Abstract
Aim: Viral etiology of respiratory infections is not well understood in Saudi Arabia. This study was conceptualized to understand viral etiology in children with acute respiratory tract infections (ARTI) from Riyadh. Patients & methods: Respiratory viruses were detected by real-time PCR in nasopharyngeal aspirates or swab from 580 children aged ≤5 years. Results: Respiratory viruses were detected in 64% of the samples with 6% mixed viral infections. Respiratory syncytial virus, adenovirus, influenza, parainfluenza and human metapneumovirus infections accounted for 42, 20, 16, 12 and 10%, respectively. Maximum prevalence (37%) was among the lowest age group followed by 30% among the 7- to 12-month age group. Conclusion: The prevalence and determinants of viral etiology are in line with the previous report from the region. No major shift in the viral etiologies was observed in the 2-year study period.
Acute respiratory tract infections (ARTIs) are major cause of morbidity and mortality among children worldwide. Viruses contribute considerably to ARTIs, followed by bacteria and fungi, which contribute to lower levels. In developing countries, the viral infections account for 40–90% of ARTIs [Citation1–3]. Children younger than 5 years are highly susceptible to infections, and millions die annually due to ARTIs [Citation4]. Respiratory viruses are prevalent worldwide, and their transmission and prevalence is affected by geographic, demographic, socioeconomic and environmental factors [Citation5,Citation6]. The frequently detected respiratory viruses, respiratory syncytial virus (RSV) and influenza virus (flu) are estimated to cause approximately 39 and 25 million episodes, and approximately 58,000 and 76,000 deaths annually, respectively [Citation7]. The global burden estimates of parainfluenza virus (PIV), adenovirus (AdV) and human metapneumovirus (HMPV) have not been reported to date. In addition, three deadly coronaviruses – severe acute respiratory syndrome-related coronavirus (SARS-CoV), Middle East respiratory syndrome-related coronavirus (MERS-CoV) and novel coronavirus (SARS-CoV-2) – were also reported in 2002, 2012 and 2019, respectively, that originated from China (SARS-CoV, SARS-CoV-2) and Saudi Arabia (MERS-CoV), and spread worldwide [Citation8–10].
The etiology of respiratory infections is complex, and the detection of causative agent is difficult due to lack of specific clinical symptoms associated with the causative agent and presence of multiple infections or mixed infections [Citation11,Citation12]. In Saudi Arabia, ARTIs lead to approximately 5 million cases annually which was 15.4% of the population in 2013 [Citation13]. The etiology of these infections remain uncharacterized due to selective focus of studies on annual Hajj pilgrimage [Citation14–16] and focus on selective viral pathogen [Citation17–20].
The etiology of ARTIs, circulation pattern and evolution of the infective agents in Saudi Arabia is affected by the presence of 11.9 million foreign workforce employees from ≥100 countries [Citation14] and the huge annual gatherings of ≥10 million Hajj pilgrims and Umrah gatherings at Mecca and Medina from 184 countries. Movement of the workforce to and from their respective destinations to Saudi Arabia and due to close proximity of the pilgrims during Hajj and Umrah pilgrimages leads to the exchange and inoculation of the masses with new strains of infective agents. During the process, a viral agent may undergo mutation and recombination, giving rise to a potent new viral agent capable of infecting other individuals. This process of renewing the pool of circulating respiratory viruses takes place every year. The agents can further be passed on to the healthy individuals on their return to their respective native countries [Citation18–21].
Despite the significant risk of respiratory infections to Saudi population, there is gap of knowledge on prevalence of different respiratory viruses. A few reports have been published on this subject from different Saudi Arabian cities (Riyadh, Abha, Jazan and Najran) [Citation20–26]. The central region of Saudi Arabia (Riyadh region) is densely populated comprising a mixed population of locals and immigrants. The interaction among them can affect the transmission pattern of the respiratory viruses. The studies conducted in Riyadh region reported few viruses with limited information on seasonal distribution of viruses [Citation20,Citation23,Citation27]. To gain a better understanding of the local epidemiology for prevention and control of the respiratory infections, we determined the distribution and prevalence of RSV, Flu, PIV, AdV and MPV in the present study.
Materials & methods
Sample collection
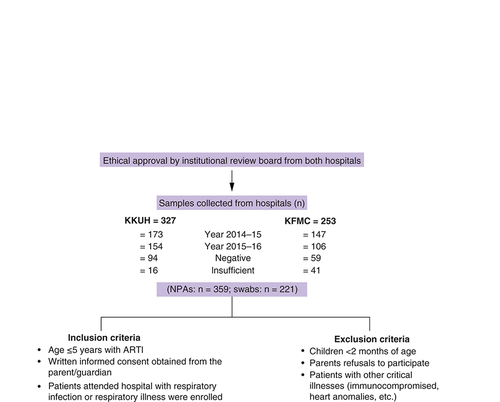
The study was conducted at King Khalid University Hospital (KKUH) and KFMC, Riyadh, Saudi Arabia. Riyadh is in the central region and is the capital of Saudi Arabia. These hospitals provide healthcare services to patients from the northern region of Riyadh. Samples were collected from the children’s hospital and pediatrics department at KFMC and KKUH, respectively. Children ≤5 years who visited hospital for the first time with acute respiratory illness and respiratory infection (fever, cough, sore throat, wheezing or apnea) were enrolled from the emergency department (ED) or from the ward between July 2014 to June 2016, following WHO criteria [Citation28]. Pediatricians examined children with ARTI, and clinical data were recorded in pro forma. Trained nurses and technicians were employed to collect nasopharyngeal aspirates ([NPAs] n = 359) or swabs (n = 221) from the patients in 1-ml viral collection tubes (UTM Copan, Brescia, Italy). The samples were transported to the laboratory on ice, processed and stored in aliquots of 500 μl at -80°C until RNA was extracted.
