Abstract
Aim: There is limited information regarding the treatment and outcomes of early stage triple-negative breast cancer (esTNBC) in real-world settings in Japan. Materials & methods: Retrospective analyses of the Medical Data Vision database assessed treatment patterns, healthcare resource utilization (HCRU), patient characteristics, outcomes and prognostic factors among four groups (neoadjuvant therapy+surgery+adjuvant therapy; neoadjuvant therapy+surgery; surgery+adjuvant therapy; surgery only) of esTNBC patients. Results: Treatment patterns, HCRU and demographics varied among the four groups. HCRU was greater and prognosis tended to be worse in the neoadjuvant+surgery+adjuvant therapy group. Conclusion: Our results provide insights into the treatment practices, HCRU and prognosis of esTNBC in Japan. The treatment practices were heterogeneous, reflecting the decision-making process in Japan during the study period.
Plain language summary
Triple-negative breast cancer (TNBC) is a cancer type that does not express three biomarkers (estrogen receptors, progesterone receptors and human epidermal growth factor receptor 2), which results in a lack of targeted treatment strategies. Early stage TNBC (esTNBC) is mainly treated by anticancer drugs before (neoadjuvant) and/or after (adjuvant) surgery and adjuvant radiotherapy. New therapies including an immune checkpoint inhibitor which helps better immune system and a PARP inhibitor which helps repair DNA damage were approved for esTNBC in 2022 in Japan, and they are expected to change the treatment options for TNBC. However, there are limited data about the treatment patterns, healthcare resource utilization (HCRU) and outcomes for esTNBC in real-world clinical practice in Japan. Therefore, a hospital-based administrative database was analyzed to understand the treatment patterns for patients with esTNBC in Japan, the HCRU, treatment outcomes (overall survival and event free survival), and the associated factors. Patients received a large variety of treatments before and after surgery. Patients who received both neoadjuvant and adjuvant therapies tended to have more severe disease and required greater HCRU, and their outcomes were worse than patients who received neoadjuvant treatment only, adjuvant treatment only or neither neoadjuvant nor adjuvant treatment. Our findings will help us understand how new treatments will impact the treatment practices and patient outcomes in the future.
Tweetable abstract
We investigated the treatment sequences, healthcare resource utilization and outcomes to provide insight into the management of triple-negative breast cancer in real-world settings in Japan.
#breastcancer
#realworld
Worldwide, it is estimated there are approximately 2.3 million new cases of breast cancer annually, leading to nearly 700,000 deaths [Citation1]. In Japan, it was reported that 97,812 patients were diagnosed with breast cancer in 2019, and breast cancer accounted for approximately 10% of all cancer-related deaths [Citation2,Citation3]. Approximately 13.8% of all breast cancer cases in Japan are triple-negative breast cancer (TNBC), which is considered to have a poor prognosis with high recurrence rates and low survival rates [Citation4]. TNBC can be associated with greater healthcare resource utilization (HCRU) compared with hormone receptor (HR)- or human epidermal growth factor receptor 2 (HER2)-positive breast cancer [Citation5–9].
The mainstay treatment options for early stage TNBC (esTNBC) have been surgery, chemotherapy (neoadjuvant and/or adjuvant) and adjuvant radiotherapy (RT). New treatment options, including olaparib as adjuvant therapy for early HER2-negative patients with germline BRCA mutations and pembrolizumab as a neoadjuvant and adjuvant therapy for TNBC, were approved in Japan in 2022. Prior to their wider clinical use, the collection of benchmark data can help us to understand the clinical situation about the treatment of esTNBC. Some previous real-world studies using the Japanese Breast Cancer Registry in Japan did not focus on TNBC; therefore, this study was performed to obtain benchmark data that can be utilized to investigate the changes in real-world treatment patterns and outcomes following the introduction of new treatments for esTNBC into clinical practice in Japan.
Methods
Ethics
This retrospective study utilized de-identified, anonymized claims data. According to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects, institutional review board approval and patient informed consent were not required for this observational study because it used an existing hospital claims database without primary data collection.
Study database
The Medical Data Vision (MDV) claims database (Medical Data Vision Co., Ltd, Tokyo, Japan; www.mdv.co.jp/ebm/), which collates hospital-based medical claims and health insurance claims data were analyzed. To date, the MDV has accumulated data for over 42 million patients, across 474 acute hospitals throughout Japan (as of December 2022) including 235 Designated Regional Cancer Center or hospitals. The database contains information that has been anonymously processed, as classified under the amended Act on the Protection of Personal Information 2003, before submission to the MDV database for processing.
The hospital-based data includes all health claims regarding inpatient care at the participating hospitals under a diagnosis procedure combination per-diem payment system. All diagnoses are coded using the International Classification of Diseases, 10th revision (ICD-10), disease names are coded using Japanese Disease Name Codes, and procedures are coded using Japanese Procedure Codes. The database also includes data on prescriptions (including generic drug names), outpatient visits and other procedures performed in outpatient settings at the same hospital.
