which is SN38 [Citation31]. In addition, DXd exhibits high membrane permeability which enables it to enter surrounding cells and exert a cytotoxic effect in tumor cells which may not necessarily express TROP2, a process known as the “bystander effect” [Citation27,Citation29].
(A) Design of datopotamab deruxtecan monoclonal antibody and (B) proposed mechanism of action of datopotamab deruxtecan.
Panel A previously presented at the 2019 World Conference on Lung Cancer [Citation32]. Panel B reproduced with kind permission of the authors with permission from [Citation33].
DXd: Deruxtecan; IgG1: Immunoglobulin G1; mAb: Monoclonal antibody; TROP2: Trophoblast cell surface antigen 2.
![Figure 1. Overview of the monoclonal antibody datopotamab deruxtecan. (A) Design of datopotamab deruxtecan monoclonal antibody and (B) proposed mechanism of action of datopotamab deruxtecan.Panel A previously presented at the 2019 World Conference on Lung Cancer [Citation32]. Panel B reproduced with kind permission of the authors with permission from [Citation33].DXd: Deruxtecan; IgG1: Immunoglobulin G1; mAb: Monoclonal antibody; TROP2: Trophoblast cell surface antigen 2.](/cms/asset/285463d0-b7e5-44c0-a6d3-35cb164e6f55/ifon_a_12333896_f0001.gif)
Preliminary results from the phase I TROPION-PanTumor01 (NCT03401385) trial have demonstrated encouraging preliminary efficacy and a manageable safety profile of Dato-DXd in patients with non-small-cell lung cancer (NSCLC) or TNBC [Citation34–36]. Recently updated data from the TNBC group reported an objective response rate (ORR) of 32% (14 of 44 patients) and a disease control rate of 80% (35/44) in patients with metastatic TNBC who progressed on standard therapies; in patients with metastatic TNBC, and no prior treatment with a topoisomerase I inhibitor-based ADC, the ORR was 44% (12/27) [Citation34]. The most common grade ≥3 treatment-emergent adverse events in TNBC patients from TROPION-PanTumor01 were stomatitis (11%), fatigue (7%) and decreased lymphocyte count (7%). In the phase I/II BEGONIA trial (NCT03742102) involving patients with unresectable locally advanced or metastatic TNBC, the confirmed ORR in patients receiving Dato-DXd + durvalumab was 74% (39/53) comprising four complete responses and 35 partial responses [Citation37]. Responses were durable, with 82% of responders remaining in response at data cut-off. The combination of Dato-DXd and durvalumab also demonstrated a manageable safety profile.
TROPION-Breast02 is a phase III study to further evaluate the safety and efficacy of Dato-DXd monotherapy versus an ICC in patients previously untreated for locally recurrent inoperable or metastatic TNBC who are not candidates for PD-1/PD-L1 inhibitor therapy.
Study design
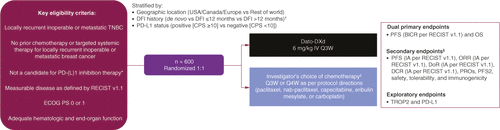
TROPION-Breast02 (NCT05374512) is an ongoing international, phase III, open-label, randomized clinical trial (). The study started enrolling patients in May 2022, and the final primary analysis date is estimated to be in December 2025. Approximately 600 eligible patients will be randomized 1:1 to receive either Dato-DXd 6 mg/kg iv. every 3 weeks (Q3W) or ICC (). Randomization will be stratified according to geographic location (USA/Canada/EU vs Rest of World), PD-L1 status (as assessed by a central laboratory) through combined positive score (CPS; CPS ≥10 is positive vs CPS <10 is negative), and disease-free interval (DFI) history (de novo vs prior DFI ≤12 months vs prior DFI >12 months). DFI is defined as the time between completion of treatment with curative intent (either surgery or last dose of systemic anticancer therapy) and first documented local or distant disease recurrence. Adjuvant radiation therapy is not considered treatment with curative intent for the purpose of calculating.