Nucleic acid extraction
The 500-μl aliquots were vortexed, and viral nucleic acid was extracted from samples using kits from Qiagen (CA, USA) following the manufacturer’s instructions. Briefly, the samples were thawed and lysed with 500 μl of lysis buffer. One volume ethanol was added to disperse any visible precipitate, and column binding was carried out by passing lysate to the columns by centrifugation at 12,000 × g for 30 s. Columns were washed with 500 μl wash buffers with ethanol and centrifugation at 12,000 × g for 15 s. Columns were dried, and nucleic acid was eluted in 50 μl DNase/RNase-free water by centrifugation for 2 min at ≥12,000 × g at room temperature.
Virus detection
The kits used for the detection of viruses were obtained from Fast Track Diagnostics (Malta). Influenza A (FLU-A), B (FLU-B) and RSV were detected using FLU/HRSV kit; parainfluenza virus 1, 2, 3 and 4 by HPIV kit; and HAdV and HMPV were detected using an HAdV/HMPV kit. All the kits were real-time PCR based and use TaqMan technology for detection. The samples were reported as insufficient when the specimen was <500 μl in volume with low viral load or low genetic material. Human GAPDH was used as a control. Reaction with negative GAPDH results or higher Ct value for GAPDH were repeated with higher volume of eluted RNA. During this process, due to low amount of viral genetic material left, the reaction for the detection of all the reported viruses in a specimen could not be carried out. Therefore, the sample was reported as insufficient and not considered for analysis.
The runs were carried out in Applied Biosystems (MA, USA) 7500 Real-Time PCR System in a 25-ul reaction mixture under recommended reaction conditions by the manufacturer. Each reaction contained 1.5 μl primer/probe mix, 12.5 μl 2× RT-PCR buffer, 1 μl 25× RT-PCR enzyme mix and 10 μl of purified RNA. Each run had a positive, negative and internal control. Presence of a viral agent was detected by an increase in fluorescence observed from the relevant dual-labeled probe. RSV, FLUA and FLUB pathogen presence was detected by increase in fluorescence at 550, 520 and 610 nm. Presence of HPIV1, 2, 3 and 4 pathogens was detected by an increase in fluorescence at 610, 550, 520 and 670 nm, respectively. For HAdV and HMPV pathogens, an increase in fluorescence was observed at 550 and 670 nm.
Statistical analysis
The data were analyzed using Microsoft Excel 2010. All the data are categorical and presented in the tables as frequency (%). The difference between the distributions in gender among different age groups () was done by chi-square test. For data in , the distribution of viral etiological agents among different age groups was presented as frequency (percentage of positives of a particular viral agent). Odds ratio (OR) and 95% CI of having viral agents (total) in lower age groups was calculated by chi-square test taking the highest age group (37–60 months) as reference. p < 0.05 was considered as significant for all tests.
Table 1. Age-wise distribution of viral infections with clinical manifestation of the study patients and positive percentage.
Table 2. Distribution of viral etiological agents among different age groups and gender.
Results
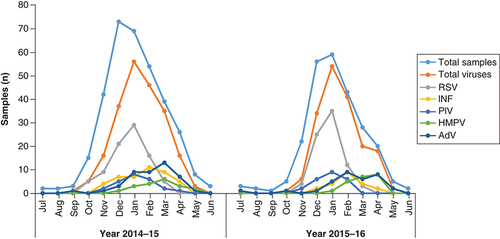
A total of 580 NPAs and swabs were collected for the study from KKUH (327) and KFMC (253) over 2 years. A total of 320 samples were collected in 2014–15 and 260 samples in 2015–16. Of these, 153 (26.4%) samples were negative, 57 (9.8%) were insufficient and 371 (64%) showed the presence of viral nucleic acid (). Yearly trends of total ARI and viral infections during 2014–15 and 2015–16 were similar (). High infections were observed during November–April while a very low percentage of infections were observed during summer, from May to September, in both years (). Circulation of different respiratory viruses during the 2-year period is shown in .
The clinical manifestation of the study patients is provided in the . Mean patient age was 7 months (standard deviation ± 2.7 months), and male to female ratio was 1.48 (59.5%). Presentation of respiratory infections were more frequent among lower age groups and decreased with age. Maximum respiratory infections (37.4%) were reported among the lowest age group (0–6 months) followed by 30.2% among the 7- to 12-months age group. The other age groups had ARTI prevalence between 16.6% to 5.9% (). No significant difference in clinical presentation was observed among age groups (p = 0.99) (). High viral etiology (45.7 and 26.5%) among lower age groups (0–6 and 7–12 months) indicate high viral infections among these groups, as shown by higher OR vales of 14.1 and 6.1, respectively (). Infection among the higher age groups was low (OR: 2.58 and 1.65). A significant number of infections was found among the lower age groups (p < 0.001) ().
