Abstract
This review highlights two rare entities that are predominantly seen in children: hepatic mesenchymal hamartoma (HMH) and undifferentiated embryonal sarcoma of the liver (UESL). HMH is a benign lesion predominantly seen in the first 2 years of life, while UESL is malignant and usually identified in patients between 6 and 10 years of age. UESL may arise in the background of HMH, and the association has been supported by similar chromosomal aberrations (19q13.4). The diagnosis of both lesions is primarily based on histologic evaluation, as the clinical and radiological features are not always typical. The clinicopathologic characteristics, pathogenesis, differential diagnoses and treatment for both lesions are discussed.
Hepatic mesenchymal hamartoma
Hepatic mesenchymal hamartoma (HMH) is the second most common benign liver tumor in children after infantile hemangioma [Citation1]. The lesion makes up 8% of all pediatric liver tumors, and 80% of the cases occur within the first 2 years of life [Citation2]. Prenatal and adult cases of HMH have also been described [Citation3–Citation6]. In pediatric series, HMH shows a slight male predominance [Citation7]. Meanwhile, adult HMH is more frequently seen in females [Citation5].
Patients with HMH generally present with abdominal distention and/or upper abdominal mass [Citation2,Citation8]. The right lobe of the liver is more frequently involved in the pediatric age group, whereas adults show a similar involvement of both lobes [Citation5]. The serum AFP levels are typically normal, although mild elevations are occasionally observed [Citation1]. The lesion tends to increase in size during the first several months and subsequently may either stabilize, continue to grow or regress [Citation1].
Prenatal HMH is usually detected by ultrasound in the third trimester. These lesions have been associated with polyhydramnios, fetal hydrops, intrauterine fetal demise and preterm labor [Citation3,Citation9]. In neonates, HMH may cause life-threatening abdominal distension and respiratory distress [Citation8].
Pathogenesis
Recurrent genetic alterations identified in HMH include androgenetic-biparental mosaicism (ABM) and chromosomal rearrangements which result in activation of chromosome 19q microRNA cluster (C19MC) [Citation10]. ABM, as seen in placental mesenchymal dysplasia and Beckwith-Wiedemann syndrome, is marked by paternal uniparental disomy. Meanwhile, sporadic HMH lesions are frequently associated with a translocation involving MALAT1 gene at chromosome 11q13 and C19MC gene at 19q13.4 [Citation11]. CM19C is exclusively expressed in the placenta and maternally imprinted. It encodes a cluster of 46 microRNAs from the paternal allele. In addition, El Demellawy et al. recently described an atypical HMH with a tandem triplication of chromosomal segment at chromosome 1q44 [Citation12].
Radiologic findings
The classic appearance of HMH on ultrasound is a complex cystic mass with internal septations; the cystic portions are anechoic or nearly anechoic with echogenic septa [Citation13,Citation14]. On CT and MRI, HMH demonstrates a complex cystic mass with septal and solid stromal enhancement. On MRI, high signal intensity of cystic components on T2-weighted images, with variable intensity on T1-weighted images is observed for the proteinaceous cyst fluid [Citation13,Citation14].
Pathologic findings
HMH may be pedunculated and its size varies greatly, ranging from a few centimeters up to greater than 30 cm [Citation2]. Although typically regarded as a solitary lesion, multifocal cases with satellite nodules have been reported [Citation7,Citation8,Citation15]. The cut surface shows solid and cystic components in varying proportions; these cystic structures do not communicate directly with the biliary tree and contain yellowish fluid with occasional gelatinous material. Significant necrosis or calcification is not commonly seen [Citation8].
Histologically, HMH is characterized by a lobular growth of myxomatous connective tissue containing scattered bland stellate-shaped mesenchymal cells (A). Branching bile ducts similar to ductal plate malformation are also present (B & ). Cystic degeneration and extramedullary hematopoiesis are occasionally seen; the larger cystic structures are usually not lined by epithelium (pseudocysts). Entrapped hepatocytes are often identified in the periphery of the lesion (D). Wu et al. argued that the intralesional hepatocytes are most likely to be derived from common progenitor cells and are a component of HMH rather than entrapped nonneoplastic hepatocytes [Citation16]. The unusual presence of immature hepatocytes with extensive steatosis has also been reported [Citation12]. Meanwhile, osseous metaplasia has been described in a case of recurrent HMH [Citation17]. Atypical mitoses and infiltrative growth pattern are not identified in HMH [Citation2].
