Abstract
Mouse double minute 2 homolog (MDM2) is a key negative regulator of the tumor suppressor p53. Blocking the MDM2–p53 interaction, and restoring p53 function, is therefore a potential therapeutic strategy in MDM2-amplified, TP53 wild-type tumors. MDM2 is amplified in several tumor types, including biliary tract cancer (BTC), pancreatic ductal adenocarcinoma (PDAC), lung adenocarcinoma and bladder cancer, all of which have limited treatment options and poor patient outcomes. Brigimadlin (BI 907828) is a highly potent MDM2–p53 antagonist that has shown promising activity in preclinical and early-phase clinical studies. This manuscript describes the rationale and design of an ongoing phase IIa/IIb Brightline-2 trial evaluating brigimadlin as second-line treatment for patients with advanced/metastatic BTC, PDAC, lung adenocarcinoma, or bladder cancer.
Plain language summary
Brightline-2: a phase IIa/IIb trial of brigimadlin (BI 907828) in advanced BTC, PDAC, or other solid tumors
In some types of cancer, including cancers of the bile duct, pancreas, bladder and lung, the number of copies of a gene called MDM2 is abnormally increased (MDM2 amplification). MDM2 usually regulates p53, a protein that stops cancer cells from growing uncontrollably. When MDM2 is amplified, the cell makes too much of the MDM2 protein, which prevents p53 from stopping cancer growth. Blocking the interaction between MDM2 and p53 may allow p53 to do its job again and stop cancer cells from growing.
Brightline-2 is a clinical trial that is currently in progress. This trial is assessing the efficacy and safety of an investigational drug, brigimadlin (or BI 907828), in patients with selected advanced or metastatic cancers. To be included, patients must have advanced biliary tract cancer, pancreatic ductal adenocarcinoma, bladder cancer, or lung adenocarcinoma. The tumor must show amplification of MDM2 when tested by a laboratory. Patients will take a 45 mg tablet of brigimadlin by mouth, once every 3 weeks. In this trial, researchers are investigating the ability of the drug to shrink tumors, the side effects of the drug, and the impact of the drug on a patients' quality of life.
The goal of this trial is to assess the potential of brigimadlin as a new treatment option for patients with advanced biliary tract cancer, pancreatic ductal adenocarcinoma, bladder cancer, or lung adenocarcinoma.
Clinical Trial Registration: NCT05512377 (ClinicalTrials.gov)
Background
Patients with advanced or metastatic biliary tract cancer (BTC) or pancreatic ductal adenocarcinoma (PDAC) generally have a poor prognosis, with a median survival of ≈1 year. Patients with lung adenocarcinomas or bladder cancer have similarly poor survival rates.
Current first- and second-line treatments for these conditions include chemotherapy; however, the benefit – especially for second-line chemotherapy – is modest.
The MDM2 gene encodes a negative regulator of the p53 tumor suppressor protein. Antagonism of Mouse double minute 2 homolog (MDM2) can restore p53 activity and represents a novel therapeutic strategy.
MDM2 is amplified in various solid tumors, including BTC (5–8%), PDAC (1%), lung adenocarcinoma (5%) and bladder cancer (8%).
Brigimadlin (BI 907828)
Brigimadlin (or BI 907828) is an oral, MDM2–p53 antagonist that inhibits the interaction between MDM2 and p53.
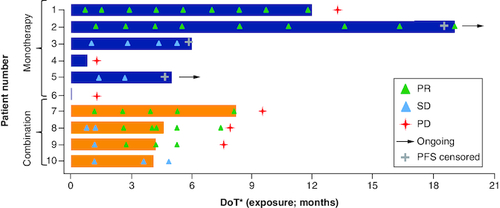
In a subgroup of ten patients with advanced MDM2-amplified BTC from two ongoing phase Ia/Ib trials, brigimadlin (with or without the anti-PD-1 inhibitor ezabenlimab) showed early signs of activity, with 50% of patients demonstrating a partial response.
In these two phase Ia/Ib trials, brigimadlin was associated with a manageable safety profile, both as monotherapy and in combination with ezabenlimab.
Brightline-2 trial
This is an ongoing phase IIa/IIb, open-label, single-arm, multicenter trial with brigimadlin in patients with BTC, PDAC, or other solid tumors.