Table 1. Study interventions.
*PD-L1 negative, previous PD-1/PD-L1 inhibitor therapy for early-stage breast cancer, comorbidities precluding PD-1/PD-L1 inhibitor therapy, or PD-L1 positive with no regulatory access to PD-1/PD-L1 inhibitor therapy (no regulatory approval in country).
†DFI defined as the time between completion of treatment with curative intent (either surgery or last dose of systemic anticancer therapy) and first documented local or distant disease recurrence. Adjuvant radiation therapy is not considered treatment with curative intent for the purpose of calculating. No minimum DFI was specified; patients could have had any length of time between curative-intent treatment and disease recurrence.
‡If no prior taxane, or prior taxane in the (neo)adjuvant setting and DFI >12 months, paclitaxel or nab-paclitaxel; if prior taxane and DFI ≤12 months: capecitabine, carboplatin, or eribulin mesylate.
§Response assessment: scan Q6W for 48 weeks, then Q9W until RECIST v1.1, regardless of study intervention, discontinuation or start of subsequent anticancer therapy.
BICR: Blinded independent central review; CPS: Combined positive score; Dato-DXd: Datopotamab deruxtecan; DCR: Disease control rate; DFI: Disease-free interval; DoR: Duration of response; ECOG PS: Eastern Cooperative Oncology Group Performance Status; IA: Investigator’s assessment; iv.: Intravenous; ORR: Objective response rate; OS: Overall survival; PD-1: Programmed cell death protein-1; PD-L1: Programmed cell death-ligand 1; PFS: Progression-free survival; PFS2: Time to second progression or death; PRO: Patient-reported outcome; Q3/4/6/9W: Every 3/4/6/9 weeks; RECIST v1.1: Response Evaluation Criteria in Solid Tumours version 1.1; TNBC: Triple-negative breast cancer; TROP2: Trophoblast cell surface protein 2.
The study is being performed in accordance with the Declaration of Helsinki, International Council for Harmonisation/Good Clinical Practice, and applicable regulatory requirements; all patients will provide written, informed consent.
Eligibility criteria
Inclusion and exclusion criteria for TROPION-Breast02 patients are presented in & . In summary, patients aged ≥18 years with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1 are eligible for enrolment. Disease characteristics include histologically or cytologically documented locally recurrent inoperable or metastatic TNBC (according to the current American Society of Clinical Oncology/College of American Pathologists guidelines definition, ER <1%, PgR <1%, HER2 0–1+, or 2+ with FISH ratio <2) and ≥1 measurable lesion per Response Evaluation Criteria in Solid Tumours version 1.1 (RECIST 1.1). Patients must not have had prior therapy for metastatic or locally recurrent inoperable breast cancer, be eligible for one of the ICC treatment options, and be ineligible for PD-1/PD-L1 inhibitor therapy (as defined in ). There is no minimum DFI required following completion of therapy for early breast cancer with curative intent; though the number of patients with DFI ≤12 months was capped at 20% of randomized patients. Patients with a history of previously treated neoplastic spinal cord compression or clinically inactive brain metastases, who require no treatment with corticosteroids or anticonvulsants, may be included in the study if they have recovered from any acute toxic effects of radiotherapy after a minimum 2 week washout period.
Table 2. Key inclusion criteria.
Table 3. Key exclusion criteria.
End points
The dual primary end points are PFS by blinded independent central review (BICR) and OS. PFS is defined as time from randomization until progression per RECIST 1.1, or death due to any cause; OS is defined as time from randomization until death due to any cause.