Hepatic mesenchymal hamartoma is histologically characterized by (A) a multinodular growth of myxomatous mesenchymal stroma with intervening fibrous septa (hematoxylin & eosin, 4×); (B) each nodule consists of a bland spindle cell proliferation with scattered malformed bile ducts (hematoxylin & eosin, 10×). (C) Some of the nodules demonstrate a florid bile duct proliferation with scattered lymphocytic infiltrate (hematoxylin & eosin, 10×). (D) Entrapped island of hepatocytes (arrow) is occasionally identified in the periphery of the lesion (hematoxylin & eosin, 10×).
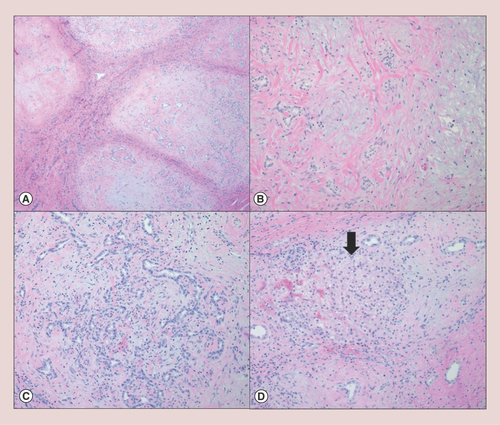
Diagnosis of HMH is generally made on the basis of hematoxylin and eosin alone. Immunohistochemical stains may be utilized to exclude other diagnostic considerations; the biliary epithelium and entrapped hepatocytes in HMH are immunoreactive for cytokeratin, while the pseudocysts and mesenchymal cells are positive for vimentin [Citation2]. Additionally, the mesenchymal cells can be immunoreactive to smooth muscle actin, α-1-antitrypsin and desmin [Citation8]. Fine needle aspirations are of limited value. The cytologic findings of HMH include clusters of cuboidal epithelial cells, spindle stromal cells, hepatocytes and loose fragments of myxoid connective tissue; however, it is difficult to exclude other entities such as hepatoblastoma and malignant mesenchymal neoplasms on a limited sample [Citation8].
Differential diagnosis
The radiologic diagnostic considerations include hepatic lesions with varying amounts of solid and cystic components, such as hepatoblastoma, undifferentiated embryonal sarcoma of the liver (UESL), hemangioma, biliary cystadenoma, teratoma and hepatic hydatid cyst [Citation2,Citation8]. Histologically, HMH should be distinguished from mixed epithelial-mesenchymal hepatoblastoma and UESL [Citation2].
Syndromic associations
Beckwith-Wiedemann syndrome (BWS), an overgrowth syndrome due to abnormal regulation of genes involving chromosome 11p15 and characterized by macroglossia, abdominal wall defects, ear abnormalities and hemihypertrophy, is associated with an increased risk for embryonic tumors [Citation18]. The most frequent tumors seen in BWS patients include Wilms tumor and hepatoblastoma. Rare cases of HMH in the setting of BWS have been reported [Citation19,Citation20]. In the setting of increased serum AFP levels, the lesions may be mistaken for hepatoblastoma.
The association between HMH and DICER1 syndrome was recently described by Apellaniz-Ruiz et al. [Citation21]. DICER1 syndrome is a tumor predisposition syndrome characterized by several dysontogenetic cystic conditions, such as pleuropulmonary blastoma and cystic nephroma [Citation22]. DICER1 mutations dysregulate microRNAs mimicking the effect of C19MC activation [Citation21]. However, the pathologic diagnosis of the lesions in this initial report was questioned, as the features described were not fully representative of HMH [Citation21,Citation23]. It is possible that these lesions represent a distinct hepatic manifestation of DICER1 mutations.
Association with other lesions
Placental mesenchymal dysplasia is characterized by stem villous cystic dilation, placentomegaly and vascular abnormalities [Citation24]. The association between HMH and placental mesenchymal dysplasia was initially described by Alwaidh et al. [Citation25]. Both entities share ABM as an underlying mechanism; paternal uniparental disomy results in imbalanced expression of imprinted loci in androgenetic cells which may lead to abnormal tissue phenotypes [Citation26]. Comorbidities of HMH and placental mesenchymal dysplasia have been associated with adverse fetal outcome.