Eligible patients are adults with locally advanced or metastatic MDM2-amplified, TP53 wild-type BTC, PDAC, lung adenocarcinoma, or bladder cancer with at least one measurable target lesion, Eastern Cooperative Oncology Group performance status of 0 or 1, and who have received appropriate prior standard of care therapy.
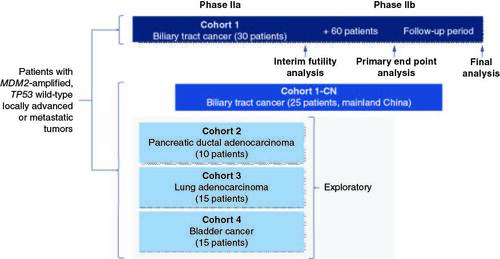
The phase IIa part of the trial will include five cohorts with the following estimated patient numbers: Cohort 1 (BTC; n = 30), Cohort 1-CN (BTC in mainland China; n = 25), Cohort 2 (PDAC; n = 10), Cohort 3 (lung adenocarcinoma; n = 15) and Cohort 4 (bladder cancer; n = 15). All patients will receive brigimadlin 45 mg orally once every 3 weeks, with up to two dose reductions allowed.
An interim futility analysis will be performed for the first 30 patients in Cohort 1 only. A non-binding futility boundary of objective response rate = 20% is planned for the interim futility analysis. The total sample size for Cohort 1 will be ∼90 patients.
The primary end point is objective response rate based on central independent review. Secondary end points include duration of response, progression-free survival, overall survival, disease control, patient-reported outcomes, treatment-emergent adverse events and the occurrence of treatment-emergent adverse events leading to discontinuation of brigimadlin.
Inactivation of the tumor suppressor protein p53 is an important mechanism by which tumors promote their survival and proliferation [Citation1]. Inactivation of p53 may occur due to a mutation of the TP53 gene, which encodes p53, or amplification of mouse double minute 2 homolog (MDM2), which encodes MDM2, an endogenous negative regulator of p53 [Citation1]. Blocking the MDM2–p53 interaction in MDM2-amplified, TP53 wild-type tumors, and restoring p53 activity, therefore represents a potential therapeutic strategy. MDM2 is amplified in various solid tumor types, including biliary tract cancer (BTC; 5–8%) [Citation2,Citation3], pancreatic ductal adenocarcinoma (PDAC; 1%) [Citation4], lung adenocarcinoma (5%) [Citation4] and bladder cancer (8%) [Citation4].
BTC is associated with ∼85,000 deaths per year globally and, despite recent treatment advances, prognosis is poor, with a median overall survival of less than 12 months [Citation5,Citation6]. Current systemic treatments for patients with advanced-stage BTC include chemotherapy, immunotherapy and targeted therapy [Citation7]; chemo-immunotherapy is increasingly considered the first-line standard of care. This recommendation is based on results from the phase III TOPAZ-1 and KEYNOTE-966 trials, which demonstrated that combining first-line chemotherapy with the immune checkpoint inhibitors durvalumab [Citation8] and pembrolizumab [Citation9], respectively, in patients with untreated advanced BTC was associated with improved survival compared with gemcitabine/cisplatin chemotherapy alone. Due to advances in the development of targeted therapies, molecular profiling has become an important tool in the second-line treatment of patients with advanced BTC [Citation10]. It has been estimated that targetable mutations may be present in up to 40–50% of intrahepatic cholangiocarcinomas and 15–20% of extrahepatic cholangiocarcinomas. Effective therapies are already approved for several alterations, including FGFR2 fusions and IDH1 mutations, and further potential targets are emerging [Citation11]. For most patients the second-line treatment option is chemotherapy and clinical benefit is limited [Citation12,Citation13]; patients with advanced BTC who progress on first-line therapy generally have a poor prognosis, with a median survival of ∼6 months [Citation12].
PDAC accounts for ∼3% of all cancers and it is among the leading causes of cancer death [Citation14,Citation15]. The 5-year survival rate is less than 10% and it is usually diagnosed at the advanced stage [Citation16]. Chemotherapy remains the standard of care for patients with unresectable or metastatic PDAC. Median survival of patients receiving subsequent therapies following disease progression on first-line therapy is ∼6 months [Citation17–19]. Almost 50% of pancreatic tumors harbor a theoretically actionable mutation, with the main driver mutation being KRAS [Citation20]. Molecular profiling is strongly recommended for pancreatic adenocarcinomas [Citation21]. The efficacy of therapeutic options after failure of first-line therapy is also limited for patients with other MDM2-amplified solid tumors. For example, advanced lung adenocarcinoma without actionable driver mutations, and bladder cancer that is refractory to platinum-based chemotherapy (±PD-1/PD-L1 inhibitors) have a poor prognosis [Citation22–25].