Secondary end points include: ORR defined as the proportion of patients with a confirmed complete or partial response (by BICR/investigator assessment per RECIST 1.1); duration of response (time from first confirmed response to progression); disease control rate at 12 weeks defined as the percentage of patients with confirmed complete or partial response, or stable disease (by BICR/investigator assessment per RECIST 1.1); PFS by investigator assessment per RECIST 1.1; time to second progression (PFS2), defined as the time from randomization to the earliest sign of progression following subsequent therapy, or death; patient-reported outcomes including time to deterioration from baseline in pain, physical functioning, breast and arm symptoms, global health score/quality of life, and time to first and second subsequent therapies. Pharmacokinetics and immunogenicity of Dato-DXd will also be assessed. For immunogenicity, patient samples will be assessed for the presence of detectable anti-Dato-DXd antibodies. Safety and tolerability will be measured in terms of adverse events (AE; graded by National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE] version 5.0), ECOG PS, clinical and biochemical assessments, as well as ophthalmologic assessments.
Study assessments & procedures
Tumor assessments per RECIST 1.1 will be undertaken with either computed tomography as the preferred method, or via MRI scans. Baseline tumor imaging will be conducted up to 28 days before treatment initiation. Further images will be taken every 6 weeks from randomization for 48 weeks, then every 9 weeks until radiological disease progression occurs per investigator assessment, with an additional follow-up assessment after progression. For assessment of PFS2 after objective progression, the patient’s progression status will be recorded every 3 months according to local practice. A second progression event must have occurred during or after treatment with a subsequent anticancer therapy.
All patients will have baseline central nervous system imaging and whole-body skeletal scintigraphy as part of baseline screening. Mandatory follow-up scans will be implemented for any patient randomized with brain metastasis at baseline, per the tumor imaging schedule until radiological progression, per RECIST 1.1.
Patient-reported outcomes will be assessed via several different quality of life questionnaires. In addition, patients will be invited to report the occurrence and severity of signs or symptoms commonly associated with stomatitis. As a precautionary measure, all patients will be started on a mandatory daily oral care routine that will be maintained until 28 days after the last dose. For patients randomized to Dato-DXd, a steroid-containing mouthwash (dexamethasone, if available) is highly recommended and prophylactic cryotherapy should also be considered.
Safety, tolerability & immunogenicity
Safety, tolerability and immunogenicity will be assessed continuously throughout the study period from 28 days before treatment until the last safety follow-up. AEs will be coded using the most recent version of National Cancer Institute Medical Dictionary for Regulatory Activities (MedDRA) and graded using NCI CTCAE version 5.0. Information on ECOG PS, vital signs, body weight and physical examinations will be collected, and laboratory, ophthalmologic and cardiac assessments will be undertaken. All patients will receive ophthalmologic examinations at baseline, as clinically indicated, and at the end of treatment, with additional examinations every 3 cycles for patients randomized to Dato-DXd. A study-specific standardized ophthalmologic assessment form is provided to capture the results of selected ophthalmologic examinations. Furthermore, patients will be monitored for potential development of interstitial lung disease (ILD)/pneumonitis; where suspected, treatment with Dato-DXd will be interrupted while a full evaluation is conducted. Pharmacokinetic parameters will be assessed from samples taken before and after Dato-DXd infusions on the first day of cycle 1, 2, 4 and 8 of treatment; a final sample will be collected at the end of treatment visit. Immunogenicity will be assessed on the first day of cycle 1, 2, 4, 6 and 8, and every 4 cycles thereafter, with additional samples collected at the end of treatment visit and the first safety follow-up.
Statistical methods
Approximately 800 patients will be screened to achieve approximately 600 patients randomized (1:1) to a treatment group. This sample size is designed to characterize differences in the dual primary end points (PFS by BICR and OS) between the Dato-DXd and ICC arms in the intention-to-treat (ITT) population. A multiple testing procedure with an alpha-exhaustive recycling strategy will be implemented. The overall alpha for the study is 0.05, which is split with 0.01 to evaluate PFS and 0.04 to evaluate OS. If the PFS dual primary analysis crosses the efficacy threshold, the 1.0% type I error allocated to the PFS end point will be reallocated to the OS end point for a total type I error of 5.0%.