The health insurance claims data are derived from approximately 104 health insurance societies covering approximately 5.4 million patients. This database included all claims submitted to these insurance societies by the healthcare providers when their subscribers visit and receive care. The health insurance claims data were only utilized for certain analysis, as described in the HCRU section of the Results as Supplemental Data.
The hospital claims data and health insurance claims data were analyzed independently. These databases are not linked, and even though there is a possibility that some patients were included in both databases, they cannot be distinguished from each other.
Patients
Using the hospital claims data, data were extracted for patients who satisfied the following criteria to identify those with esTNBC: diagnosis of breast cancer (ICD-10 code C50) between 1 April 2008 and 31 August 2015 (index period); age ≥18 years at diagnosis, no recorded prescription for anti-HER2 or hormone therapy; record of first breast cancer surgery in the index period documenting Stage I, II, IIIA or IIIB (index surgery), but prior to diagnosis of Stage IV breast cancer.
For the health insurance claims data, the eligibility criteria were diagnosis of breast cancer (ICD-10: C50) between 1 April 2012 and 31 December 2015 (index period); age ≥18 years at diagnosis; no recorded prescription for anti-HER2 or hormone therapy; surgery code K476 for breast cancer in the index period and first surgery performed after diagnosis but prior to recurrence (index surgery).
All of the patients who met those criteria were defined as an early disease stage with operable TNBC in this study.
In both databases, for (1), the index period was chosen to allow a potential follow-up of ≥4 years for all accrued patients in the dataset extracted for this study before the approval of atezolizumab for PD-L1-positive inoperable or recurrent TNBC in August 2019 in Japan [Citation10]. For (3), the anti-HER2 and hormone therapies are listed in Supplementary Table 1. K476 is the procedure code for malignant breast tumor surgery.
The identified records of anticancer drugs for the breast cancer treatment were grouped as regimen names of systemic anticancer therapies. Together with records of RT and surgeries in the database, their chorological relation to the index surgery were determined. Neoadjuvant therapy was defined as any systemic anticancer therapy recorded within 3 months before the date of index surgery. Adjuvant therapy was defined as any systemic anticancer therapy recorded within 3 months after index surgery. RT recorded within 6 months after the last date of adjuvant therapy or the date of index surgery (whichever came last) was considered as adjuvant RT. The patients were divided into four groups according to the use and sequence of treatments in the database (Supplementary Figure 1):
Group 1: Neoadjuvant therapy, surgery and adjuvant therapy
Group 2: Neoadjuvant therapy and surgery (no adjuvant therapy)
Group 3: Surgery and adjuvant therapy
Group 4: Surgery only
The following patient demographic and clinical characteristics are available in the databases and were summarized: age, sex, BMI (calculated from the height and weight in the database), disease stage, comorbidities, concomitant medications, pregnancy status and smoking index.
HCRU data included hospitalization, outpatient visits and RT recorded during the following periods: from the start date of neoadjuvant treatment to the date before index surgery; from the date of index surgery to the start date of advanced TNBC, death or end of record for Groups 1 and 2; and from the date of index surgery until the start of new anticancer therapy or RT ≥6 months after the end of adjuvant therapy or the date of index surgery (defined as ‘recurrence’; Supplementary Figure 1), death, or end of record in Groups 3 and 4. Only hospitalizations associated with breast cancer treatment were counted, but included index surgery, neoadjuvant therapy and adjuvant therapy. The duration of hospitalization was defined as the date of discharge – date of hospitalization. Hospitalization was counted as 0 day for patients with a record of death on the day of hospitalization and patients who were discharged on the day of admission to hospital. HCRU data were analyzed and presented using appropriate statistics.
The distribution and flow of therapies in Groups 1–3, including index surgery and RT, were plotted using Sankey diagrams.
The post-surgical overall survival (psOS) and post-surgical EFS (psEFS) were analyzed using the Kaplan–Meier method. psOS was defined as the time from the date of index surgery until death or the end of the patient’s records, whichever occurred first. For patients who received neoadjuvant therapy (Groups 1 and 2), OS was also calculated as the time from the start of neoadjuvant therapy until death or the end of the patient’s records, whichever occurred first. psEFS was defined as the time from the date of index surgery until recurrence, death, or the end of patient’s records, whichever occurred first. For patients in Groups 1 and 2, EFS was also calculated from the start of neoadjuvant therapy until recurrence, death or the end of the patient’s records, whichever occurred first.
Multivariable Cox regression was conducted to investigate potential prognostic factors for psOS and psEFS, using two models for each outcome. The first model included group (Groups 1–4), age group (≥18 to <50, ≥50 to <65, ≥65 to <75 and ≥75 years), height, body weight, BMI, pregnancy status, smoking index and stage at initial diagnosis (II or III). In the second model, stepwise variable selection method was applied to enter and remove covariates from the model using a significance level of 0.05.