In this article, we present the background, rationale and design of the ongoing Brightline-2 phase IIa/IIb trial in patients with advanced MDM2-amplified, TP53 wild-type BTC, PDAC, or other solid tumors who will receive the MDM2–p53 antagonist brigimadlin (BI 907828) as second-line therapy.
Brightline-2 trial
Background & rationale
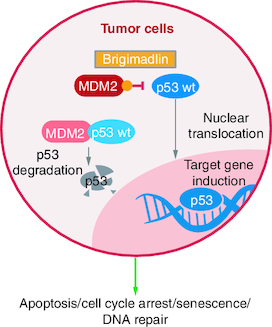
Brigimadlin is a highly potent MDM2 antagonist that blocks the interaction between p53 and its negative regulator MDM2. The subsequent restoration of p53 activity and thus p53 target gene induction, thereby results in cell cycle arrest or apoptosis in TP53 wild-type tumor cells () [Citation26]. Early preclinical studies demonstrated encouraging antitumor activity in vivo, providing a strong rationale for clinical investigation of brigimadlin.
Figure 1. Brigimadlin mechanism of action in MDM2-amplified, TP53 wild-type tumor cells.
MDM2: Mouse double minute 2 homolog; p53: Protein 53; wt: Wild-type.
In phase I clinical development, brigimadlin is being investigated in two ongoing phase Ia/Ib clinical trials in patients with solid tumors, as monotherapy (NCT03449381) and in combination with the anti-PD-1 inhibitor ezabenlimab (NCT03964233) [Citation27,Citation28]. In the monotherapy trial, patients received oral brigimadlin once every 3 weeks (q3w); in phase Ia, the recommended dose for expansion was selected as 45 mg q3w; phase Ib is ongoing. In the combination trial, patients received oral brigimadlin q3w in combination with ezabenlimab 240 mg q3w. Among ten patients with BTC treated in these two ongoing trials, five patients achieved a partial response (two in the monotherapy trial and three in the combination trial) and three patients achieved stable disease as best response () [Citation28]. A partial response was also seen in a patient with PDAC in the monotherapy trial. Across both trials, brigimadlin was associated with a manageable safety profile. In phase Ia of the monotherapy trial, the most common any-grade treatment-emergent adverse events (TEAE) were nausea (74%) and vomiting (52%), and the most common grade ≥3 TEAEs were thrombocytopenia (26%) and neutropenia (24.1%); 35% of phase Ia patients had an AE of any-cause that led to a dose reduction, and 6% had an AE of any-cause that led to study discontinuation [Citation27]. In early results from the combination trial, the most common any-grade TEAEs were also nausea (73%) and vomiting (53%), and the most common grade ≥3 TEAEs were thrombocytopenia (27%) and anemia (20%) [Citation28]. This phase I clinical evidence supports the ongoing investigation of brigimadlin in patients with selected MDM2-amplified, TP53 wild-type solid tumors, including BTC and PDAC, in the Brightline-2 trial (NCT05512377).
Trial objectives
The primary objective of Brightline-2 is to evaluate the efficacy of brigimadlin, as assessed by the proportion of patients with an objective response (OR). Secondary objectives are to evaluate the efficacy of brigimadlin in terms of median estimates of duration of response (DOR), progression-free survival (PFS), overall survival (OS) and health-related quality of life (Cohort 1 only). Additional objectives are to explore further measures of efficacy as well as patient-reported outcomes (PRO), pharmacokinetic (PK) parameters and biomarkers.
