Abstract
Aim: Human papillomavirus (HPV) is the most common cause of high-grade lesions and carcinogenesis such as cervical intraepithelial neoplasia (CIN) and cervical cancer (CC). The prevalence and genotype distribution of HPV infection varies greatly in different geographical areas. Patients & methods: This study enrolled 92,932 patients from January 2017 to December 2021 and analyzed the prevalence and distribution of HPV genotypes. Results: 18038 (19.41%) specimens were HPV-positive. No significant difference in infection rates between men and women (19.05 vs 19.41%). The most prevalent HPV subtypes are HPV52, HPV58, HPV16, HPV53, HPV51 and HPV81. Single infection of HPV has dominated in HPV-positive patients. Conclusion: Our results show that the prevalence and distribution of HPV subtypes have obvious region-specific and age-specific characteristics.
Human papillomavirus (HPV) was originally called the ‘human warts virus’ because it can cause genital warts and laryngeal papillomatosis [Citation1]. In the 1970s, Harald Zur Hausen isolated HPV 16 and 18 from cervical cancer (CC) biopsy samples and proposed their possible carcinogenic effects in subsequent studies [Citation2–7]. Recently, HPV has been increasingly confirmed to exhibit carcinogenic potential in cervical cancer, anogenital malignancies, upper respiratory tract cancers and tumors in other sites [Citation8–10]. HPV is mainly transmitted through sexual contact, but there are also non-sexual transmission routes, including horizontal transmission, self-inoculation and vertical transmission [Citation11]. The horizontal transmission of HPV mainly refers to fingers, fomites and mouth to skin contact (other than sexual) [Citation12]. Recently, self-inoculation has been reported as a potential HPV transmission route in children and females without a history of sexual activity [Citation13]. Transmission of HPV from mother to newborn is known as vertical transmission.
Most HPV-infected women and men have no obvious clinical symptoms. However, recurrent or persistent HPV infection is considered to be the most common cause of high-grade lesions and carcinogenesis such as cervical intraepithelial neoplasia (CIN), CC and other malignancies [Citation14,Citation15]. According to the carcinogenicity for CC, HPV subtypes can be divided into high-risk HPV (HR-HPV), potentially high-risk HPV (PHR-HPV) and low-risk HPV (LR-HPV). In 2012, the International Agency for Research on Cancer (IARC) designated the subtypes of HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, HPV66 and HPV68 as high-risk HPVs [Citation16]. Infection with HR-HPV or PHR-HPV is the main risk for cancers, while an LR-HPV infection usually leads to genital condyloma acuminatum. However, more and more HPV subtypes have been confirmed to be closely related to other cancers such as head and neck cancer, oral squamous cell carcinoma, bladder cancer, skin cancer and penile cancer in men. Among the common HPV subtypes, HPV16, HPV18, HPV33, HPV35 and HPV56 have been reported to be associated with head and neck cancer [Citation17]; HPV16 and HPV18 are associated with oral squamous cell carcinoma [Citation18]; HPV6, HPV 11, HPV16, HPV 18, HPV 31 and HPV 33 are associated with bladder cancer [Citation19]; while infection with HPV5, HPV8, HPV 15, HPV17, HPV20, HPV24, HPV36 and HPV38 increases the risk of skin cancer [Citation20]. Male infection with HPV6, HPV 11, HPV 16 and HPV18 has also been found to be associated with penile cancer [Citation21]. Evidence shows that the incidence of HPV infections in males has risen in recent years [Citation22,Citation23]. Therefore, it is important to detect HPV genotypes not only in females, but also in males, to prevent and treat cervical cancer.