The final PFS analysis for superiority will be performed after approximately 422 PFS events (by BICR) across the Dato-DXd and ICC treatment groups (70% maturity). This assumes a HR of 0.6 for a true PFS treatment effect between Dato-DXd and ICC, and a median PFS in the ICC group of 5.6 months to provide >99% power to demonstrate statistical significance at the 2-sided alpha level of 1.0%. The final OS analysis for superiority will be performed when approximately 340 OS events have occurred in the ITT population across the Dato-DXd and the ICC arms (57% maturity) and assumes a median OS of 20.6 months for the ICC arm.
Conclusion
TROP2-directed ADCs represent a novel and potentially effective avenue by which to localize cytotoxic therapy to the intended site of action in patients with TNBC. Trials are ongoing in advanced/metastatic TNBC with other TROP2-directed ADCs, including SKB264, which is moving into phase III development at the time of writing, and sacituzumab govitecan in combination with immunotherapies such as anti-PD-(L)1 agents, targeted agents or chemotherapy. Evaluations in patients with earlier stages of TNBC are also underway.
The ongoing phase III TROPION-Breast02 trial is evaluating the efficacy and safety of Dato-DXd versus ICC in patients previously untreated for locally recurrent inoperable or metastatic TNBC who are not candidates for PD-1/PD-L1 inhibitor therapy. It will build on interim data from the TROPION-PanTumor01 study [Citation34–36], which showed that Dato-DXd exhibits promising antitumor activity and a manageable safety profile in patients with refractory metastatic TNBC. These data will also complement and support the role of Dato-DXd in breast cancer as results become available from the BEGONIA trial [Citation37] (arm 7; NCT03742102), which is evaluating Dato-DXd in combination with durvalumab in metastatic TNBC, the ongoing TROPION-Breast01 study (NCT05104866) [Citation34] of Dato-DXd in patients with HR+/HER2– breast cancer, and the recently initiated TROPION-Breast03 trial (NCT05629585) which will evaluate Dato-DXd with or without durvalumab in patients with stage I–III TNBC who have residual invasive disease in the breast and/or axillary lymph nodes at surgical resection following neoadjuvant therapy.
In patients with TNBC and no actionable biomarkers, single-agent chemotherapy has been the only available treatment choice. An emerging role for TROP2-directed therapies in this setting supports the potential for Dato-DXd to offer an important new treatment option. As such, TROPION-Breast02 aims to demonstrate improved outcomes and quality of life in these patients with a high unmet need.
Background
Triple-negative breast cancer (TNBC) is characterized by a lack of actionable treatment targets and an aggressive disease course, resulting in early local recurrence, a high risk of metastasis and poor 5-year survival rates.
Despite recent advances in treatment following the introduction of immunotherapies for programmed death-ligand 1 (PD-L1) positive tumors, and poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors for those with germline BRCA mutations, chemotherapy remains the mainstay of first-line treatment for most patients and the prognosis for those diagnosed with locally recurrent inoperable or metastatic TNBC continues to be poor.
Antibody drug conjugates (ADC), that deliver a cytotoxic payload linked to a tumor-directed antibody, have rapidly emerged as a new modality for the delivery of cytotoxic therapy in advanced breast cancer. One potential candidate for tumor-directed therapy is trophoblast cell surface antigen 2 (TROP2), a transmembrane protein associated with proliferation, migration and invasion of cancerous cells that is often highly expressed on solid tumors, including TNBC.
Datopotamab deruxtecan (Dato-DXd) is an investigational TROP2-directed ADC that consists of a humanized anti-TROP2 IgG1 monoclonal antibody conjugated to a potent topoisomerase I inhibitor via a stable, cleavable linker. The phase I TROPION-PanTumor01 (NCT03401385) trial has demonstrated encouraging preliminary efficacy and a manageable safety profile for Dato-DXd in patients with TNBC.