Design
Trial design
Brightline-2 (NCT05512377) is an open-label, single-arm, multicenter, international, seamless phase IIa/IIb trial (). The phase IIa part of the trial will include five cohorts of patients with advanced or metastatic solid tumors: BTC (Cohort 1 and Cohort 1-CN [patients from mainland China only]), PDAC (Cohort 2), lung adenocarcinoma (Cohort 3) and bladder cancer (Cohort 4). All patients will receive brigimadlin 45 mg orally once every 3 weeks (i.e., on day 1 of 3-week cycles), with no control arm. An interim futility analysis will be performed for the first 30 treated patients in Cohort 1. A non-binding futility boundary of confirmed objective response rate (ORR) = 20% is planned for the interim futility analysis. Enrolment of patients to Cohort 1 will continue while the analysis takes place. If Cohort 1 passes the futility boundary, these 30 patients will enter the phase IIb part of the trial; the total sample size for Cohort 1 will be ≈90 patients. The planned sample size for Cohort 1-CN is approximately 25; and for Cohorts 2, 3 and 4 is approximately 10, 15 and 15 patients, respectively. Entry into phase IIb of the trial was not preplanned for these cohorts as part of the trial design, although a futility analysis will be conducted to determine the next steps.
Figure 3. Brightline-2 trial design.
CN: China; MDM2: Mouse double minute-2 homolog; TP53: Tumor protein 53.
Treatment with brigimadlin will continue until disease progression according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1), unacceptable toxicity, or withdrawal of consent, although treatment beyond progression will be allowed if the investigator judges that this would provide continued clinical benefit to the patient. Events that require discontinuation include: Common Terminology Criteria for Adverse Events v5.0 Grade 4 adverse events (with the exception of myelosuppressive adverse events such as neutropenia, thrombocytopenia, and anemia, for which targeted treatment options and blood transfusion are available); adverse events that cause a dose delay and do not recover within 63 days; patient requires more than two dose reductions, unless the investigator deems treatment continuation beneficial, and a third dose reduction is agreed between the investigator and the sponsor. Dosing of brigimadlin may be delayed or reduced if neutrophil or platelet counts are reduced and do not recover within specified timeframes. Brigimadlin 45 mg may be reduced to 30 mg and then 20 mg, if necessary; dose reductions to 10 mg are permitted for responding patients if agreed with the sponsor. All dose reductions will be permanent.
Eligibility criteria
Key inclusion and exclusion criteria are shown in . In brief, the trial will include adult patients with locally advanced or metastatic, MDM2-amplified, TP53 wild-type BTC, PDAC, lung adenocarcinoma, or urothelial bladder cancer who have received appropriate prior standard of care (SoC) therapy. Patients must have at least one measurable target lesion according to RECIST v1.1, Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function. Patients will be excluded if they have previously received MDM2–p53 or MDMX (MDM4)–p53 antagonists, have active bleeding or significant risk of hemorrhage, had recent (or have imminently planned) major surgery, or have other malignancies that may affect the trial outcome.
Table 1. Selected inclusion and exclusion criteria.
End points & outcomes
The primary end point is OR based on central independent review according to RECIST v1.1. OR is defined as the best overall response of confirmed complete response or confirmed partial response from the time of treatment initiation until disease progression, death from any cause, or other reasons such as withdrawal of consent.
Secondary end points are based on central independent review, where applicable, and include: DOR, defined as the time interval from first documented confirmed OR until disease progression or death among patients with confirmed OR; PFS, defined as the time from starting treatment until tumor progression, according to RECIST v1.1, or death from any cause; OS, defined as the time interval from starting treatment until death from any cause; disease control (DC), defined as the proportion of patients with a best overall response of complete response, partial response, or stable disease according to RECIST v1.1; TEAEs, and the occurrence of TEAEs leading to discontinuation of brigimadlin, during the on-treatment period. Secondary end points reflecting PROs will be assessed for Cohort 1 and 1-CN only and include the change from baseline in European Organisation for Research and Treatment Quality of Life Questionnaire (EORTC QLQ)-C30 physical functioning, fatigue and role functioning domain scores, as well as the change from baseline in EORTC QLQ-BIL21 tiredness domain score.
Further end points include: measures of efficacy based on investigator assessment; PK measures during the first treatment cycle; biomarker studies (considered as exploratory end points); and PROs as per the following measures: change in EORTC QLQ-C30 and EORTC IL46 (item 168) scores; changes in Patient Global Impression Scale and Patient Global Impression of Change scores; change in EORTC QLQ-BIL21 score (Cohort 1 and Cohort 1-CN only); change in EORTC QLQ-PAN26 scores (Cohort 2 only); changes in Non-Small-Cell Lung Cancer Symptom Assessment Questionnaire scores (Cohort 3 only).