HPV prevalence and genotype distribution in different countries and geographical regions are substantially varied. It has been reported that HPV58 and HPV52 were the most common subtypes in Asians [Citation24]. However, in China, a recent meta-analysis study demonstrated the prevalence of HPV genotypes HPV16 (34.56–36.61%), HPV58 (14.36–15.90%), HPV52 (14.01–15.53%) and HPV33 (7.31–8.48%) among women with cervical lesions. In addition, differences in prevalence and distribution of HPV genotypes were observed in different geographical regions of China. At present, relevant studies have been conducted in Beijing [Citation25], Hengyang [Citation26], Nanjing [Citation27], Yangzhou [Citation28] and Chongqing [Citation29].
Here, this study retrospectively analyzed the prevalence and distribution of HPV genotypes in 92,932 patients in central Shanghai from January 2017 to December 2021, providing references for early prevention and treatment of cervical cancer, male HPV detection and application of a HPV vaccine in Shanghai city.
Materials & methods
Reagents & instruments
The nucleic acid extraction kit and HPV genotyping detection kit were purchased from Ningbo Hershi Gene Technology Co., LTD (Ningbo, China). DNA extraction instrument (model: Smart LabAssist-32) was purchased from Taiwan Dot Nanotechnology Co., Ltd, and the PCR amplification instrument (model: A300) was purchased from Hangzhou Langji Scientific Instrument Co., LTD (Hangzhou, China). HPV genotyping was performed using a Beckman Coulter GenomeLab GeXP genetic analysis system (CA, USA).
Patients & specimen collection
Clinical specimens were collected from 92,932 outpatients at Changning Maternal and Infant Health Hospital from January 2017 to December 2021. For female patients, cervical exfoliated cell samples were scraped by a cytobrush and stored in a cell preservation solution. For male patients, exfoliated cells are usually collected from the penile head, inner prepuce plate and around the coronal sulcus. All protocols were approved by the ethics committee of Changning Maternal and Infant Health Hospital and patients involved in this study gave their informed consent.
DNA extraction
Ningbo Health DNA extraction kits (Ningbo Health Gene Technology Co., Ltd) were used to extract DNA from the exfoliated cells. According to the manufacturer’s instructions, the sample of exfoliated cells was swirled on the vortex mixer for 10 s. Then, 1 ml of the cell preservation solution sample was transferred into a 1.5 ml Eppendorf tube and centrifuged at 12,000 × g for 5 min. The supernatant was carefully removed and 200 μl 1× PBS buffer was added into the tube. The subsequent DNA extraction procedures were completed on the automatic DNA extraction instrument.
PCR amplification
The extracted DNA was used as a PCR reaction template. The PCR amplification system contained an 11 μl DNA template, 9 μl HPV PCR mix and 2 μl Taq DNA polymerase. PCR amplification procedures were as follows: pre-denaturation at 94°C for 8 min; denaturation at 94°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 1 min, repeated for 35 cycles; and final extension at 72°C for 1 min. Stored amplification products were used for capillary electrophoresis.
Capillary electrophoresis
28.7 μl SLS loading buffer, 0.3 μl DNA Size Standard-400, 1 μl PCR product and one drop of mineral oil were added to each sample well. Then, 220 μl of separation buffer was added to the appropriate position on the 96-well separation plate. All operations were carried out strictly in accordance with the manufacturer’s instructions. The HPV genotyping method is based on sequencing technology, the gold standard for genotyping. The detection results were consistent with the sequencing (data not shown).
Statistical analysis
The statistical analysis was performed using SPSS version 16.0 statistical software (SPSS Inc., IL, USA). The chi-square test (χ2 test) was used to compare count data between groups. p-values less than 0.05 were considered to be statistically significant.