The rare association between HMH and infantile hemangioma, the most common benign liver tumor in children, has been reported in single case reports and small case series [Citation27–Citation29]. This uncommon occurrence should be distinguished from HMH with prominent vascular components, as patients with hepatic infantile hemangioma may benefit from beta-blocker treatment [Citation8,Citation28]. Histologically, infantile hemangioma is characterized by back-to-back capillaries lined by plump, benign-appearing endothelial cells. The diagnosis of infantile hemangioma can be confidently made on the basis of GLUT1-positive endothelial cells.
Malignant transformation
Reports of UESL and angiosarcoma arising in HMH have been well documented [Citation30–Citation35]. Clinical and histological evidence has strongly suggested that UES can develop within a pre-existing HMH. Malignant transformation may occur several years after an incomplete resection of the lesions [Citation8]. Similar cytogenetic abnormalities have also been identified in HMH and UESL [Citation8,Citation33,Citation35,Citation36]. In practice, it is important to sample different portions of HMH to rule out area(s) of malignant transformation.
Treatment
Surgical resection is the mainstay treatment for HMH, although recurrence may occur in the setting of positive resection margins [Citation1,Citation2,Citation17]. Liver transplantation may be considered in unresectable cases [Citation37,Citation38]. Ultrasound-guided percutaneous cyst aspiration has been shown to improve the clinical course of prenatal cases [Citation2,Citation39]. Ultrasound-guided intraoperative aspiration of cystic fluid may also facilitate surgical excision of HMH cases with massive cystic components [Citation40]. In asymptomatic patients, careful monitoring may be appropriate as a subset of HMH lesions spontaneously regress [Citation2].
Undifferentiated embryonal sarcoma
Undifferentiated embryonal sarcoma (UESL) is a rare tumor, with an estimated incidence of one case per million people per year [Citation41]. Despite the low overall incidence, UESL is the most common sarcoma and the third most common hepatic malignancy in the pediatric population after hepatoblastoma and hepatocellular carcinoma (HCC) [Citation42,Citation43]. Most cases of UESL are diagnosed in the first decade of life, between 6 and 10 years of age, but several case reports have been described in adults and even elderly patients [Citation36,Citation41,Citation44–Citation48]. UESL shows no sex predilection in children and a slight female predominance in adults [Citation41,Citation45].
The clinical presentation of UESL often includes abdominal pain, fever and hepatomegaly, and only few patients are asymptomatic at diagnosis [Citation44,Citation45]. The lesion usually presents as a large (≥10 cm), solitary nodule in the right liver lobe; multifocal disease and involvement of the left lobe are less frequent [Citation36,Citation41–Citation45]. Extrahepatic spread is observed in 5–15% of all the patients and common metastatic site include the lung, diaphragm, heart and peritoneum [Citation41,Citation45,Citation49–Citation51]. Distant metastases are more common in adults than in pediatric patients [Citation45]. There is no known association between UESL and any tumor predisposition syndrome.
Pathogenesis
UESL derives from the mesenchyme, although a more precise histogenesis and its cell of origin are still to be defined. The carcinogenic mechanisms of UESL are also unclear. Genome-wide molecular analyses in a large cohort and/or experimental validation of putative molecular drivers are still lacking. Nevertheless, comparative genomic hybridization (CGH) studies suggested a role for chromosomal instability and showed that copy number alterations are common in UESL [Citation52,Citation53]. There were no copy number changes specific to UESL, which may suggest some degree of intertumor heterogeneity; recurrent events which have been reported in UESL include gains in chromosomes 1q, 5p, 6q and losses in chromosome 14, 9p and 11p [Citation52,Citation53].
UESL may also arise within HMH or demonstrate focal regions of HMH-like histology [Citation54–Citation56]. In such cases, genomic rearrangements that are typical of HMH including add(19)(q13.4) and t(11;19)(q11;q13.3–13.4) are also shared by the malignant (or more undifferentiated) regions of the tumor [Citation54,Citation56]. The findings suggest a genetic continuum between these lesions, in other words, some cases of HMH may indeed undergo malignant transformation into UESL. However, the absence of the HMH-associated genomic rearrangements in de novo UESL and the differences in DNA ploidy between the HMH and UESL regions in some of the bi-phenotypic tumors support additional molecular events being necessary for malignant transformation of HMH [Citation52–Citation54].