Coinfections were observed in 21 samples, and single-viral infection was detected in 350 samples. Single-viral infections and coinfections were observed in 89.3% (350) and 10.7% (42), respectively, of all infections (). RSV was the most frequently found virus accounting to 41.8% of the infections followed by AdVs, reaching to 19.6% of all the infections (). INF, PIV and MPV were detected in 16.3, 11.9 and 10.2% of the infections. Single-viral infection was observed in 89.3% of the samples, and the remaining samples (10.7%) showed coinfection with two viruses. Among RSV cases, 93.9% of the samples had single-viral infection, and 6.1% of the samples were infected with two viruses. FLU single-viral infections accounted for 79.7% of cases, and 20.3% had double infections. FLUA was observed in 68.7% of the total FLU infections, and FLUB was observed in the remaining samples (31.3%). FLUA and FLUB had the same ratio of 80% and 20% in single infection and coinfections. PIV single-viral infections were observed in 89.4% of the infections, and coinfections were seen in 10.6% of the infections. Single-viral infections among PIV1, PIV2 and PIV3 were in 91.7%, 83.3% and 78.6%, respectively, and coinfections were seen in 8.3, 16.7 and 21.4%, respectively. All PIV4-infected samples had single-viral infection, and no coinfections were observed with it. Single-viral infections were seen in 85% and coinfections in 15% of the HMPV-infected samples. AdV coinfections were the second most common coinfections (10.4%), and 89.6% AdV infections were single-viral infections.
Table 3. Distribution of viral etiological agents and coinfections.
ARTI: Acute respiratory tract infections; KFMC: King Fahad Medical City KKUH: King Khalid University Hospital; NPAs: Nasopharyngeal aspirates.
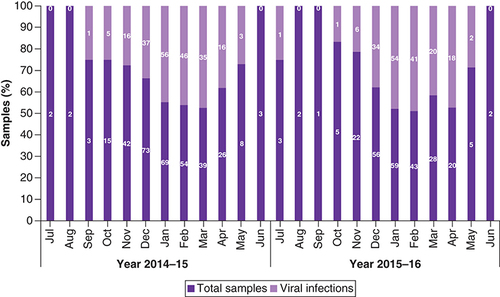
Total sample collected from both the hospitals. Total viruses refers to the total number of viruses detected in samples and counted twice for double viral infections.
AdV: Adenovirus; HMPV: Human metapneumovirus; INF: Influenza; PIV: Parainfluenza virus; RSV: Respiratory syncytial virus.
Discussion
ARTIs are the major cause of morbidity and mortality in children [Citation29]. Most ARTIs among children aged <5 years are caused by viruses [Citation30]. In the present study, respiratory viral infections were recorded in 371 out of 580 patients with 64% of the specimens infected with at least one virus. This percentage is in accordance with the previous reported range between 43 and 95% worldwide [Citation1,Citation22,Citation23,Citation25,Citation31–37] and also in range (between 48 and 92%) with the earlier reports from Saudi Arabia [Citation13,Citation20,Citation23,Citation25]. The varied percentage of viral infections in the literature may be attributed to the heterogeneous study population, genetic differences between them, different presentation of patients, varied numbers of viruses included in the study and different methods used for virus detection [Citation1,Citation22,Citation25,Citation38,Citation39].
A higher proportion of respiratory infections (61%) was reported in the age group <1 year followed by the 1- to 2-year (15%) and 3- to 5-year (14%) age groups. Similar infection rates were also reported in earlier studies from Saudi Arabia and other countries where higher proportions of infection were seen in children <1 year [Citation20,Citation23–25,Citation36,Citation40–43]. The lower age groups are prone to infections, perhaps because of low viral clearance due to underdeveloped immunity, weakening maternal antibodies to prevent viral infections after 6 months of life, unhygienic living conditions and higher pathogen exposure [Citation2,Citation39,Citation44,Citation45]. Furthermore, higher infection rates among infants are reported because of the anxiety of the parents to seek immediate healthcare for an ill infant. In agreement with previous studies [Citation20,Citation46–49], we also observed that boys were more vulnerable (59.5%) to ARTIs than girls.
Accurate and fast detection of an infecting viral pathogen has recently been achieved through real-time PCR-based Fast Track diagnostic kits (Fast Track Diagnosis, Luxembourg) [Citation50,Citation51]. In this study, RSV was the most common viral pathogen, the main causative agent of ARTIs and a major contributor of children hospitalization [Citation52–54]. In a previous study from Riyadh, RSV was also shown to be the predominant virus, circulating between 2005 and 2010, and accounted with high prevalence of 95.5% of infections [Citation23]. High prevalence (97.4%) was also reported by another author from Riyadh [Citation27]. Other studies from the region reported a lower RSV prevalence of 16.5 and 22.4% [Citation20,Citation22]. Studies from other regions of Saudi Arabia (Jazan and Najran) reported RSV prevalence of 13.6 and 30.3% [Citation24,Citation25]. The varied prevalence of RSV is dependent mostly on geographic and chronological factors, but the varied prevalence in the same region may be due to bias in sample collection and the time of collection.
Adenovirus also causes ARTI and has been associated with bronchiolitis. Its prevalence is shown to be 11 and 27.3% in studies from Saudi Arabia [Citation21,Citation22,Citation25,Citation26]. A low (0.3%) prevalence was also reported from Saudi Arabia [Citation23]; however, this low prevalence was due to the study limitations, low sample size and the study being a retrospective. Another study reported 5.7% AdV infection in ARTI but included patients of all age groups [Citation24]. AdV was the second highest prevalent pathogen (19.6%) observed in our study. The prevalence is higher than that from any previous report from the region. Worldwide, the percentage varies from 3 to 12% in pediatric ARTI [Citation55–57]. Higher prevalence (48%) of AdV is also seen in Taiwan [Citation58]. Such higher discrepancy in AdV detection rates can be explained by different climatic and geographic conditions of the regions. In Saudi Arabia, the adenovirus infections are reported throughout the year, with higher prevalence in the summer [Citation22], which is similar to the prevalence seen in Beijing and Guangzhou, China [Citation59–61].