TROPION-Breast02 (NCT05104866)
TROPION-Breast02 (NCT05374512) is an ongoing international, phase III, open-label, randomized clinical trial to further evaluate the safety and efficacy of Dato-DXd monotherapy versus investigator’s choice of chemotherapy (ICC) in patients previously untreated for locally recurrent inoperable or metastatic TNBC who are not candidates for PD-1/PD-L1 inhibitor therapy.
Approximately 600 eligible patients will be randomized 1:1 to receive either Dato-DXd 6 mg/kg intravenously every 3 weeks or ICC.
Outcomes
The dual primary end points are progression-free survival by blinded independent central review, and overall survival.
Conclusion
The results of the phase III TROPION-Breast02 study will help define the role of Dato-DXd in patients previously untreated for locally recurrent inoperable or metastatic TNBC with no actionable biomarkers.
Author contributions
Conception and design: MJ Maxwell, P Vukovic, D Mapiye, P Schmid, RA Dent, J Cortés; Manuscript writing, final approval of manuscript, accountable for all aspects of the work: all authors.
Financial & competing interests disclosure
The TROPION-Breast02 trial (NCT05374512) is sponsored by AstraZeneca. In July 2020, Daiichi-Sankyo entered into a global development and commercialization collaboration with AstraZeneca for datopotamab deruxtecan (Dato-DXd).
RA Dent has received consulting fees from AstraZeneca, Merck Sharp & Dohme (MSD), Roche, Pfizer, Eisai, Novartis, and Lilly in the last 24 months. DW Cescon has received consulting fees from AstraZeneca, Exact Sciences, Eisai, Gilead, GlaxoSmithKline (GSK), Lilly, Merck, Novartis, Pfizer, and Roche in the last 24 months; research funding to institution from AstraZeneca, Gilead, GSK, Inivata, Knight Therapeutics, Merck, Pfizer, and Roche; patent (US62/675,228) for methods of treating cancers characterized by a high expression level of spindle and kinetochore associated complex subunit 3 (ska3) gene. T Bachelot has received consulting fees from Seagen, Novartis, Daiichi-Sankyo, AstraZeneca, Pfizer, and Lilly in the last 24 months. KH Jung has received consulting fees from Daiichi-Sankyo, Everest Medicine, MSD, Novartis, Pfizer, and Roche in the last 24 months. Z-M Shao has no conflicts of interest to disclose. S Saji has received consulting fees from AstraZeneca, Chugai, Eli Lilly, Kyowa Kirin, MSD, Ono, and Pfizer; conducted contracted research for AstraZeneca, Chugai, Daiichi-Sankyo, MSD, and Taiho; received fees for non-CME services directly from commercial interest or their agents from AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi-Sankyo, Eli Lilly, Eisai, Kyowa Kirin, MSD, Novartis, Ono, Pfizer, and Taiho in the last 24 months. TA Traina has received consulting fees from AstraZeneca, Daiichi-Sankyo, Gilead, Pfizer, Novartis, and Genentech (DSMB); conducted contracted research for AstraZeneca, Astellas Pharma, Genentech, Roche, Daiichi-Sankyo, and Ayala Pharmaceuticals in the last 24 months. P Vukovic is an employee and ownership interest holder of AstraZeneca. D Mapiye is an employee of AstraZeneca. MJ Maxwell is an employee of AstraZeneca. P Schmid has received consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Puma, Roche, Eisai, and Celgene; conducted contracted research for Astellas, AstraZeneca, Genentech, Novartis, OncoGenex Pharmaceuticals, Roche, and Medivation in the last 24 months. J Cortés has received consulting fees from Roche, Celgene, Cellestia, AstraZeneca, Seattle Genetics, Daiichi-Sankyo, Erytech, Athenex, Polyphor, Lilly, MSD, GSK, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, and Reveal Genomics; conducted contracted research for Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GmbH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffmann-La Roche, Guardant Health, MSD, Pfizer, Piqur Therapeutics, and Puma on behalf of Queen Mary University of London; received fees for non-CME services received directly from commercial interest or their agents from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, MSD, and Daiichi-Sankyo; ownership interest in MedSIR, Nektar Pharmaceuticals, and Leuko stocks in the last 24 months; holds patents WO 2014/199294 A and US 2019/0338368 A1. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Catherine Crookes of Ashfield MedComms (Macclesfield, UK), an Inizio company, and was funded by AstraZeneca.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Data sharing statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Additional information
Funding
References
- Sung H , FerlayJ, SiegelRLet al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249 (2021).