Specific point mutations have also been implicated in UESL development. Hu et al. and Lepreux et al. reported mutations in the DNA-binding domain of the TP53 gene, but only few cases were analyzed [Citation52,Citation57]. Kim et al. performed whole exome sequencing and in silico predictions in one UESL sample and suggested somatic mutations in WDR25, CMTM1 and DNAH17, among others, as putative drivers in this cancer [Citation58]. However, evaluation of additional cases and experimental validation of some of these mutations is necessary.
Radiologic findings
On ultrasounds, UESL appear as a solid mass, predominantly hyperechoic, with focal anechoic, cystic portions. Some cases may be predominantly anechoic (cystic), mimicking benign tumors [Citation59–Citation62]. Computed tomography (CT) often shows a single, well-demarcated and predominantly hypoattenuated, cystic mass, with internal septations [Citation63]. Presence of serpiginous vessels within tumors on CT scans of pediatric patients have been described as suggestive of UESL [Citation60,Citation61]. Of note, the discrepancy between the cystic appearance on CT scans and solid appearance on ultrasounds was also emphasized as a potential diagnostic clue for UESL [Citation60,Citation64,Citation65]. Finally, on magnetic resonance imaging (MRI), the lesion presents as a well-demarcated mass, hyperintense on T2 and hypointense on T1. MRI is regarded as the preferred pre-operative exam due to its accuracy in detecting vascular invasion, biliary obstruction and hilar adenopathy [Citation66].
Pathologic findings
Grossly, UESL typically presents as a large, spherical and well-demarcated mass located in the right liver lobe. Due to its predominantly expansive rather than infiltrative growth pattern, UESL often has clear tumor borders, and presents with a fibrous pseudocapsule in the interface between the tumor and the adjacent parenchyma. The cut surface usually reveals a yellow to tan, heterogenous tumor with glistening solid regions alternating with cystic areas. Necrotic and hemorrhagic tissue, clotted blood and gelatinous material (that corresponds to areas of myxoid histology) are also often detected [Citation36,Citation44,Citation67,Citation68].
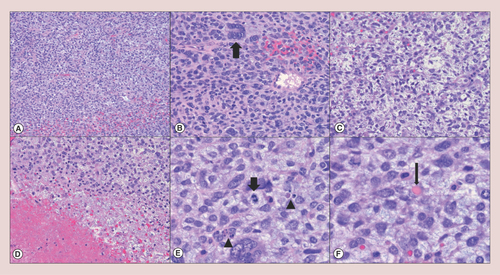
Histologically, UESL shows solid/irregular tumor architecture. Hypercellular sheets of highly pleomorphic tumor cells, necrosis, high mitotic index, frequent atypical mitoses and apoptotic bodies are important microscopic features of this tumor and indicate fast cellular turnover (A–E). In accordance, the proliferative index of UESL by Ki67 immunohistochemistry is usually high (≥30%) [Citation69,Citation70]. Cytologically, UESL is enriched for spindle or stellate-shaped cells with ill-defined borders and inconspicuous nucleoli. Multinucleated giant cells, aberrant nuclei and eosinophilic hyaline globules (F) may also be identified during microscopic studies and are important diagnostic clues of UESL [Citation36]. The edge of the tumor is often demarcated by fibrous connective tissue (corresponding to the pseudocapsule observed by gross examination) and the adjacent liver parenchyma is usually compressed, but normal. Sometimes, clusters of normal hepatocytes and dilated biliary ducts may be detected between the fibrous pseudocapsule and the cancer cells [Citation44,Citation69].
Undifferentiated embryonal sarcoma of the liver generally shows (A) hypercellular sheets of tumor cells (hematoxylin & eosin, 4×); (B) the neoplastic cells are highly pleomorphic with an increased nuclear to cytoplasmic ratio and hyperchromatic nuclei; tumor giant cells (thick arrow) are frequently identified (hematoxylin & eosin, 10×). (C) The lesional cells are focally elongated/spindled with a loose, myxoid stroma (hematoxylin & eosin, 10×). (D) Necrosis (lower left) is occasionally seen (hematoxylin & eosin, 10×). (E) Mitotic figures (thick arrow) and apoptotic bodies (arrowheads) are readily identified (hematoxylin & eosin, 20×). (F) Eosinophilic hyaline globules (thin arrow), which are PAS-positive and diastase-resistant, may be observed in the neoplastic cell cytoplasm and extracellular matrix (hematoxylin & eosin, 20×).