Results
The overall infection of HPV
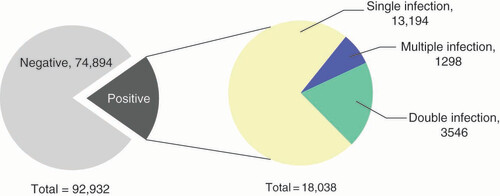
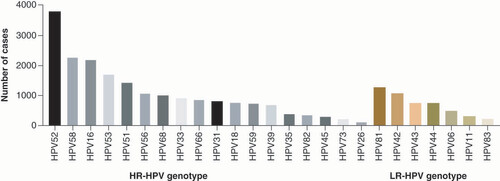
In this study, 19.41% (18,038/92,932) of patients demonstrated HPV infection (). The HPV infection rate in male and female patients was 19.05% (144/756) and 19.41% (17,894/92,176), respectively, with no significant difference between men and women (χ2 = 0.06; p = 0.80 > 0.05). Among HPV-positive cases, the infection rate of HR-HPV was 16.61% (15,438/92,932) and the infection rate of LR-HPV was 4.82% (4482/92,932). The most common genotypes of HPV included five HR-HPV which were HPV52 (4.08%), HPV58 (2.45%), HPV16 (2.38%), HPV53 (1.84%) and HPV51 (1.56%), and one LR-HPV that was HPV81 (1.37%) ().
Multiple HPV infections
The rates of total, single, double and multiple HPV infections were analyzed over the calendar year from 2017 to 2021. Over the past five years, the total and single HPV infection rates showed an increasing trend and significant differences were found over the calendar year (linear-by-linear association test) (). However, in the past 5 years, the double and multiple HPV infection rates exhibited no significant differences ().
Table 1. The rates of single, double and multiple human papillomavirus infection from 2017 to 2021.
Annual analysis of HPV subtype infection
To further analyze the annual HPV subtype infections, we discovered there were significant differences in HPV16, HPV18, HPV26, HPV33, HPV35, HPV39, HPV53, HPV56, HPV68, HPV73, HPV43, HPV81 and HPV83 infection based on the year (linear-by-linear association test, p < 0.05) (). Moreover, the infection rates of HPV16, HPV26, HPV11 and HPV83 subtypes decreased with the year (gamma value < -0.100), while HPV39 infection showed an uptrend (gamma value > 0.100) ().
Table 2. Distribution of human papillomavirus subtypes infection in different years.
Age distribution of HPV subtypes infection
In the present research, we analyzed the distribution of 25 HPV genotypes among HPV-infected individuals. Among 17894 female and 144 male patients, the proportion of HPV16, HPV33, HPV52 and HPV58 in HPV-positive women was significantly higher than that in men, while the percentage of HPV39, HPV82 and HPV43 was significantly lower than that in men (p < 0.05) ().
Table 3. The infection of human papillomavirus subtypes in females and males.
HPV-positive females were further divided into six groups according to age to analyze and compare the differences in the distribution of HPV subtypes in different age groups. As shown in , the total HPV-infected females aged <20, 20–29, 30–39, 40–49, 50–59 and >60 years old were 118 (0.66%, 118/17,894), 3559 (19.89%, 3559/17,894), 6905 (38.59%, 6905/17,894), 3635 (20.31%, 3635/1789), 2259 (12.62%, 2259/17,894) and 1418 (7.92%, 1418/17,894), respectively. The distribution of HPV16, HPV18, HPV33, HPV45, HPV51, HPV52, HPV53, HPV58, HPV59, HPV66, HPV82, HPV6, HPV11, HPV42, HPV44, HPV81 and HPV83 was significantly different among women of different age groups (p < 0.05) (). Moreover, the distribution of HPV18, HPV45, HPV51, HPV59, HPV66, HPV82, HPV6, HPV44 and HPV83 infection among females <20 years old was significantly higher than that in other age groups (p < 0.05) (). In contrast, the distribution of HPV52, HPV53 and HPV42 was obviously lower in women aged below 20 than in other age groups (p < 0.05) (). In addition, this study indicated that the distribution of HPV18, HPV45, HPV51, HPV59, HPV82, HPV6, HPV11, HPV44 and HPV83 showed a greater trend in the younger population, while HPV44 and HPV81 were more frequent in the older population ().
Table 4. Distribution of human papillomavirus subtypes in female patients in different age groups.