Influenza is a worldwide problem, with an annual infection rate of 20–30% in children [Citation62]. The burden due to influenza in our study (16.3%) was in accordance with the other studies in the region (7.2–19.5%) [Citation20,Citation22,Citation25]. Another report included higher age groups and showed a prevalence of 15.9% [Citation24]. A prevalence of 15.1% in Kuwait studies is also in line with these findings [Citation63]. Lower prevalence (6%) and higher prevalence (36.1%) were reported from Fujian province in China [Citation37] and Indonesia [Citation64], respectively. A meta-analysis of influenza virus reported a burden of 7.0% in children with ALRI [Citation65] and is lower than in Riyadh. PIV prevalence was also in range (12%) with that reported from the region (1.2–18%). Four of the studies reported prevalence below 6.3% [Citation20,Citation22,Citation23,Citation25], and one study [Citation26] reported a higher prevalence of 18%. Worldwide PIV prevalence is 2.7% of the ALRI patients [Citation65]. Kuwait also reported lower prevalence of 1.7% [Citation63]. A study reported PIV-2 and PIV-3 prevalence of 5.6 and 0.6%, respectively [Citation66]. In our study, the percentage of different subtypes from PIV-1 to 4 were 3, 1.3, 3 and 4%. Variable percentage of PIV1–4 were also reported to be between 0.3 and 13% in other research [Citation22,Citation26]. HMPV was least prevalent during our study period accounting for 10.2% of all the cases. Its prevalence, reported earlier from the region, ranged between 4.2 and 12%, and our data is in line with these reports [Citation13,Citation18,Citation20,Citation22,Citation25]. In a neighboring country, Kuwait, the HMPV prevalence was 6.2% among all hospitalized patients [Citation63]. China reported a low prevalence of 2.7% [Citation67]. Variability observed in the prevalence of influenza, PIV and HMPV may be due to differences in the patient inclusion criteria, sample size, period and duration of study and diagnostic methods employed.
Coinfections were low in the study and observed to be in range (5–10%) with the coinfections reported worldwide using real-time PCR [Citation37,Citation39,Citation68,Citation69]. A study in China identified viral coinfections in 13.2% of the samples [Citation70]. In another study, viral mixed infections were observed in 8% of samples [Citation41]. A study of virus-infected ARTI children aged up to 8 years showed 14.5% of mixed viral infections [Citation71]. However, a higher percentage (15.3%) of coinfections in total infections and 21.9% among virus-positive ALRI was reported in a meta-analysis of various studies [Citation65]. These differences in mixed-viral prevalence may be attributed to environmental and regional differences, panel of respiratory viruses tested and different virus detection techniques used. In the present study, the viruses implicated in coinfections include RSV, AdV, INF, HMPV and PIV. RSV + INF-A coinfections were the highest followed by RSV + AdV coinfections. Earlier studies reported RSV and AdV as the leading viruses involved in coinfections among children [Citation40,Citation42]. AdV coinfections in this study are on the upper limit, and its higher prevalence may facilitate other viral infections [Citation22,Citation42]. Virus with higher prevalence in coinfections suggest their facilitation of infection or colonization by other virus in some patients. The remaining coinfections ranged between 4.8 to 9.5%, and no coinfection was observed with PIV4. Coinfections have also been reported in an earlier study (6.7%), with RSV and HMPV accounting for 44.4% of the total coinfections [Citation25]. No coinfection with RSV and HMPV were reported [Citation63,Citation72], but others report coinfections rates of 5–14% [Citation73–75]. PIV and flu are the most prevalent viruses in coinfections involving children with RTI [Citation70]. A higher coinfection percentage of 17.1% was reported from Korea and may be due to the larger sample size tested [Citation76]. Further, a higher coinfection rate of 20.3% with RSV and other viruses among children has also been reported [Citation40]. The coinfections in children may be due to underdeveloped immunity of the infants and their lack of earlier exposure to pathogens, which could increase their chances of susceptibility to a mixed infection [Citation77]. Viral coinfections may also be attributed to asymptomatic persistence or the shedding of virus among children [Citation33]. The effect of coinfections in patient health is not clear, and various studies have shown that coinfections are less likely to contribute to disease severity and hospitalization [Citation11,Citation78–80]; however, other studies report high fever, longer hospital stay, high pneumonia incident, more hypoxia and high antibiotic use with coinfections [Citation81–83]. It has also been suggested that sequential infections within a short period of time with viral shedding in the late phase of previous infection may explain coinfection detection in the same specimen. However, more comprehensive, longitudinal studies need to be conducted for better understanding of the clinical impact of the coinfections. Moreover, multiplex respiratory virus assays may be used and will be helpful to elucidate and better interpret coinfections. These assays are cheaper and will allow more people to perform studies and accumulate data.