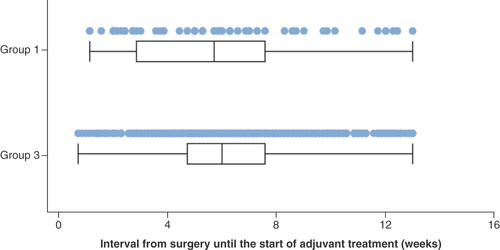
The interval from the date of index surgery to the date of the first record of adjuvant therapy was calculated (in days) for Groups 1 and 3.
All statistical analyses were performed using SAS release 9.4 (SAS Institute Inc., NC, USA).
Results
Patients
Of 182,172 patients with a diagnosis of breast cancer in the index period (1 April 2008 to 31 December 2015), 3925 satisfied the eligibility criteria and were included in the study. Most of reasons for ineligibilities were the absence of staging information, as summarized in the patient disposition diagram (Supplementary Figure 2). The median (range) age of the total population was 63.0 (25–100) years and 99.7% of patients were female (). 47.9, 43.8 and 8.3% of patients were diagnosed with Stage I, II and III breast cancer, respectively. Most patients had a T stage of T1 (52.0%) or T2 (38.6%), and N stage of N0 (78.5%) or N1 (17.8%).
Table 1. Baseline patient demographics and characteristics.
Nearly all patients had an M stage of M0 (99.3%). Two patients had M1 (locally advanced breast cancer) at their index surgery of breast cancer after their records of suspected metastasis as disease names in addition to their first diagnosis of breast cancer. 24 patients had MX (not evaluable).
The median observation period was 1486 days (range 2–3779 days).
Groups 1–4 comprised 58 patients, 367 patients, 799 patients and 2701 patients, respectively. There were some differences in patient characteristics among the four groups (). In particular, Group 4 tended to be older, and had greater proportions of patients with Stage I breast cancer, T1 stage, or node-negative (N0) breast cancer. By comparison, Group 1 had greater proportions of patients with Stage III, T3, or node-positive (N1–N3) breast cancer.
Regarding the health insurance claims data, of 23,886 patients with a diagnosis breast cancer recorded in the index period (1 April 2012 to 31 December 2015), 272 satisfied the eligibility criteria and were included in the analyses, as summarized in the patient disposition diagram (Supplementary Figure 3). Groups 1–4 comprised three, 25, 57 and 187 patients, respectively. Because of the small numbers of patients available, the data were not analyzed in detail.
Treatment patterns
The treatment patterns in Groups 1–3 are shown in , respectively, as Sankey diagrams.
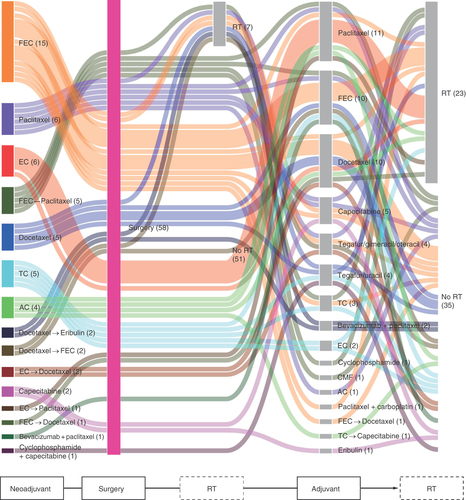
Group 1: Neoadjuvant therapy, surgery and adjuvant therapy.
AC: Doxorubicin+cyclophosphamide; CMF: Cyclophosphamide+methotrexate+5-fluorouracil; EC: Epirubicin+cyclophosphamide; FEC: 5-fluorouracil+epirubicin+cyclophosphamide; RT: Radiotherapy; TC: Docetaxel+cyclophosphamide.
AC: Doxorubicin+cyclophosphamide; EC: Epirubicin+cyclophosphamide; FEC: 5-fluorouracil+epirubicin+cyclophosphamide; RT: Radiotherapy; TAC: Docetaxel+doxorubicin+ cyclophosphamide; TC: Docetaxel+cyclophosphamide.
AC: Doxorubicin+cyclophosphamide; CMF: Cyclophosphamide+methotrexate+5-fluorouracil; EC: Epirubicin+cyclophosphamide; FEC: 5-fluorouracil+epirubicin+cyclophosphamide; RT: Radiotherapy; TAC: Docetaxel+doxorubicin+ cyclophosphamide; TC: Docetaxel+cyclophosphamide.
In Group 1 (n = 58; ), 5-fluorouracil+epirubicin+cyclophosphamide (FEC) was the most common neoadjuvant therapy, being used in 15 patients. RT was administered after index surgery but before adjuvant therapy in seven patients. The most common adjuvant therapies were paclitaxel (n = 11) and FEC (n = 10). RT was administered after starting adjuvant therapy in 23 patients. The most common sequences were FEC→FEC→RT, FEC→paclitaxel→RT, and epirubicin+cyclophosphamide (EC)→paclitaxel→RT.