Distribution of HPV subtypes in different diseases
To analyze the distribution characteristics of HPV subtypes in different diseases, we divided HPV-infected patients into five groups according to the final diagnosis: Normal condition, Inflammation, CIN1, CIN2/3 and Cancer. The distribution of HPV subtypes was different in the different disease groups. There were significant differences in the distribution of HPV16, HPV18, HPV52, HPV56, HPV58, HPV73, HPV42 and HPV83 in patients with different diseases (p < 0.05), and the infection rate of HPV73 and HPV83 decreased with the aggravation of cervical lesions (gamma value < -0.100) ().
Table 5. Distribution of human papillomavirus subtypes according to the final diagnosis.
Discussion
HPV is a circular, closed and double-stranded DNA virus that usually infects human mucosal and skin epithelial cells [Citation25,Citation30]. To date, over 220 subtypes of HPV have been identified (https://pave.niaid.nih.gov). The prevalence and subtype distribution of HPV has obvious regional characteristics. This study analyzed the prevalence and genotype distribution of HPV among patients in the Changning District, Shanghai. Our data revealed the average HPV infection was 19.41% in patients admitted at Changning Maternity and Infant Health Hospital over the past 5 years. The total HPV infection rate in this study was higher than that in Taiyuan (8.92%) [Citation31], Hengyang (10.16%) [Citation26], Zhengzhou (12.09%) [Citation32] and Xinjiang (14.02%) [Citation33], but lower than that in Zhejiang (22.3%) [Citation34], Hangzhou (22.41%) [Citation35], Shandong (28.4%) [Citation36] and Sichuan (31.5%) [Citation37]. Differences in HPV infection rates were partly related to population samples and geographical differences in China. In addition, we also found the total and single HPV infection rates in the Changning area showed an increasing trend over the past five years, whereas there was no significant change in double and multiple HPV infection rates. Our findings are consistent with those of other Chinese scholars [Citation25,Citation38,Citation39].
In view of the significant differences in the prevalence of HPV subtypes among the population, we further analyzed the characteristics of HPV subtype infection in the Changning area. Our data indicated that the most prevalent HPV subtypes were HPV52, HPV58 and HPV16 in the central area of Shanghai, which was different from the dominant HPV subtypes in western Shanghai [Citation40,Citation41]. In other cities in China, the prevalent HPV subtypes also varied greatly. For example, HPV52, HPV16 and HPV58 were the most common subtypes in Yunnan and Huzhou [Citation42,Citation43], HPV16, HPV52 and HPV58 in Beijing [Citation44], HPV16, HPV58 and HPV52 in Hunan [Citation26], HPV16, HPV52 and HPV18 in Hangzhou [Citation35], and HPV52, HPV16 and HPV53 in Taizhou and Xi’an [Citation45,Citation46].
Evidence showed that the prevalence of HPV subtypes is changing over time, possibly in part due to HPV vaccination in the population. In recent years, there have been different trends in the prevalence of different HPV subtypes. Our data suggested the infection rates of two HR-HPV (HPV16 and HPV26) and two LR-HPV (HPV11 and HPV83) exhibited a downward trend, while HPV39 infections showed an uptrend. Therefore, it has great significance to analyze the annual prevalence and distribution of HPV for formulating vaccination strategies.
An important factor related to HPV infection may be the age of patients. In our study, HPV-positive women aged 30–39 years accounted for the largest proportion (38.59%) of HPV infections and those younger than 20 years had the lowest proportion (0.66%). Our study shows that the HPV infection rate of young women is lower than that of older women, which is inconsistent with previous reports [Citation25,Citation47,Citation48]. This may be related to the smaller sample of young women in this study and the lower HPV detection rate among young women. In addition, our data suggested young women may be more susceptible to HPV18, HPV45, HPV51, HPV59, HPV66, HPV82, HPV6, HPV44 and HPV83. However, women over 40 years of age are more susceptible to HPV52, HPV53 and HPV81. In addition, the lower prevalence of HPV16 and 18 subtypes in women over 60 years old may be related to their decreased sexual behavior and regular HPV screening in Shanghai. Therefore, it is necessary to develop precise HPV vaccination strategies for women of different ages.