Immunohistochemical studies are nonspecific for the histogenesis of UESL; there is also no isolated marker conclusive for the diagnosis of this cancer. Therefore, broad immunohistochemical panels are often necessary to rule out differential diagnoses (discussed in the section Differential diagnosis) and to increase the diagnostic accuracy of UESL [Citation69,Citation71]. Most cases of UESL are positive for Vimentin, alpha-1 antytrypsin and CD68 [Citation69–Citation72]. Other common – but slightly less prevalent – markers include BCL2, CD10 and CD56 [Citation36,Citation47,Citation69,Citation70,Citation72,Citation73]. UESL is usually negative for HepPar1, AFP, alpha smooth muscle actin, myogenin, synaptophysin, CD117, HMB-45 and CD34 [Citation69,Citation70,Citation72]. Staining for pan-cytokeratins and Glypican 3 should be carefully interpreted. In fact, cytokeratins AE1/AE3, CAM5.2 and OSCAR may show focal or dot-like expression in UESL, which could suggest the diagnosis of carcinoma [Citation72]. Similarly, Glypican 3, which is often expressed in hepatocellular carcinoma and hepatoblastoma, stains close to half of the cases of UESL [Citation74]. Therefore, these markers should not be used to guide the differential diagnosis between these lesions.
Differential diagnosis
In the pediatric population, UESL should be mainly distinguished from embryonal rhabdomyosarcoma (of the biliary tree), hepatoblastoma and HMH [Citation36]. Embryonal rhabdomyosarcoma depicts areas showing rhabdomyoblastic differentiation and are usually positive for immunohistochemical markers of skeletal muscle differentiation including myoD1 and myogenin. Hepatoblastoma usually develops in younger patients (<5 years); these tumors show embryonal or fetal liver epithelium that can be distinguished from UESL by careful histological analysis. Furthermore, the mesenchymal component in hepatoblastomas usually lack the degree of cytological atypia observed in UESL [Citation15]. Finally, HMH also develops in younger patients (<2 years); the gross appearance of these tumors is predominantly cystic, while morphological anaplasia and atypical mitoses are not usually identified [Citation2,Citation8].
In adults, the main differential diagnoses of UESL include sarcomatoid HCC, melanoma, gastrointestinal stromal tumors, angiomyolipoma and other high-grade sarcomas. HCC usually develops in a background of chronic liver injury. Thus, careful evaluation of the clinical history and histological analysis of the adjacent liver parenchyma should guide the diagnosis. Furthermore, sarcomatoid HCC may also present with areas of classic hepatocellular (nonsarcomatoid) differentiation [Citation75]. Melanomas are morphologically heterogeneous tumors and distinction from UESL solely on histological grounds may be challenging. Presence of melanin pigment and expression of melanocyte markers (e.g., SOX10, HMB-45 and Melan A) and S100 may aid the diagnosis [Citation76,Citation77]. Gastrointestinal stromal tumors are usually less anaplastic and express DOG1, CD117 (C-kit) and/or CD34 [Citation78]. Hepatic angiomyolipoma, predominantly seen in females, is characterized by the presence of thick-walled blood vessels, smooth muscle (myoid cells) and mature adipose tissue histologically [Citation79]. The lesional cells are generally immunoreactive for HMB45, Melan A, desmin and smooth muscle actin. High-grade sarcomas may be distinguished based on histological or immunohistochemical evidence of specific cell lineage differentiation (e.g., adipocytes in liposarcomas; vascular spaces in angiosarcoma). The clinicopathologic characteristics of UESL and its differential diagnoses are summarized in .
Table 1. Clinicopathologic characteristics of undifferentiated embryonal sarcoma of the liver and its differential diagnoses.
Treatment & prognosis
The outcome of patients with UESL treated with surgical intervention is generally favorable, with a 5-year overall survival superior to 70% [Citation45]. A pooled analysis of multiple cases of UESL showed that partial hepatectomy is the most common surgical intervention in this cancer reported in the literature. Patients with negative resection margins and those that received adjuvant chemotherapy following partial hepatectomy showed improved overall- and recurrence-free survival rates. Meanwhile, large tumor size (>10 cm) and extrahepatic dissemination did not significantly correlate with the survival rates in patients submitted to partial hepatectomy. These results suggest that patients with UESL should be considered for surgical intervention followed by adjuvant chemotherapy regardless of tumor stage [Citation45]. Furthermore, patients with unresectable tumors seemed to benefit from neoadjuvant chemotherapy followed by tumor resection, and cases refractory to neoadjuvant chemotherapy may benefit from liver transplantation [Citation41,Citation45,Citation80].