Trial procedures
TP53 wild-type and MDM2 amplification status will be assessed (tissue-based next-generation sequencing only, not liquid biopsy) prior to enrolment to the study and will be confirmed retrospectively using archival tumor samples (); in addition, exploratory, longitudinal ctDNA analyses will be conducted while patients are on treatment to monitor the evolution of TP53 mutational status. Patients will have the option to provide a fresh tumor sample at the end of treatment. Tumor response will be evaluated according to RECIST v1.1 at baseline and every 6 weeks starting 6 weeks after Cycle 1 Day 1 and continuing until disease progression. Imaging of all known or suspected sites of disease using an appropriate method (e.g., computerized tomography scan, magnetic resonance imaging, ultrasound) will be conducted. Safety will be assessed using physical examination, measurement of vital signs, laboratory parameters, electrocardiograms and monitoring of adverse events. PK parameters of brigimadlin will be determined after single-dose oral administration in Cycle 1. Blood sampling for PK analysis will be conducted on days 1, 8 and 15 of cycle 1, and days 1 and 2 of cycle 2, with further sampling on day 1 of selected subsequent cycles. Samples will also be taken for exploratory biomarker analyses, including circulating tumor DNA, circulating micro RNAs and circulating proteins (e.g., cytokines, chemokines, hormones).
Statistical analysis
The primary end point of OR will be analyzed in terms of ORR. For Cohort 1, this will be evaluated by two statistical objectives. The first statistical objective is to show the superiority of brigimadlin over historical benchmark from current available treatment options (i.e., vs ORR of 5% with FOLFOX). The second statistic objective is to show definitive clinically meaningful efficacy of brigimadlin (defined as ORR ≥23.3%). The interim futility analysis will be performed for Cohort 1 once 30 patients have been followed for ≥12 weeks or have two post-baseline tumor assessments or have discontinued tumor assessment earlier. A non-binding futility boundary of ORR = 20% is planned for the interim futility analysis. Overall, 90 patients are considered sufficient for Cohort 1. With 90 patients, the one-sided error of falsely observing an ORR passing the critical value of 23.3% will be controlled under 0.1% if the true underlying ORR is 5%. Even under the scenario where the true underlying ORR is 15%, the probability of observing an ORR ≥23.3% will be less than 2%. Assuming ORR ≥30%, the probability of observing an ORR ≥23.3% will be more than 88%.
Ethical considerations
The trial is being carried out in compliance with the protocol, the ethical principles laid down in the Declaration of Helsinki, and in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice, relevant standard operating procedures of the sponsor, plus all other local regulatory requirements.
Conclusion
This paper details the design of Brightline-2 (NCT05512377), a phase IIa/IIb trial that aims to evaluate the efficacy of brigimadlin in a large group of patients with previously treated MDM2-amplified TP53 wild-type, advanced or metastatic BTC, PDAC, lung adenocarcinoma, or bladder cancer. All four tumor types are associated with poor survival rates, and treatment options are limited, particularly after first-line therapy [Citation12,Citation16,Citation22–25]. MDM2 amplification is found in BTC, PDAC, lung adenocarcinomas and bladder cancer [Citation2–4], making antagonism of the MDM2–p53 interaction an attractive therapeutic strategy.
Brigimadlin is an oral MDM2–p53 antagonist that is in clinical development across a number of solid tumors. Following receipt of a Fast Track Designation for dedifferentiated liposarcoma, the US FDA has recently granted Orphan Drug Designation to brigimadlin for the treatment of BTC. Early-phase clinical data from a small number of patients indicate that brigimadlin has antitumor activity in patients with MDM2-amplified, TP53 wild-type BTC and PDAC [Citation27,Citation28].
The Brightline-2 trial is actively recruiting patients and aims to demonstrate the efficacy of second-line (or above) brigimadlin as a new therapeutic strategy for patients with selected unresectable or metastatic MDM2-amplified, TP53 wild-type tumors, including BTC, PDAC, lung adenocarcinoma and bladder cancer.