Persistent or repeated HPV infection is an important risk factor for cervical cancer and precancerous lesions [Citation15,Citation49]. Infection with HPV subtypes varies at different stages of disease progression. The infection rate of HPV16, HPV18 and HPV52 was significantly higher in the CC group, and the infection rate of HPV58 was higher in the CIN2/3 group. Additionally, the prevalence of HPV73 and HPV83 infection decreased with increasing cervical lesions. Since HPV16, HPV18 and HPV52 are typical HR-HPV subtypes, persistent infection of HR-HPV has been considered an independent risk factor for cervical cancer in women. Our findings are consistent with previous reports. Moreover, in this study, there were only 18 cases of cervical cancer with HPV multi-infection. However, due to the small sample size, no reliable conclusions can be drawn. Our results suggested that infection and prevalence of HPV subtypes differ in their prevalence and role in the disease process. Regular analysis of HPV infection and subtype distribution is necessary to understand human infection and develop vaccines.
In the present study, we also found the proportion of HPV16, HPV33, HPV52 and HPV58 in HPV-infected females was significantly higher than that in men, while the proportion of HPV39, HPV82 and HPV43 was significantly lower than in men. Due to the difference in the physiological structure of the male and female reproductive systems, the infection rate of HPV16, HPV52 and other HPV subtypes in males is relatively low. However, the reasons for the higher prevalence of HPV39, HPV82 and HPV43 in men are not clear. Given the small number of male patients, no further analysis of HPV subtype infection was performed by disease and age.
Although this study retrospectively analyzed and discovered the characteristics of HPV infection and subtype distribution in 92,932 patients in central Shanghai, there are still some unavoidable limitations. First of all, we lack more detailed demographic and clinical data, such as the patient’s sexual behavior, clinicopathological diagnosis data, clinical outcomes, etc., which makes it impossible to analyze the sexual behavior characteristics, clinicopathological grade characteristics and clinical outcomes of HPV-infected patients. Secondly, this study did not explore potential risk factors such as smoking, alcohol consumption and immune status for HPV infection. Moreover, the sample size of cervical cancer patients was very small, only 100 cases, resulting an inability to draw reliable conclusions from the the analysis of HPV infection and subtype distribution of cervical cancer patients. In follow-up research, we will pay attention to these factors and HPV vaccination status.
Conclusion
In conclusion, the present study exhibited a high prevalence of HPV52, HPV58, HPV16, HPV53, HPV51 and HPV81 in the Changning area of Shanghai, China. Single HPV infections were dominant in patients admitted to Changning Maternity and Infant Health Hospital. There was no significant difference in the infection rates between men and women. The prevalence and distribution of HPV subtypes have obvious region-specific and age-specific characteristics. It is necessary to investigate and analyze the subtype distribution of HPV infection in this region to prevent and treat cervical cancer and related diseases, develop targeted HPV vaccines and promote the screening of male patients.
This study retrospectively analyzed the prevalence and distribution of HPV genotypes in 92,932 patients in central Shanghai from January 2017 to December 2021, providing references for early prevention and treatment of cervical cancer, male HPV detection and application of HPV vaccine in Shanghai city.
Among all specimens, 18038 (19.41%) were HPV-positive. There was no significant difference in infection rates between men and women (19.05 vs 19.41%). The most prevalent HPV subtypes were HPV52, HPV58, HPV16, HPV53, HPV51 and HPV81. Single infection of HPV dominated in HPV-positive patients. The infection rates of HPV16, HPV26, HPV11 and HPV83 subtypes decreased with the year, while HPV39 infection showed an uptrend.