Respiratory viruses show seasonal prevalence [Citation84–86]. We observed peak activity of the viruses during winter season and least viral incidents in summer. These findings are consistent with earlier reports from Saudi Arabia [Citation13,Citation20,Citation22,Citation23]. In our study, higher prevalence of viruses, including RSV and HMPV, was observed in winter and FLU in February and March. Brittain-Long et al. reported similar prevalence [Citation84]. Previous studies also show higher RSV incidents from December to February [Citation22] and December to March [Citation20]. HMPV has a similar yearly pattern of prevalence to RSV but a low occurrence [Citation20,Citation22], and is also observed in our study. Influenza infections peak in February and March in Saudi Arabia. Almost all the respiratory viruses show seasonal variation except AdV and to some extent, parainfluenza virus, which show prevalence throughout the year [Citation84]. The seasonal activity of respiratory viruses in regions adjacent to Saudi Arabia, such as United Arab Emirates, Oman and Kuwait, show a similar pattern [Citation63,Citation87,Citation88]. In other parts of the world, such as Taiwan, China and Indonesia, respiratory viral infections are common in summer/autumn, winter and rainy seasons, respectively [Citation57,Citation58,Citation89]. This is due to their different geographic locations and climatic conditions [Citation90,Citation91]. Aerosols are the mode of respiratory virus transmission, and therefore, in high-humidity environments in tropical countries such as Indonesia, the aerosol of respiratory viruses remains longer and thus increases viral infections. In dry and hot climates during the summer in Middle Eastern region, viruses do not survive due to rapid aerosol evaporation, and thus prevalence during summer is low.
The strength of an epidemiology study lies in the detection of a complete range of respiratory viruses and their correlation with clinical manifestations in single infection and coinfection. Although our results are consistent with the results of previous studies, there are limitations. The data for this study were procured from two hospitals and the small sample size may not represent the entire pediatric population of the area. Future studies must focus on a larger, more scattered pediatric population to confirm the reported observations. The lack of testing measures for potential secondary bacterial pathogens, such as Mycoplasma pneumoniae and Streptococcus pneumoniae, should be noted. Also to be considered is the difference in sensitivity and specificity of RT-PCR kits for different genes in different targeted pathogens. ARI specimens were not collected from all patients with respiratory symptoms due to duty shifts of the hospital staff, and some respiratory viruses were excluded in our testing, such as the MERS. There is also a lack of data on the severity of infections. Finally, the clinical presentation of patients was not assessed or compared with the identified respiratory viral pathogens.
Conclusion
There was not a significant difference in prevalence and predominance of different viruses (RSV, AdV, INF, PIV and HMPV) compared with previous reports from the region. All viruses were involved in coinfections with other viruses except PIV4, which showed no coinfection. Seasonality of the different determinants of ARTI were also similar to the previous reports. Therefore, it appears that there is no major yearly shift in the viral etiologies of ARTI in the Riyadh region of Saudi Arabia during study period.
Viral etiologies of the acute respiratory tract infections (ARTIs) are not well understood in the Riyadh region of Saudi Arabia.
This study was conceptualized to understand viral etiology in children with ARTIs.
Prevalence and determinants of viral etiology in the present study were in line with the previous studies from the Riyadh region.
Seasonal prevalence of different viruses was similar to the earlier reports.
Coinfections were low in the study compared with earlier reports but in range (5–10%) with the coinfections reported from around the world using real-time PCR.
No major yearly shift in the viral etiologies of ARTI occur in the Riyadh region of Saudi Arabia.
Author contributions
A Ahmed: conceptualization, data curation, formal analysis, funding acquisition, writing of original draft. A Ahmed, K Mobaireek, AA AlSaadi: investigation, data collection, methodology, visualization. A Ahmed, MM Alsenaidy: supervision, project administration, resources.
Ethical conduct of research
The authors state that they have obtained approval from the institutional review boards of College of Medicine, King Saud University (approval no. E-14-1155) and King Fahad Medical City (approval no. 14-279). Consent was obtained from the parents/guardians of the children, and demographic data were obtained from records.
Acknowledgments
The authors are grateful to Dr Salman Alamery and Kaiser Ahmed Wani for their valuable contribution toward reviewing the manuscript and statistical analysis.
Financial & competing interests disclosure
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, for funding work through the Research Group Project no. RG-1439-74. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Van Der Zalm MM , Van EwijkBE, WilbrinkBet al. Respiratory pathogens in children with and without respiratory symptoms. J. Pediatr., 154(3), 396–400, 400.e391 (2009).
- Huijskens EG , BiesmansRC, BuitingAGet al. Diagnostic value of respiratory virus detection in symptomatic children using real-time PCR. Virol. J., 9, 276 (2012).
- Kwofie TB , AnaneYA, NkrumahBet al. Respiratory viruses in children hospitalized for acute lower respiratory tract infection in Ghana. Virol. J., 9, 78 (2012).
- Rudan I , Boschi-PintoC, BiloglavZet al. Epidemiology and etiology of childhood pneumonia. Bull. World Health Organ., 86(5), 408–416 (2008).
- Amugsi DA , AborigoRA, OduroARet al. Socio-demographic and environmental determinants of infectious disease morbidity in children under 5 years in Ghana. Glob. Health Action, 8, 29349 (2015).
- Pica N , BouvierNM. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol., 2(1), 90–95 (2012).
- Troeger C , BlackerB, KhalilIaet al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis., 18(11), 1191–1210 (2018).
- Zaki AM , Van BoheemenS, BestebroerTMet al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med., 367(19), 1814–1820 (2012).
- Drosten C , GuntherS, PreiserWet al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med., 348(20), 1967–1976 (2003).
- Chan JF , KokKH, ZhuZet al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect., 9(1), 221–236 (2020).
- Kouni S , KarakitsosP, ChraniotiAet al. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin. Microbiol. Infect., 19(8), 772–777 (2013).
- Martin ET , KuypersJ, WaldA, EnglundJA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir. Viruses, 6(1), 71–77 (2012).