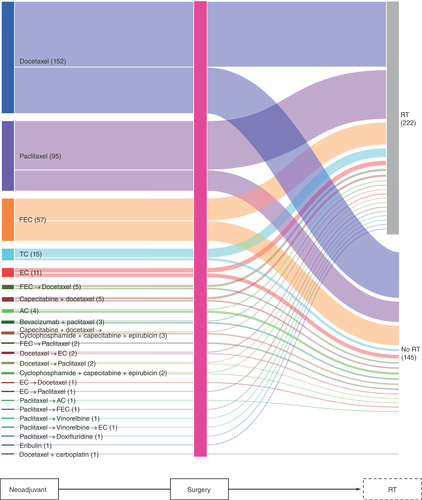
In Group 2 (n = 367), docetaxel (n = 152) and paclitaxel (n = 95), and FEC (n = 57) were the three most common neoadjuvant therapies (). RT was administered for 222 patients.
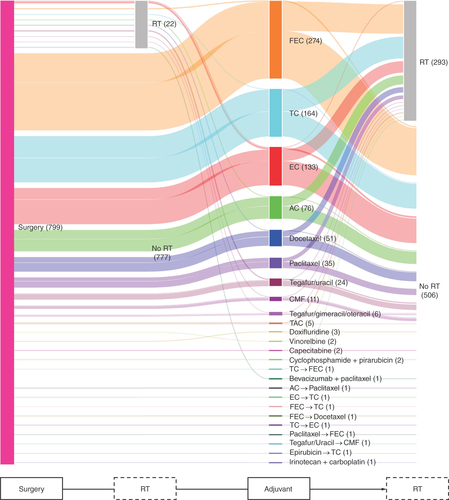
In Group 3 (n = 799; ), the three most frequently used adjuvant therapies were FEC (n = 274), docetaxel+cyclophosphamide (TC; n = 164) and EC (n = 133). Oral agents included the use of tegafur/uracil (UFT) in 24 patients and tegafur/gimeracil/oteracil (TS-1) in six patients. RT was administered for 22 patients prior to adjuvant therapy and for 293 patients after starting adjuvant therapy.
Among 2701 patients in Group 4 who underwent index surgery (without neoadjuvant or adjuvant therapy), RT was administered for 494 (18.3%).
HCRU
The total observation periods (100 patient-weeks) in Groups 1–4 and in the total population were 123.42, 782.70, 1821.81, 4307.68 and 7035.61, respectively (), and were used to calculate the HCRU-related events in each group. The median observation times per patient were 1691, 1724, 1745, 1337 and 1486 days, respectively.
Table 2. Healthcare resource utilization outcomes.
The median length of each hospital stay was 8.0 days in Groups 1 and 2 and 6.0 days in Groups 3 and 4. The mean length of each hospital stay was 10.6, 10.1, 7.1 and 7.4 days in Groups 1–4, respectively. At least one record of an outpatient visit was found for over 94% of patients in Groups 1, 2 and 3, compared with 18.5% in Group 4. The number of outpatient visits per 100 patient-weeks were 12.18, 8.79, 7.16 and 2.70 in Groups 1–4, respectively. Less than 4% of patients in each group required emergency hospitalizations associated with breast cancer treatment, with an event rate of ≤0.03-times/100 patient-weeks.
Seven patients (12.1%) in Group 1 and 32 patients (8.7%) in Group 2 experienced hospitalization in the pre-surgical period (). The length of hospital stay was shorter in the pre-surgical period than in the post-surgical period in both groups (Group 1: 2.9 vs 11.3 days; Group 2: 4.7 vs 10.7 days). The number of outpatient visits per 100 patient-week was greater in the pre-surgical period than in the post-surgical period in both groups (Group 1: 32.13 vs 11.15; Group 2: 35.24 vs 7.35), reflecting the outpatient visits associated with neoadjuvant therapy in both groups and those associated with adjuvant therapy in Group 1. The number of outpatient visits per 100 patient weeks in the post-surgical period was 11.15 and 7.16 in Groups 1 and 3, respectively, and were 7.35 and 2.70 in Groups 2 and 4, respectively.
All records of RT were found in the post-surgical adjuvant period in Groups 1 and 2.
At least one record of RT in either inpatient or outpatient settings was found for 28 (48.3%), 222 (60.5%), 314 (39.3%) and 494 (18.3%) patients in Groups 1–4, respectively. Among patients with at least one record of RT, the median number of administered RT per patient was 25 in all four groups () and ranged from 1 to 80-times in the total population. The number of administered RT per 100 patient-weeks was 12.98, 11.67, 10.30 and 11.39 in Groups 1–4, respectively.