- Palma G , FrasciG, ChiricoAet al. Triple negative breast cancer: looking for the missing link between biology and treatments. Oncotarget.6(29), 26560–26574 (2015).
- Yao Y , ChuY, XuB, HuQ, SongQ. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci. Rep.39(6), BSR20190288 (2019).
- Bianchini G , BalkoJM, MayerIA, SandersME, GianniL. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol.13(11), 674–690 (2016).
- Wolff AC , HammondMEH, AllisonKHet al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch. Pathol. Lab. Med.142(11), 1364–1382 (2018).
- Moy B , RumbleRB, ComeSEet al. Chemotherapy and targeted therapy for patients with human epidermal growth factor receptor 2-negative metastatic breast cancer that is either endocrine-pretreated or hormone receptor-negative: ASCO Guideline update. J. Clin. Oncol.39(35), 3938–3958 (2021).
- European Society for Medical Oncology (ESMO) . ESMO Metastatic Breast Cancer Living Guidelines. https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guidelines/triple-negative-breast-cancer (Accessed 26July2022).
- Rozenblit M , HuangR, DanzigerNet al. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J. Immunother. Cancer.8(2), e001558 (2020).
- Emens LA , AdamsS, BarriosCHet al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol.32(8), 983–993 (2021).
- Schmid P , AdamsS, RugoHSet al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med.379(22), 2108–2121 (2018).
- Cortés J , RugoHS, CesconDWet al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med.387(3), 217–226 (2022).
- Rossari JR , Metzger-FilhoO, PaesmansMet al. Bevacizumab and breast cancer: a meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J. Oncol.2012, 417673 (2012).
- O’shaughnessy J , MilesD, GrayRJet al. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC). J. Clin. Oncol.28(Suppl. 15), 1005 (2010).
- Sasich LD , SukkariSR. The US FDAs withdrawal of the breast cancer indication for Avastin (bevacizumab). Saudi. Pharm. J.20(4), 381–385 (2012).
- Ye F , HeM, HuangLet al. Insights into the impacts of BRCA mutations on clinicopathology and management of early-onset triple-negative breast cancer. Front. Oncol.10, 574813 (2020).
- Gonzalez-Angulo AM , TimmsKM, LiuSet al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin. Cancer Res.17(5), 1082–1089 (2011).
- Robson M , ImSA, SenkusEet al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med.377(6), 523–533 (2017).
- Litton JK , RugoHS, EttlJet al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med.379(8), 753–763 (2018).
- Brufsky A , ValeroV, TiangcoBet al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res. Treat.133(3), 1067–1075 (2012).
- Park IH , ImSA, JungKHet al. Randomized open label phase III trial of irinotecan plus capecitabine versus capecitabine monotherapy in patients with metastatic breast cancer previously treated with anthracycline and taxane: PROCEED Trial (KCSG BR 11–01). Cancer Res. Treat.51(1), 43–52 (2019).
- Perez EA , PatelT, Moreno-AspitiaA. Efficacy of ixabepilone in ER/PR/HER2-negative (triple-negative) breast cancer. Breast Cancer Res. Treat.121(2), 261–271 (2010).