- Fagbo SF , GarbatiMA, HasanRet al. Acute viral respiratory infections among children in MERS-endemic Riyadh, Saudi Arabia, 2012–2013. J. Med. Virol., 89(2), 195–201 (2017).
- Balkhy HH , MemishZA, BafaqeerS, AlmuneefMA. Influenza a common viral infection among Hajj pilgrims: time for routine surveillance and vaccination. J. Travel Med., 11(2), 82–86 (2004).
- Mandourah Y , Al-RadiA, OcheltreeAHet al. Clinical and temporal patterns of severe pneumonia causing critical illness during Hajj. BMC Infect. Dis., 12, 117 (2012).
- Barasheed O , RashidH, AlfelaliMet al. Viral respiratory infections among Hajj pilgrims in 2013. Virol. Sin., 29(6), 364–371 (2014).
- Almajhdi FN , Al-JarallahA, ElaeedMet al. Prevalence of respiratory syncytial virus infection in Riyadh during the winter season 2007–2008 and direct risk factors impact. Int. J. Virol. J., 5(4), 154–163 (2009).
- Al Hajjar S , AlThawadi S, AlSeraihi Aet al. Human metapneumovirus and human coronavirus infection and pathogenicity in Saudi children hospitalized with acute respiratory illness. Ann. Saudi Med., 31(5), 523–527 (2011).
- Abdel-Moneim AS , KamelMM, Al-GhamdiAS, Al-MalkyMI. Detection of bocavirus in children suffering from acute respiratory tract infections in Saudi Arabia. PLoS One, 8(1), e55500 (2013).
- Amer HM , AlshamanMS, FarragMAet al. Epidemiology of 11 respiratory RNA viruses in a cohort of hospitalized children in Riyadh, Saudi Arabia. J. Med. Virol., 88(6), 1086–1091 (2016).
- Al-Hajjar S , AkhterJ, AlJumaah S, HussainQadri SM. Respiratory viruses in children attending a major referral centre in Saudi Arabia. Ann. Trop. Paediatr., 18(2), 87–92 (1998).
- Eifan SA , HanifA, AljohaniSM, AtifM. Respiratory tract viral infections and coinfections identified by Anyplex II RV16 detection kit in pediatric patients at a Riyadh tertiary care hospital. Biomed. Res. Int., 2017, 1928795 (2017).
- Bukhari EE , ElhazmiMM. Viral agents causing acute lower respiratory tract infections in hospitalized children at a tertiary care center in Saudi Arabia. Saudi Med. J., 34(11), 1151–1155 (2013).
- Abdulhaq AA , BasodeVK, HashemAMet al. Patterns of human respiratory viruses and lack of MERS-coronavirus in patients with acute upper respiratory tract infections in southwestern province of Saudi Arabia. Adv. Virol., 2017, 4247853 (2017).
- Al-Ayed MS , AsaadAM, QureshiMA, AmeenMS. Viral etiology of respiratory infections in children in southwestern Saudi Arabia using multiplex reverse-transcriptase polymerase chain reaction. Saudi Med. J., 35(11), 1348–1353 (2014).
- Al-Shehri MA , SadeqA, QuliK. Bronchiolitis in Abha, Southwest Saudi Arabia: viral etiology and predictors for hospital admission. West African J. Med., 24(4), 299–304 (2005).
- Albogami SS , AlotaibiMR, AlsahliSAet al. Seasonal variations of respiratory viruses detected from children with respiratory tract infections in Riyadh, Saudi Arabia. J. Infect. Public Health, 11(2), 183–186 (2018).
- WHO . Integrated management of childhood illness handbook. (2005).
- Walsh MP , SetoJ, LiuEBet al. Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J. Clin. Microbiol., 49(10), 3482–3490 (2011).
- Bellau-Pujol S , VabretA, LegrandLet al. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J. Virol. Methods, 126(1–2), 53–63 (2005).
- Shafik CF , MoharebEW, YassinASet al. Viral etiologies of lower respiratory tract infections among Egyptian children under five years of age. BMC Infect. Dis., 12, 350 (2012).
- Niang MN , DiopOM, SarrFDet al. Viral etiology of respiratory infections in children under 5 years old living in tropical rural areas of Senegal: The EVIRA project. J. Med. Virol., 82(5), 866–872 (2010).
- Bicer S , GirayT, ColDet al. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital. J. Pediatr., 39, 22 (2013).
- Martinez-Roig A , SalvadoM, Caballero-RabascoMAet al. Viral coinfection in childhood respiratory tract infections. Arch. Bronconeumol, 51(1), 5–9 (2015).
- Tran DN , TrinhQD, PhamNTet al. Clinical and epidemiological characteristics of acute respiratory virus infections in Vietnamese children. Epidemiol. Infect., 144(3), 527–536 (2016).
- Wen S , LvF, ChenXet al. Application of a nucleic acid-based multiplex kit to identify viral and atypical bacterial aetiology of lower respiratory tract infection in hospitalized children. J. Med. Microbiol., 68(8), 1211–1218 (2019).
- Huang H , ChenS, ZhangX, HongLet al. Detection and clinical characteristics analysis of respiratory viruses in hospitalized children with acute respiratory tract infections by a GeXP-based multiplex-PCR assay. J. Clin. Lab. Anal. doi:10.1002/jcla.23127e23127 (2019) ( Epub ahead of print).
- Del Valle Mendoza J , Cornejo-TapiaA, WeilgPet al. Incidence of respiratory viruses in Peruvian children with acute respiratory infections. J. Med. Virol., 87(6), 917–924 (2015).