EFS & OS
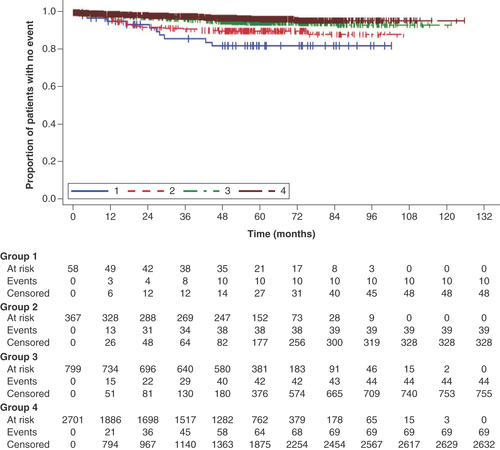
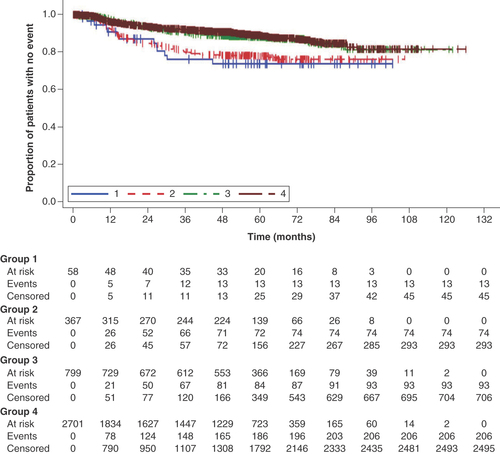
psOS and OS tended to track lower in Group 1 than in the other groups ( & Supplementary Figure 4). psEFS tended to track lower in Groups 1 and 2 than in Groups 3 and 4, indicating patients who received neoadjuvant therapy showed worse psEFS than those who did not, but the curves for EFS were similar between Groups 1 and 2 ( & Supplementary Figure 5).
Post-surgical overall survival was calculated as the time from the date of initial surgery until death or the end of record, whichever occurred first.
Group 1: neoadjuvant therapy, surgery and adjuvant therapy; Group 2: neoadjuvant therapy and surgery (no adjuvant therapy); Group 3: surgery and adjuvant therapy; Group 4: surgery.
Post-surgical event-free survival was calculated as the time from the date of surgery until the start of new anticancer therapy or radiotherapy, death, or the end of record, whichever occurred first.
Group 1: neoadjuvant therapy, surgery and adjuvant therapy; Group 2: neoadjuvant therapy and surgery (no adjuvant therapy); Group 3: surgery and adjuvant therapy; Group 4: surgery.
Prognostic factors
The multivariable Cox regression analysis of psOS showed that all variables included in the first model were significantly associated with psOS (). After applying the stepwise variable selection method, group, age-group and stage at initial diagnosis remained as independent prognostic factors.
Table 3. Multivariable Cox regression analysis of post-surgical overall survival.
Among the variables included in the first model for psEFS, Group 2, age ≥50 to <65 years, BMI (height and body weight) and stage at initial diagnosis were independent prognostic factors (). After applying stepwise variable selection, group and stage at initial diagnosis remained as independent prognostic factors.
Table 4. Multivariable Cox regression analysis of post-surgical event-free survival.
Interval from surgery to the start of adjuvant therapy
The median interval from the date of index surgery to the start of adjuvant therapy was 39 days (range 7–90 days) in Group 1 and 41 days (range 4–90 days) in Group 3 ().
Discussion
These retrospective analyses of the MDV hospital claims database provide insights into the treatment practices, particularly the use of neoadjuvant and adjuvant therapies and RT, as well as HCRU, for patients with esTNBC for the period 2008–2019, before the first approval of an immuno-oncology drug for breast cancer in Japan, focusing on four groups of patients according to their treatment records (Group 1: neoadjuvant therapy, surgery and adjuvant therapy; Group 2: neoadjuvant therapy and surgery; Group 3: surgery and adjuvant therapy; Group 4: surgery only).
In this study, esTNBC was defined as a recorded diagnosis of breast cancer (ICD-10 code C50.x) with initial breast cancer surgery documenting disease stage of I–III without a prescription of an anti-HER2 agent or hormone therapy. Unlike TNBC defined by pathological data, as in clinical practice, it is possible that not all patients had TNBC. The patients who received neoadjuvant therapy were younger than who received adjuvant therapy only or those who underwent surgery only. Similar findings were reported in a US study, where the mean age was 52.1 years among esTNBC patients who received neoadjuvant therapy [Citation1]. By comparison, the patients who received adjuvant therapy only or surgery only in this study were older, in their late 50s to early 60s. Over half of the patients in Group 4 presented at Stage I with high proportions of N0 and M0, and few had Stage III breast cancer, while Group 1 comprised higher proportions of patients with Stage III, T3/T4 or N2/N3, which is appropriate considering this group of patients underwent neoadjuvant therapy surgery and adjuvant therapy. This implies that Group 4 comprised more patients who were considered as candidates for surgery only, especially older patients who may be less likely to tolerate adjuvant or neoadjuvant chemotherapy. These characteristics and treatment patterns of esTNBC patients are broadly consistent with earlier studies in Japan [Citation11–15].