Among 17894 female and 144 male patients, the proportion of HPV16, HPV33, HPV52 and HPV58 in HPV-positive women was significantly higher than in men, while the percentage of HPV39, HPV82 and HPV43 was significantly lower than in men. The distribution of HPV18, HPV45, HPV51, HPV59, HPV66, HPV82, HPV6, HPV44 and HPV83 infection among females under 20 years old was significantly higher than that in other age groups. In contrast, the distribution of HPV52, HPV53 and HPV42 was obviously lower in women aged below 20 than in other age groups.
There were significant differences in the distribution of HPV16, HPV18, HPV52, HPV56, HPV58, HPV73, HPV42 and HPV83 in patients with different diseases, and the infection rate of HPV73 and HPV83 decreased with the aggravation of cervical lesions.
There was a high prevalence of HPV52, HPV58, HPV16, HPV53, HPV51 and HPV81 in the Changning area of Shanghai, China. Single HPV infections were dominant in patients admitted to Changning Maternity and Infant Health Hospital.
There was no significant difference in the infection rates between men and women. The prevalence and distribution of HPV subtypes have obvious region-specific and age-specific characteristics.
Financial & competing interests disclosure
This work was partially funded by the National Natural Science Foundation of China (no. 81972709 and no. 82211530434), the Foundation of Changning District Medical Doctor Innovative Talent Base, Shanghai, China (no. RCJD2021B08) and the Internal Foundation of Changning Maternity and Infant Health Hospital (no. 2023Y-7). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Ntanasis-Stathopoulos I , KyriazoglouA , LiontosM , DimopoulosMA , GavriatopoulouM. Current trends in the management and prevention of human papillomavirus (HPV) infection. J. BUON25(3), 1281–1285 (2020).
- Zur Hausen H , GissmannL , SteinerW , DippoldW , DregerI. Human papilloma viruses and cancer. Bibl. Haematol. (43), 569–571 (1975).
- Bosch FX , DurstM , SchwarzE , BoukampP , FusenigNE , ZurHausen H. The early genes E6 and E7 of cancer associated human papilloma viruses as targets of tumor suppression?Behring Inst. Mitt. (89), 108–121 (1991).
- Dust K , CarpenterM , ChenJCet al. Human papillomavirus 16 E6 and E7 oncoproteins alter the abundance of proteins associated with DNA damage response, immune signaling and epidermal differentiation. Viruses14(8), (2022).
- Durst M , GissmannL , IkenbergH , ZurHausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl Acad. Sci. USA80(12), 3812–3815 (1983).
- Vieira GV , SomeraDos Santos F , LepiqueAPet al. Proteases and HPV-induced carcinogenesis. Cancers (Basel)14(13), (2022).
- Bhattacharjee R , DasSS , BiswalSSet al. Mechanistic role of HPV-associated early proteins in cervical cancer: Molecular pathways and targeted therapeutic strategies. Crit. Rev. Oncol. Hematol.174, 103675 (2022).
- Chelimo C , WouldesTA , CameronLD , ElwoodJM. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J. Infect66(3), 207–217 (2013).
- Berman TA , SchillerJT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer123(12), 2219–2229 (2017).
- Sofiani VH , VeisiP , RukerdMRZ , GhaziR , NakhaieM. The complexity of human papilloma virus in cancers: a narrative review. Infect Agent Cancer18(1), 13 (2023).
- Petca A , BorislavschiA , ZvancaME , PetcaRC , SandruF , DumitrascuMC. Non-sexual HPV transmission and role of vaccination for a better future (review). Exp. Ther. Med.20(6), 186 (2020).
- Gallay C , MirandaE , SchaeferSet al. Human papillomavirus (HPV) contamination of gynaecological equipment. Sex Transm. Infect92(1), 19–23 (2016).
- Mammas IN , DalianisT , DoukasSGet al. Paediatric virology and human papillomaviruses: an update. Exp. Ther. Med.17(6), 4337–4343 (2019).