- Suryadevara M , CummingsE, BonvilleCAet al. Viral etiology of acute febrile respiratory illnesses in hospitalized children younger than 24 months. Clin. Pediatr. (Phila), 50(6), 513–517 (2011).
- Harada Y , KinoshitaF, YoshidaLMet al. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr. Infect. Dis. J., 32(5), 441–445 (2013).
- Sentilhes AC , ChoumlivongK, CelhayOet al. Respiratory virus infections in hospitalized children and adults in Lao PDR. Influenza Other Respir. Viruses, 7(6), 1070–1078 (2013).
- Cantais A , MoryO, PilletSet al. Epidemiology and microbiological investigations of community-acquired pneumonia in children admitted at the emergency department of a university hospital. J. Clin. Virol., 60(4), 402–407 (2014).
- Trenholme AA , BestEJ, VogelAM, StewartJM, MillerCJ, LennonDR. Respiratory virus detection during hospitalisation for lower respiratory tract infection in children under 2 years in South Auckland, New Zealand. J. Paediatr. Child Health, 53(6), 551–555 (2017).
- Van Der Zalm MM , UiterwaalCS, WilbrinkBet al. Respiratory pathogens in respiratory tract illnesses during the first year of life: a birth cohort study. Pediatr. Infect. Dis. J., 28(6), 472–476 (2009).
- Gulen F , YildizB, CicekCet al. Ten year retrospective evaluation of the seasonal distribution of agent viruses in childhood respiratory tract infections. Turk. Pediatri. Ars., 49(1), 42–46 (2014).
- Khor CS , SamIC, HooiPSet al. Epidemiology and seasonality of respiratory viral infections in hospitalized children in Kuala Lumpur, Malaysia: a retrospective study of 27 years. BMC Pediatr., 12, 32 (2012).
- Ahmed A , HaiderSH, ParveenSet al. Co-circulation of 72bp duplication group A and 60bp duplication group b respiratory syncytial virus (RSV) strains in Riyadh, Saudi Arabia during 2014. PLoS One, 11(11), e0166145 (2016).
- Al-Hassinah S , ParveenS, SomilyAMet al. Evolutionary analysis of the ON1 genotype of subtype a respiratory syncytial virus in Riyadh during 2008–16. Infect. Genet. Evol., 79, 104153 (2020).
- Richter J , PanayiotouC, TryfonosCet al. Aetiology of acute respiratory tract infections in hospitalised children in Cyprus. PLoS One, 11(1), e0147041 (2016).
- Chen Y , CuiD, ZhengSet al. Simultaneous detection of influenza A, influenza B, and respiratory syncytial viruses and subtyping of influenza A H3N2 virus and H1N1 (2009) virus by multiplex real-time PCR. J. Clin. Microbiol., 49(4), 1653–1656 (2011).
- Malhotra B , SwamyMA, ReddyPVet al. Evaluation of custom multiplex real-time RT-PCR in comparison to fast-track diagnostics respiratory 21 pathogens kit for detection of multiple respiratory viruses. Virol. J., 13, 91 (2016).
- Bhuiyan MU , SnellingTL, WestRet al. The contribution of viruses and bacteria to community-acquired pneumonia in vaccinated children: a case-control study. Thorax, 74(3), 261–269 (2019).
- Wang H , ZhengY, DengJet al. Prevalence of respiratory viruses among children hospitalized from respiratory infections in Shenzhen, China. Virol. J., 13, 39 (2016).
- Krishnan A , KumarR, BroorSet al. Epidemiology of viral acute lower respiratory infections in a community-based cohort of rural north Indian children. J. Glob. Health, 9(1), 010433 (2019).
- Sandkovsky U , VargasL, FlorescuDF. Adenovirus: current epidemiology and emerging approaches to prevention and treatment. Curr. Infect. Dis. Rep., 16(8), 416 (2014).
- Yao LH , WangC, WeiTLet al. Human adenovirus among hospitalized children with respiratory tract infections in Beijing, China, 2017–2018. Virol. J., 16(1), 78 (2019).
- Zhao Y , LuR, ShenJet al. Comparison of viral and epidemiological profiles of hospitalized children with severe acute respiratory infection in Beijing and Shanghai, China. BMC Infect. Dis., 19(1), 729 (2019).
- Lin CY , HwangD, ChiuNCet al. Increased detection of viruses in children with respiratory tract infection using PCR. Int. J. Environ. Res. Public Health, 17(2), (2020).
- Fillatre A , FrancoisC, SegardCet al. Epidemiology and seasonality of acute respiratory infections in hospitalized children over four consecutive years (2012–2016). J. Clin. Virol., 102, 27–31 (2018).
- Wu PQ , ZengSQ, YinGQet al. Clinical manifestations and risk factors of adenovirus respiratory infection in hospitalized children in Guangzhou, China during the 2011–2014 period. Medicine (Baltimore), 99(4), e18584 (2020).
- Zou L , ZhouJ, LiHet al. Human adenovirus infection in children with acute respiratory tract disease in Guangzhou, China. APMIS, 120(8), 683–688 (2012).
- WHO . Fact sheets. Influenza (seasonal) (2019). http://www.who.int/mediacentre/factsheets/fs211/en/
- Essa S , OwayedA, AltawalahHet al. Mixed viral infections circulating in hospitalized patients with respiratory tract infections in kuwait. Adv. Virol., 2015, 714062 (2015).