The considerable diversity in the treatment sequences in the neoadjuvant and adjuvant periods () may reflect the lack of specific targeted therapies for TNBC and the broad range of chemotherapy regimens suggested for initial treatment in the Japanese Breast Cancer Society clinical practice guidelines [Citation16], the choice of which depends on the physician’s individual opinion or institutional recommendations. Some patients received FEC as both neoadjuvant and adjuvant therapy, possibly because a cycle of FEC was interrupted by surgery, rather than separate FEC treatments in the neoadjuvant and adjuvant periods. Monotherapy with docetaxel or paclitaxel () is not typically considered as neoadjuvant therapy, and it is most likely that these drugs were captured as a part of EC, AC or FEC sequential regimens because of the definition of neoadjuvant period, and some components of adjuvant therapy started after 3 months of initial surgery were not captured due to the study definitions. Oral tegafur/uracil are not commonly used as adjuvant therapy in other countries, but they are available in Japan and were used in this study population.
Greater HCRU burden was observed for patients who underwent neoadjuvant and /or adjuvant therapy than those who had surgery only: longer hospital stay, higher proportion of patients with RT and greater number of outpatient visits per 100 patient-weeks. Hospitalization was common throughout the observation period, occurring in over one-quarter of patients. The median number of RTs per patient was 25 in all four groups among patients received RT, concordant with the standard RT regimen (50 Gy/25-times) in clinical practice; however, the proportions of patients received RT in each four groups seems to be lower than expected in clinical practice. This could be explained by the fact that the hospital claims data cannot track patients across different hospitals or clinics. Analyses of the health insurance claims data, which can track patients across multiple institutions as long as the patients continue the same employee insurance plan, showed approximately 10% greater proportions of patients with RT than in the hospital claims data in the corresponding groups (data not shown) suggesting that some patients possibly underwent RT at a hospital other than where surgery was performed, such as at core institutions in the same regional area, although the sample size was only 272 patients.
The Kaplan–Meier plots showed trends for shorter survival times after surgery (psOS and psEFS) for Group 1 than in the other groups. Because Group 1 had the highest proportion of Stage III disease and lowest proportion of Stage I disease, the patients in this group had more severe disease that necessitated a combination of neoadjuvant and adjuvant therapies, and it may be assumed that the treatments were chosen based on the patient’s disease condition. Consequently, their prognosis was worse than that of the patients in the other groups. This interpretation is also consistent with the treatment sequence and HCRU burden, including the length of hospitalization and the number of outpatient visits. In addition, our multivariable analysis revealed that group (representing the type of therapies received), age-group and stage at diagnosis were prognostic factors for psOS and psEFS.
Approximately three quarters of patients who received adjuvant therapy actually started it within 8 weeks of surgery, which seems to be in accordance with the Japan Society of Clinical Oncology Clinical Practice Guidelines [Citation17]. To our knowledge, this is the first report describing the timing of initiation with adjuvant therapies in relation to the surgery in real-world practice. However, because some adjuvant therapies, if delayed beyond 3 months after surgery, were not included in this analysis, this interval to the initiation of adjuvant therapy would have been underestimated.
Although the current findings are limited to the Japanese setting, benchmark studies in the US previously revealed that various treatments were used in clinical practice which heterogeneity was dependent on the stage of esTNBC, the rates of early relapse and metastasis remained high, resulting in poor survival, and HCRU burden was greater during the neoadjuvant therapy period than in the adjuvant period [Citation18]. Our observation as well showed that HCRU tended to be greater among patients in Groups 1 and 2: the length of hospital stay was also longer in Groups 1 and 2 than in Group 3. It is notable that fewer patients experienced hospitalization during the neoadjuvant period in Groups 1 and 2 (12.1 and 8.7%, respectively), but their length of stay was longer (2.9 and 4.7 days on average) in our study than the forementioned study, in which about one-quarter of patients were hospitalized but the mean length was approximately 1 day [Citation18]. These findings both in the US and our study in Japan demonstrate the considerable HCRU burden during the neoadjuvant period in patients with esTNBC. Furthermore, HCRU during the post-surgical period was greater in Groups 1 and 2 than in Groups 3 and 4 in our study, consistent with the other results of our study showing that patients who underwent neoadjuvant therapies had characteristics possibly associated with higher risk of more serious disease.
Pembrolizumab, an anti-PD-1 antibody, was approved for the treatment of high-risk TNBC in combination with chemotherapy as neoadjuvant treatment and continued as a single agent as adjuvant treatment in the US based on KEYNOTE-522 trial followed by approval in Japan in September 2022. Its use for TNBC has been recommended in the National Comprehensive Cancer Network and American Society of Clinical Oncology guidelines [Citation19,Citation20], as well as in the Japanese Breast Cancer Society Guidelines [Citation3]. The current data can therefore be used as a benchmark for evaluating the use of pembrolizumab upon its introduction for esTNBC in Japan and will provide insight into the changes in treatment practices and outcomes in the near future.