- Pivot X , MarmeF, KoenigsbergR, GuoM, BerrakE, WolferA. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann. Oncol.27(8), 1525–1531 (2016).
- Okajima D , YasudaS, MaejimaTet al. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol. Cancer Ther.20(12), 2329–2340 (2021).
- Zeng P , ChenMB, ZhouLN, TangM, LiuCY, LuPH. Impact of TROP2 expression on prognosis in solid tumors: a systematic review and meta-analysis. Sci. Rep.6, 33658 (2016).
- Zhao W , KuaiX, ZhouXet al. TROP2 is a potential biomarker for the promotion of EMT in human breast cancer. Oncol. Rep.40(2), 759–766 (2018).
- Bardia A , HurvitzSA, TolaneySMet al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med.384(16), 1529–1541 (2021).
- Nakada T , SugiharaK, JikohT, AbeY, AgatsumaT. The latest research and development into the antibody-drug conjugate [fam-], trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem. Pharm. Bull (Tokyo).67(3), 173–185 (2019).
- Ogitani Y , AidaT, HagiharaKet al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res.22(20), 5097–5108 (2016).
- Ogitani Y , HagiharaK, OitateM, NaitoH, AgatsumaT. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci.107(7), 1039–1046 (2016).
- Mahmood I . Clinical pharmacology of antibody-drug conjugates. Antibodies (Basel).10(2), 20 (2021).
- Han S , LimKS, BlackburnBJet al. The potential of topoisomerase inhibitor-based antibody-drug conjugates. Pharmaceutics.14(8), 1707 (2022).
- Heist RS , SandsJM, ShimizuTet al. First-in-human phase I study of DS-1062a (TROP2 antibody-drug conjugate) in patients with advanced non-small-cell lung cancer. Presented at: Oral presentation at the World Conference on Lung Cancer (WCLC).Barcelona, Spain, 7–10 September 2019.
- Bardia A , KalinskyK, TsurutaniJet al. Datopotamab deruxtecan (Dato-DXd), a TROP2-directed antibody-drug conjugate, vs investigator’s choice of chemotherapy (ICC) in previously treated, inoperable or metastatic hormone-receptor (HR) positive, HER2-negative (HR+/HER2-) breast cancer: TROPION-Breast01. Program and Abstracts of: European Society for Medical Oncology (ESMO). Paris.France, 9–13 September 2022 ( Poster 274TiP).
- Bardia A , KropI, Meric-BernstamFet al. Datopotamab deruxtecan (Dato-DXd) in advanced triple-negative breast cancer (TNBC): updated results from the phase I TROPION-PanTumor01 study. Presented at: San Antonio Breast Cancer Symposium (SABCS).San Antonio, TX, USA, 6–10 December 2022 (Poster P6-10–03).
- Garon E , JohnsonM, LisbergAet al. TROPION-PanTumor01: updated results from the NSCLC cohort of the phase I study of datopotamab deruxtecan in solid tumors. J. Thorac. Oncol.16, S892–S893 (2021).
- Krop I , JuricD, ShimizuTet al. Datopotomab deruxtecan (Dato-DXd) in advanced/metstatic HER2 negative breast cancer: triple negative breast cancer results from the phase I TROPION-PanTumor01 study. Presented at: San Antonio Breast Cancer Symposium (SABCS).San Antonio, TX, USA, 7–10 December 2021 ( Oral GS01-05).
- Schmid P , WysockiP, MaCet al. Datopotamab deruxtecan (Dato-DXd)+durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): updated results from BEGONIA, a phase 1b/2 study. Presented at: San Antonio Breast Cancer Symposium (SABCS).San Antonio, TX, USA, 6–10 December 2022 ( Poster PD11-09).
- Allison KH , HammondMEH, DowsettMet al. Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Guideline update. Arch. Pathol. Lab. Med.144(5), 545–563 (2020).