- Adam K , PangestiKN, SetiawatyV. Multiple viral infection detected from influenza-like illness cases in Indonesia. Biomed. Res. Int., 2017, 9541619 (2017).
- Luksic I , KearnsPK, ScottFet al. Viral etiology of hospitalized acute lower respiratory infections in children under 5 years of age – a systematic review and meta-analysis. Croat. Med. J., 54(2), 122–134 (2013).
- Almajhdi FN , AlshamanMS, AmerHM. Human parainfluenza virus type 2 hemagglutinin-neuramindase gene: sequence and phylogenetic analysis of the Saudi strain Riyadh 105/2009. Virol. J., 9, 316 (2012).
- Zhu R , GuoC, ZhaoLet al. Epidemiological and genetic characteristics of human metapneumovirus in pediatric patients across six consecutive seasons in Beijing, China. Int. J. Infect. Dis., 91, 137–142 (2020).
- Renois F , TalmudD, HugueninAet al. Rapid detection of respiratory tract viral infections and coinfections in patients with influenza-like illnesses by use of reverse transcription-PCR DNA microarray systems. J. Clin. Microbiol., 48(11), 3836–3842 (2010).
- Hasman H , PachuckiCT, UnalAet al. Aetiology of influenza-like illness in adults includes parainfluenzavirus type 4. J. Med. Microbiol., 58(Pt. 4), 408–413 (2009).
- Lu Y , WangS, ZhangLet al. Epidemiology of human respiratory viruses in children with acute respiratory tract infections in Jinan, China. Clin. Dev. Immunol., 2013, 210490 (2013).
- Tecu C , MihaiME, AlexandrescuVIet al. Single and multipathogen viral infections in hospitalized children with acute respiratory infections. Roum. Arch. Microbiol. Immunol., 72(4), 242–249 (2013).
- Van Woensel JB , BosAP, LutterR, RossenJW, SchuurmanR. Absence of human metapneumovirus co-infection in cases of severe respiratory syncytial virus infection. Pediatr. Pulmonol., 41(9), 872–874 (2006).
- Williams JV , HarrisPA, TollefsonSJet al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med., 350(5), 443–450 (2004).
- Canducci F , DebiaggiM, SampaoloMet al. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J. Med. Virol., 80(4), 716–723 (2008).
- Xepapadaki P , PsarrasS, BossiosAet al. Human metapneumovirus as a causative agent of acute bronchiolitis in infants. J. Clin. Virol., 30(3), 267–270 (2004).
- Kim JK , JeonJS, KimJW, RheemI. Epidemiology of respiratory viral infection using multiplex rt-PCR in Cheonan, Korea (2006–2010). J. Microbiol. Biotechnol., 23(2), 267–273 (2013).
- Drews AL , AtmarRL, GlezenWPet al. Dual respiratory virus infections. Clin. Infect. Dis., 25(6), 1421–1429 (1997).
- Cebey-Lopez M , HerbergJ, Pardo-SecoJet al. Viral co-infections in pediatric patients hospitalized with lower tract acute respiratory infections. PLoS One, 10(9), e0136526 (2015).
- Cebey-Lopez M , HerbergJ, Pardo-SecoJet al. Does viral co-infection influence the severity of acute respiratory infection in children? PLoS One, 11(4), e0152481 (2016).
- Marcos MA , RamonS, AntonAet al. Clinical relevance of mixed respiratory viral infections in adults with influenza A H1N1. Eur. Respir. J., 38(3), 739–742 (2011).
- Esposito S , DalenoC, PrunottoGet al. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influenza Other Respir. Viruses, 7(1), 18–26 (2013).
- Franz A , AdamsO, WillemsRet al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J. Clin. Virol., 48(4), 239–245 (2010).
- Paranhos-Baccala G , Komurian-PradelF, RichardNet al. Mixed respiratory virus infections. J. Clin. Virol., 43(4), 407–410 (2008).
- Brittain-Long R , AnderssonLM, OlofssonSet al. Seasonal variations of 15 respiratory agents illustrated by the application of a multiplex polymerase chain reaction assay. Scand. J. Infect. Dis., 44(1), 9–17 (2012).
- Tang LF , WangTL, TangHF, ChenZM. Viral pathogens of acute lower respiratory tract infection in China. Indian Pediatr., 45(12), 971–975 (2008).
- Kim CK , ChoiJ, CallawayZet al. Clinical and epidemiological comparison of human metapneumovirus and respiratory syncytial virus in seoul, Korea, 2003–2008. J. Korean Med. Sci., 25(3), 342–347 (2010).
- Jeon JH , HanM, ChangHEet al. Incidence and seasonality of respiratory viruses causing acute respiratory infections in the northern United Arab Emirates. J. Med. Virol., 91(8), 1378–1384 (2019).
- Khamis FA , Al-KobaisiMF, Al-AreimiWSet al. Epidemiology of respiratory virus infections among infants and young children admitted to hospital in Oman. J. Med. Virol., 84(8), 1323–1329 (2012).
- Ge X , GuoY, ChenJ, HuR, FengX. Epidemiology and seasonality of respiratory viruses detected from children with respiratory tract infections in Wuxi, East China. Med. Sci. Monit., 24, 1856–1862 (2018).
- Monto AS . Occurrence of respiratory virus: time, place and person. Pediatr. Infect. Dis. J., 23(Suppl. 1), S58–64 (2004).
- Du Prel JB , PuppeW, GrondahlBet al. Are meteorological parameters associated with acute respiratory tract infections? Clin. Infect. Dis., 49(6), 861–868 (2009).