Limitations
Some limitations of this study warrant mention. In particular, any treatments performed at hospitals or clinical centers not registered in the MDV hospital claims database were not captured, including treatments performed at medical institutions other than the institutions where the surgery was performed, and this may have caused underestimation of the utilization of some treatments. In order to meet this limitation, the health insurance claims data, which can track patients across multiple institutions was analyzed as another type of data; however, the number of eligible patients was too small for meaningful analyses. Another limitation is that staging information was unavailable for a large proportion of patients, resulting in the small sample size. Due to the absence of imaging, pathological and genetic data, TNBC was defined as the absence of HER2- or hormone receptor-targeted therapies in the follow-up period, rather than pathology and it is possible that some of the patients had HER2-positive or hormone receptor-positive breast cancer but they either did not receive their expected targeted therapies due to their age, costs, or they declined to receive particular treatments, or they received these treatments at another medical institution. Group 4, which comprised patients who underwent surgery only, may not have received any systemic therapies for hormone receptor-positive or HER2-receptor positive breast cancer due to an early stage of breast cancer. The dates of stage information and TNM given were not necessarily the same and each information is not linked to each other. Furthermore, the definition of recurrence in this study may not capture all cases of recurrence because the database codes do not correspond to the condition of disease or recurrence but only disease names and procedures. For patient outcomes (psOS/OS and psEFS/EFS), deaths as events included only in-hospital deaths that occurred in the same institution, and the databases are not linked to the death registers in Japan. Because in-hospital death is possibly related to the patient’s disease stage and condition, survival among the four groups may not be comparable. Since patients were censored at the end of the patient’s records, the survival of some patients may be underestimated because, after the dates censored, they could have changed institutions to continue to receive treatments and not being tracked in the database. Due to the definition, any systemic anticancer therapies given earlier than 3 months before the date of initial surgery or any those after 3 months since the date of initial surgery were not included as neoadjuvant therapy or adjuvant therapy in this study, respectively; therefore, some patients might have been misclassified in the patient groups. In addition, the time period from surgery to adjuvant therapy could differ substantially across demographic and clinical characteristics of patients, which could have influenced psOS and psEFS, introducing the possibility of immortal time bias. Finally, the data cannot be directly compared among the four groups because the treatments were selected according to the patient’s condition in real world practice unlike interventional or randomized clinical trials.
Conclusion
The results of this study provide important insight into the treatment practices, HCRU and prognosis of patients with esTNBC in Japan. The treatment practices were very heterogeneous and reflect the decision-making process in Japan during the index period. HCRU tended to be greater and prognosis tended to be worse in patients who received both neoadjuvant and adjuvant therapy, consistent with the higher disease stage of these patients. These data will be valuable in the future to provide a benchmark on which the changes in treatment practices with the anticipated introduction of novel therapies can be assessed.
Triple-negative breast cancer (TNBC) accounts for approximately 13.8% of all breast cancer cases in Japan with high recurrence rates and low survival rates.
Surgery, chemotherapy (neoadjuvant and/or adjuvant) and radiotherapy have been the mainstay treatments for early stage (es) TNBC for many years; but new treatment options became available since 2022 and are expected to lead to changes in its treatment.
Currently, there are no data on the treatment practices and outcomes of esTNBC in real-world settings in Japan. Therefore, analyses of a Japanese secondary administrative database were performed.
Eligible patients were identified and categorized four groups of patients with Stage I–IIIB esTNBC: Group 1–neoadjuvant therapy, surgery and adjuvant therapy; Group 2–neoadjuvant therapy and surgery (no adjuvant therapy); Group 3–surgery and adjuvant therapy; Group 4–surgery only.
Patient characteristics varied among the four groups; Group 4 tended to be older and lower disease stages, whereas Group 1 tended to have more severe disease with higher T and N stages; Groups 2 and 3 were broadly in-between Groups 1 and 4.
Marked heterogeneity in treatment patterns within Groups 1–3 was observed, indicating highly variable treatment decisions for esTNBC in Japan.
Healthcare resource utilization tended to be greater in Group 1, and is consistent with the use of neoadjuvant and adjuvant therapy in this group.
The overall survival and event-free survival curves tended to track lower in Group 1 than in the other groups, suggesting patients in Group 1 had more severe disease necessitating neoadjuvant and adjuvant therapy.
The factors associated with post-surgical overall survival were group, age-group and stage at initial diagnosis, and the factors associated with post-surgical event-free survival were group and stage at initial diagnosis in multivariable Cox regression analysis.
This study provides important insight into the treatment practices, HCRU and prognosis of patients with esTNBC in Japan before the introduction of new treatment options.
Author contributions
H Sanno and K Taniguchi conceived the study. H Sanno, K Taniguchi and S Saji designed the study. All authors contributed to data interpretation. H Sanno wrote the first draft of the manuscript. All authors critically revised the manuscript, approved the final draft, and take accountability for the accuracy and integrity of the work.