Author contributions
All authors were involved in the conception and design of the Brightline-2 trial. All authors were involved in the drafting of the manuscript and/or critically revised the manuscript for important intellectual content. All authors gave final approval of the manuscript and agree to be accountable for all aspects of the work, which includes ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Financial disclosure
The Brightline-2 trial is funded by Boehringer Ingelheim. The authors did not receive payment related to the development of this manuscript. C Yoo has received honoraria from Servier, Bayer, AstraZeneca, Merck Sharp & Dohme, Eisai, Celgene, Bristol Myers Squibb, Ipsen, Novartis, Boryung Pharmaceuticals, Mundipharma, Boehringer Ingelheim, and Roche; and received research grants from Servier, Bayer, AstraZeneca, Ono Pharmaceuticals, Ipsen, and Boryung Pharmaceuticals. A Lamarca has consulting/advisory roles with Nutricia, Ipsen, QED Therapeutics, Roche, Servier, Boston Scientific, Albireo Pharma, and AstraZeneca/MedImmune; serves on the speakers' bureau for Merk, Ipsen, Pfizer, Novartis, Incyte, Advanced Accelerator Applications, QED Therapeutics, Servier, and AstraZeneca/MedImmune; has received travel, accommodations, and/or expenses from Abbott Nutrition, Ipsen, Pfizer, Celgene, Novartis, Advanced Accelerator Applications, Sirtex Medical, Bayer, Delcath Systems, and Mylan; and has received research funding from Ipsen and Roche. HY Choi has no interests to disclose. A Vogel has advisory council/committee roles with AstraZeneca, Amgen, BeiGene, and Böhringer Mannheim; and has received honoraria from AstraZeneca, Amgen, BeiGene, Böhringer Mannheim, Bristol Myers Squibb, BTG, Daichi-Sankyo, Eisai, GSK, Imaging Equipment Ltd (AAA), Incyte, Ipsen, Jiangsu Hengrui Medicines MSD, PierreFabre, Roche, Servier, Sirtex, Tahio, and Terumo. MJ Pishvaian has advisory roles with Astra Zeneca, Ideaya, Seattle Genetics, Merus, and Merck; has received honoraria from Pfizer and Novartis; is on the steering committee for Astellas and Trisalus; has received travel expenses from Astellas and RenovoRx; has stocks and patent at Perthera; has patent pending with Abbvie; has continuing medical education activities with Tumor Board Tuesdays and TRICC; and reports funding to the institution from Seattle Genetics, Tesaro, Arcus Bio, Ideaya, Repare Tx, Novartis, Pfizer, Merck, Tizonia, Biomed Valley Discoveries, Amgen, RenovoRx, Boehringer Ingelheim, Astellas, Hutchinson, Medipharma, Takeda, Actuate, and MEI Pharma. L Goyal has a consulting role with Abbvie. M Ueno reports grants to the institution from Taiho Pharmaceutical, AstraZeneca, Merck Biopharma, MSD, Astellas Pharma, Eisai, Ono Pharmaceutical, Incyte, Chugai Pharmaceutical, DFP Pharma, Daiichi Sankyo, Novartis, Boehringer Ingelheim, and J-pharma; and has received honoraria from Taiho Pharmaceutical, AstraZeneca, Yakult Honsha, MSD, Nihon, Servier, Ono Pharmaceutical, Incyte, Chugai Pharmaceutical, Boehringer Ingelheim, and J-Pharma. A Märten, M Teufel, and L Geng are employed by Boehringer Ingelheim. C Morizane has received honoraria from Novartis, Yakult Honsha, Teijin Pharma, Taiho, Pharmaceutical, Eisai, MSD K.K., and AstraZeneca; has grants/funds from Boehringer Ingelheim, Eisai, Yakult Honsha, ONO Pharmaceutical, Taiho Pharmaceutical, J-Pharma, AstraZeneca, Merck biopharma, Daiichi Sankyo RD, Novare, and Hitachi; and has advisory roles with Boehringer Ingelheim, Merck, Taiho Pharmaceutical, AstraZeneca, Servier, MSD K.K., and Yakult Honsha. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Jim Sinclair, PhD, and Jane Saunders, PhD, of Ashfield MedComms, an Inizio Company, and was funded by Boehringer Ingelheim.
Ethical disclosure
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Zhao Y, Yu H, Hu W. The regulation of MDM2 oncogene and its impact on human cancers. Acta Biochim. Biophys. Sin. 46(3), 180–189 (2014).
- Nakamura H, Arai Y, Totoki Y et al. Genomic spectra of biliary tract cancer. Nat. Genet. 47(9), 1003–1010 (2015).
- Bouattour M, Valle JW, Vogel A et al. Characterization of long-term survivors in the TOPAZ-1 study of durvalumab or placebo plus gemcitabine and cisplatin in advanced biliary tract cancer. J. Clin. Oncol. 41(Suppl. 4), 531 (2023).