- Zhao D , ZhangL , XieFet al. Outcomes of prior cervical cytology and HR-HPV testing in women subsequently diagnosed with CIN1, CIN2/3, and invasive cervical cancer: a 4-year routine clinical experience after implementation of systematic training and quality control programs. BMC Cancer20(1), 810 (2020).
- Branca M , CiottiM , GiorgiCet al. Up-regulation of proliferating cell nuclear antigen (PCNA) is closely associated with high-risk human papillomavirus (HPV) and progression of cervical intraepithelial neoplasia (CIN), but does not predict disease outcome in cervical cancer. Eur. J. Obstet. Gynecol. Reprod. Biol.130(2), 223–231 (2007).
- Arbyn M , TommasinoM , DepuydtC , DillnerJ. Are 20 human papillomavirus types causing cervical cancer?J. Pathol.234(4), 431–435 (2014).
- Spence T , BruceJ , YipKW , LiuFF. HPV associated head and neck cancer. Cancers (Basel)8(8), (2016).
- Melo BaC , VilarLG , OliveiraNRet al. Human papillomavirus infection and oral squamous cell carcinoma – a systematic review. Braz. J. Otorhinolaryngol.87(3), 346–352 (2021).
- Sun JX , XuJZ , LiuCQet al. The association between human papillomavirus and bladder cancer: evidence from meta-analysis and two-sample mendelian randomization. J. Med. Virol.95(1), e28208 (2023).
- Gupta R , RadyPL , DoanHQ , TyringSK. Development of a beta-HPV vaccine: Updates on an emerging frontier of skin cancer prevention. J. Clin. Virol.126, 104348 (2020).
- Anic GM , GiulianoAR. Genital HPV infection and related lesions in men. Prev. Med.53(Suppl. 1), S36–S41 (2011).
- Liu M , HeZ , ZhangCet al. Prevalence, incidence, clearance, and associated factors of genital human papillomavirus infection among men: a population-based cohort study in rural China. Cancer Epidemiol. Biomarkers Prev.23(12), 2857–2865 (2014).
- Vives A , CosentinoM , PalouJ. The role of human papilloma virus test in men: First exhaustive review of literature. Actas Urol. Esp. (Engl. Ed.)44(2), 86–93 (2020).
- Li N , FranceschiS , Howell-JonesR , SnijdersPJ , CliffordGM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int. J. Cancer128(4), 927–935 (2011).
- Zhu X , WangY , LvZ , SuJ. Prevalence and genotype distribution of high-risk HPV infection among women in Beijing, China. J. Med. Virol.93(8), 5103–5109 (2021).
- Tang SY , LiaoYQ , HuY , ShenHY , WanYP , WuYM. HPV prevalence and genotype distribution among women from Hengyang District of Hunan Province, China. Front Public Health9, 710209 (2021).
- Zhang YY , XuXQ , ZhangD , WuJ , ZhangHX. Triage human papillomavirus testing for cytology-based cervical screening in women of different ages in primary hospitals: A retrospective clinical study. Medicine (Baltimore)99(38), e22320 (2020).
- Li Y , LiuX , HanC , RenC. Prevalence and genotype distribution of high-risk human papillomavirus in 34 420 cases in Yangzhou city, Jiangsu province, China. J. Med. Virol.93(8), 5095–5102 (2021).
- Yan L , YangJ , LongX , ZhouD. Epidemiological Characteristics of human papillomavirus (HPV) infection in different groups of women in Chongqing, China. Jpn. J. Infect Dis.74(4), 369–372 (2021).
- Dunne EF , ParkIU. HPV and HPV-associated diseases. Infect Dis. Clin. North Am.27(4), 765–778 (2013).
- Yang J , WangW , WangZet al. Prevalence, genotype distribution and risk factors of cervical HPV infection in Yangqu, China: a population-based survey of 10086 women. Hum. Vaccin. Immunother.16(7), 1645–1652 (2020).
- Liu J , MaS , QinCet al. Prevalence and genotype distribution of human papillomavirus in Zhengzhou, China, in 2016. Arch. Virol.165(3), 731–736 (2020).