Financial disclosure
This study was funded by MSD KK, Tokyo, Japan. H Sanno and K Taniguchi are employees of MSD KK and holds stock or stock options in Merck & Co. Inc. Y Yoshimoto received funding from Chugai for an investigator-initiated trial (jRCTs021210010) and reports funding for an endowed chair at the Okinawa PCR-Rinsyo Center. S Saji has received grants and contract for clinical trials from Taiho, Chugai, and Daiichi Sankyo; grants from Eisai, Takeda, and Eli Lilly; contract for clinical trials from MSD and AstraZeneca; personal fees from Chugai, Kyowa Kirin, MSD, Novartis, Eisai, Takeda, Daiichi Sankyo, Eli Lilly, AstraZeneca, Pfizer, Taiho, Ono, and Nipponkayaku; and has participated on advisory boards for Chugai/Roche, AstraZeneca, Eli Lilly, Pfizer, Kyowa Kirin, and Daiichi Sankyo; and is a member of the executive boards for Japan Breast Cancer Research Group, Japanese Breast Cancer Society, Japanese Society of Medical Oncology, and Breast International Group. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
The authors thank Nicholas D Smith (EMC KK) for medical writing support, which was funded by MSD KK, Tokyo, Japan.
Ethical conduct of research
This study involved the use of de-identified claims data with all data anonymized. According to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects, institutional review board approval and patient informed consent were not required for this type of retrospective study because it used an existing hospital claims database with no primary data collection.
Data sharing statement
This study used claims data under license from Medical Data Vision Co., Ltd. The data are not publicly available. However, interested researchers may request data for research purposes directly from Medical Data Vision Co., Ltd (https://en.mdv.co.jp/).
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Supplementary document
Download MS Word (326.6 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2023-0960
References
- Sung H , FerlayJ , SiegelRLet al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249 (2021).
- National Cancer Institute Cancer Information Service . Statistics by Cancer Species—Breast. ( In Japanese). https://ganjoho.jp/reg_stat/statistics/stat/cancer/14_breast.html#anchor3 (Accessed 30January2023).
- Japanese Breast Cancer Society . Breast Cancer Clinical Practice Guidelines 2022. ( In Japanese). https://jbcs.xsrv.jp/guideline/2022/ (Accessed 30January2023).
- Kosaka Y , MinataniN , TanakaYet al. Lymph node metastasis and high serum CEA are important prognostic factors in hormone receptor positive and HER2 negative breast cancer. Mol Clin Oncol.9(5), 566–574 (2018).
- Baser O , WeiW , HenkHJ , TeitelbaumA , XieL. Patient survival and healthcare utilization costs after diagnosis of triple-negative breast cancer in a United States managed care cancer registry. Curr. Med. Res. Opin.28(3), 419–428 (2012).
- Dent R , TrudeauM , PritchardKIet al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res.13(15 Pt 1), 4429–4434 (2007).
- Li X , YangJ , PengLet al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat.161(2), 279–287 (2017).
- Lin NU , VanderplasA , HughesMEet al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer118(22), 5463–5472 (2012).
- Brandão M , MoraisS , Lopes-ConceiçãoLet al. Healthcare use and costs in early breast cancer: a patient-level data analysis according to stage and breast cancer subtype. ESMO Open.5(6), e000984 (2020).
- Chugai Pharmaceutical . TECENTRIQ® Interview Form, revised May 2022 (version 14). ( in Japanese). www.info.pmda.go.jp/go/pack/4291441A1024_1_15/ (Accessed 7December2022).
- Iwase M , AndoM , AogiKet al. Long-term survival analysis of addition of carboplatin to neoadjuvant chemotherapy in HER2-negative breast cancer. Breast Cancer Res. Treat.180(3), 687–694 (2020).
- Masuda H , MasudaN , KodamaYet al. Predictive factors for the effectiveness of neoadjuvant chemotherapy and prognosis in triple-negative breast cancer patients. Cancer Chemother. Pharmacol.67(4), 911–917 (2011).
- Nakashoji A , MatsuiA , NagayamaA , IwataY , SasaharaM , MurataY. Clinical predictors of pathological complete response to neoadjuvant chemotherapy in triple-negative breast cancer. Oncol Lett.14(4), 4135–4141 (2017).
- Yagata H , KajiuraY , YamauchiH. Current strategy for triple-negative breast cancer: appropriate combination of surgery, radiation, and chemotherapy. Breast Cancer.18(3), 165–173 (2011).
- Iwase H , KurebayashiJ , TsudaHet al. Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast Cancer.17(2), 118–124 (2010).
- Japanese Breast Cancer Society . Main combination therapies for initial treatment of breast cancer. ( In Japanese). https://jbcs.xsrv.jp/guidline/2018/index/yakubutu/app1/ (Accessed 29November2022).
- Japan Society of Clinical Oncology . Clinical Practice Guidelines 2017 for fertility preservation in childhood, adolecent, and young adult cancear patients. Breast, CQ2: is delayed chemotherapy tolerable if breast cancer patients want fertility preservation? ( In Japanese). www.jsco-cpg.jp/fertility/guideline/#III_cq02 (Accessed 29November2022).
- Haiderali A , RhodesWC , GautamSet al. Healthcare resource utilization and cost among patients treated for early-stage triple-negative breast cancer. Future Oncol.17(29), 3833–3841 (2021).
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 1, 2022. www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed 29November2022).
- Korde LA , SomerfieldMR , HershmanDL. Use of Immune Checkpoint Inhibitor Pembrolizumab in the Treatment of High-Risk, Early-Stage Triple-Negative Breast Cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol.40(15), 1696–1698 (2022).