- AACR Project Genie Consortium. AACR Project GENIE: powering precision medicine through an international consortium. J. Cancer Discov. 7(8), 818–831 (2017).
- Cancer.Net. Gallbladder cancer: statistics. www.cancer.net/cancer-types/gallbladder-cancer/statistics (21st May, 2023).
- Ghidini M, Pizzo C, Botticelli A et al. Biliary tract cancer: current challenges and future prospects. Cancer Manag. Res. 11, 379–388 (2019).
- Vogel A, Bridgewater J, Edeline J et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34(2), 127–140 (2023).
- Oh D-Y, Ruth He A, Qin S et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. N. Eng. J. Med. Evid. 1(8), EVIDoa2200015 (2022).
- Kelley RK, Ueno M, Yoo C et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet 401(10391), 1853–1865 (2023).
- Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: ready for “prime time” in biliary tract cancer. J. Hepatol. 73(1), 170–185 (2020).
- Karasic TB, Eads JR, Goyal L. Precision medicine and immunotherapy have arrived for cholangiocarcinoma: an overview of recent approvals and ongoing clinical trials. JCO Precis. Oncol. 7, e2200573 (2023).
- Lamarca A, Palmer DH, Wasan HS et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a Phase 3, open-label, randomised, controlled trial. Lancet Oncol. 22(5), 690–701 (2021).
- Yoo C, Kim K-P, Jeong JH et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, Phase 2b study. Lancet Oncol. 22(11), 1560–1572 (2021).
- Cancer.Net. Pancreatic cancer: statistics. www.cancer.net/cancer-types/pancreatic-cancer/statistics#:∼:text=How%20many%20people%20are%20diagnosed,approximately%203%25%20of%20all%20cancers (21 May 2023).
- Principe DR, Underwood PW, Korc M, Trevino JG, Munshi HG, Rana A. The current treatment paradigm for pancreatic ductal adenocarcinoma and barriers to therapeutic efficacy. Front. Oncol. 11, 688377 (2021).
- Sarantis P, Koustas E, Papadimitropoulou A, Papavassiliou AG, Karamouzis MV. Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 12(2), 173–181 (2020).
- Hayuka K, Okuyama H, Murakami A et al. Gemcitabine plus nab-paclitaxel as second-line chemotherapy following FOLFIRINOX in patients with unresectable pancreatic cancer: a single-institution, retrospective analysis. Chemotherapy 66(3), 58–64 (2021).
- Portal A, Pernot S, Tougeron D et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Brit. J. Cancer 113(7), 989–995 (2015).
- Wang-Gillam A, Li C-P, Bodoky G et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, Phase 3 trial. Lancet 387(10018), 545–557 (2016).
- Tempero MA. NCCN guidelines updates: pancreatic cancer. J. Natl Compr. Canc. Netw. 17(5.5), 603–605 (2019).
- Tempero MA, Malafa MP, Al-Hawary M et al. Pancreatic adenocarcinoma, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw. 19(4), 439–457 (2021).
- Rittmeyer A, Barlesi F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a Phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066), 255–265 (2017).
- Garon EB, Ciuleanu T-E, Arrieta O et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised Phase 3 trial. Lancet 384(9944), 665–673 (2014).
- Borghaei H, Paz-Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Eng. J. Med. 373(17), 1627–1639 (2015).
- Powles T, Rosenberg JE, Sonpavde GP et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Eng. J. Med. 384(12), 1125–1135 (2021).
- Rudolph D, Weyer-Czernilofsky U, Reschke M et al. BI-907828: a novel, and potent MDM2-p53 antagonist, acts synergistically in a triple combination with anti-PD-1 and anti-LAG-3 antibodies in syngeneic mouse models of cancer. Cancer Res. 79(Suppl. 3), 3197 (2019).
- LoRusso P, Yamamoto N, Patel MR et al. The MDM2-p53 antagonist brigimadlin (BI 907828) in patients with advanced or metastatic solid tumors: results of a Phase Ia, first-in-human, dose-escalation study. Cancer Discov. 13(8), 1802–1813 (2023).
- Yamamoto N, Tolcher AW, Hafez N et al. Efficacy and safety of the MDM2–p53 antagonist BI 907828 in patients with advanced biliary tract cancer: data from two Phase Ia/Ib dose-escalation/expansion trials. J. Clin. Oncol. 41(Suppl. 4), 543 (2023).