- Wang J , TangD , WangKet al. HPV genotype prevalence and distribution during 2009–2018 in Xinjiang, China: baseline surveys prior to mass HPV vaccination. BMC Womens Health19(1), 90 (2019).
- Yan X , ShenL , XiaoY , WangQ , LiF , QianY. Prevalence, characteristics, and distribution of HPV genotypes in women from Zhejiang Province, 2016-2020. Virol. J.18(1), 208 (2021).
- Wang L , YuC , NiXet al. Prevalence characteristics of human papillomavirus (HPV) infection among women receiving physical examinations in the Shangcheng District, Hangzhou city, China. Sci. Rep.11(1), 16538 (2021).
- Jiang L , TianX , PengDet al. HPV prevalence and genotype distribution among women in Shandong Province, China: analysis of 94,489 HPV genotyping results from Shandong’s largest independent pathology laboratory. PLOS ONE14(1), e0210311 (2019).
- Chen Z , WangQ , DingX , LiQ , ZhongR , RenH. Characteristics of HPV prevalence in Sichuan Province, China. Int. J. Gynaecol. Obstet131(3), 277–280 (2015).
- Luo LP , HeP , LiuQTet al. Prevalence and genotype distribution of HPV infection among 214,715 women from Southern China, 2012–2018: baseline measures prior to mass HPV vaccination. BMC Infect Dis.21(1), 328 (2021).
- Tang Y , ZhengL , YangS , LiB , SuH , ZhangLP. Epidemiology and genotype distribution of human papillomavirus (HPV) in Southwest China: a cross-sectional five years study in non-vaccinated women. Virol. J.14(1), 84 (2017).
- Zhang C , ZhangC , HuangJ , WuZ , MeiX , ShiW. Prevalence and genotype distribution of human papillomavirus among females in the suburb of Shanghai, China. J. Med. Virol.90(1), 157–164 (2018).
- Li H , LiP , HuangL , SunL , RenH , LiP. Prevalence characteristics of cervical human papillomavirus (HPV) infection in the Zhoupu District, Shanghai City, China. Virol. J.17(1), 84 (2020).
- Li Z , LiuF , ChengSet al. Prevalence of HPV infection among 28,457 Chinese women in Yunnan Province, southwest China. Sci. Rep.6, 21039 (2016).
- Zhu Y , QianF , ZouWet al. Prevalence and genotype distribution of human papillomavirus infection in Huzhou City, eastern China, 2018-2019. Trans R. Soc. Trop. Med. Hyg.115(1), 30–37 (2021).
- Li M , DuX , LuMet al. Prevalence characteristics of single and multiple HPV infections in women with cervical cancer and precancerous lesions in Beijing, China. J. Med. Virol.91(3), 473–481 (2019).
- Han X , SongG , LiYet al. Prevalence and genotype distribution of human papillomavirus infection among women aged 30–65 years in Xi’an, China: a population-based study of 14,655 women. Hum. Vaccin. Immunother.17(12), 5439–5446 (2021).
- Jin R , QianH , ZhangYet al. The prevalence and genotype distribution of human papillomaviruses among women in Taizhou, China. Medicine (Baltimore)98(39), e17293 (2019).
- Kim MJ , KimJJ , KimS. Type-specific prevalence of high-risk human papillomavirus by cervical cytology and age: data from the health check-ups of 7,014 Korean women. Obstet. Gynecol. Sci.56(2), 110–120 (2013).
- Wang S , WeiH , WangNet al. The prevalence and role of human papillomavirus genotypes in primary cervical screening in the northeast of China. BMC Cancer12, 160 (2012).
- Owosho AA , WileyR , StansburyT , GbadamosiSO , RyderJS. Trends in human papillomavirus-related oropharyngeal squamous cell carcinoma incidence, Vermont 1999–2013. J. Comm. Health43(4), 731–737 (2018).