Conclusion
HMH and UESL are rare conditions primarily seen in the pediatric population. Tissue diagnosis is extremely important since the clinical and radiologic presentations are not specific. In practice, pathologists should be familiar with the histologic characteristics of these lesions and recognize the limitations of ancillary studies (molecular cytogenetics and immunohistochemistry). Resection is the mainstay treatment for both entities; multimodal therapy with adjuvant chemotherapy has significantly improved the outcome of patients with UESL.
Future perspective
An understanding of the pathogenesis of HMH and UESL is still evolving. C19MC activation has recently been proposed as the unifying mechanism for most HMH cases. Meanwhile, UESL lesions demonstrate a vast variety of cytogenetic aberrations. Genome-wide molecular analyses and/or experimental validation of putative molecular drivers in a large cohort are necessary to identify distinctive molecular signatures and/or potential targets for therapeutic intervention.
Hepatic mesenchymal hamartoma (HMH) and undifferentiated embryonal sarcoma of the liver (UESL) are rare mesenchymal tumors arising predominantly in the pediatric population.
HMH is the second most common benign hepatic tumor in children, most commonly diagnosed in patients younger than 2 years of age.
Different mechanisms of HMH include androgenetic-biparental mosaicism and chromosomal aberrations which lead to chromosome 19q microRNA cluster activation.
Histologically, HMH is characterized by a lobular growth of myxomatous stroma with scattered stellate-shaped mesenchymal cells, branching bile ducts, and entrapped islands of hepatocytes.
UESL is the third most common malignant hepatic tumor in children following hepatoblastoma and hepatocellular carcinoma; the median age of diagnosis is 9 years.
UESL is characterized by sheets of highly pleomorphic neoplastic cells. Necrosis, high mitotic index, atypical mitoses, multinucleated giant cells and eosinophilic hyaline globules are frequently identified.
HMH may undergo malignant transformation into UESL, suggested by identical chromosomal abnormalities involving chromosome 19q13.4.
Surgical resections are the mainstay treatments for HMH and UESL. Chemotherapy may improve the outcome of patients with UESL. Liver transplantation may be considered in unresectable cases.
Author contributions
SN Martins-Filho drafted the manuscript, reviewed the literature and approved the final version of the manuscript. J Putra drafted the manuscript, provided the histological images, reviewed the literature and approved the final version of the manuscript.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- MeyersRL. Tumors of the liver in children. Surg. Oncol.16(3), 195–203 (2007).
- SiddiquiMA, McKennaBJ. Hepatic mesenchymal hamartoma: a short review. Arch. Pathol. Lab. Med.130(10), 1567–1569 (2006).
- LabergeJM, PatenaudeY, DesiletsVet al. Large hepatic mesenchymal hamartoma leading to mid-trimester fetal demise. Fetal Diagn. Ther.20(2), 141–145 (2005).
- MulrooneyDA, CarpenterB, GeorgieffMet al. Hepatic mesenchymal hamartoma in a neonate: a case report and review of the literature. J. Pediatr. Hematol. Oncol.23(5), 316–317 (2001).
- CookJR, PfeiferJD, DehnerLP. Mesenchymal hamartoma of the liver in the adult: association with distinct clinical features and histological changes. Hum. Pathol.33(9), 893–898 (2002).
- KlaassenZ, ParagiPR, ChamberlainRS. Adult mesenchymal hamartoma of the liver: case report and literature review. Case Rep. Gastroenterol.4(1), 84–92 (2010).
- StockerJT, IshakKG. Mesenchymal hamartoma of the liver: report of 30 cases and review of the literature. Pediatr. Pathol.1(3), 245–267 (1983).
- StringerMD, AlizaiNK. Mesenchymal hamartoma of the liver: a systematic review. J. Pediatr. Surg.40(11), 1681–1690 (2005).
- BesshoT, KubotaK, KomoriSet al. Prenatally detected hepatic hamartoma: another cause of non-immune hydrops. Prenat. Diagn.16(4), 337–341 (1996).
- KapurRP, BerryJE, TsuchiyaKD, OpheimKE. Activation of the chromosome 19q MicroRNA cluster in sporadic and androgenetic-biparental mosaicism–associated hepatic mesenchymal hamartoma. Pediatr. Dev. Pathol.17(2), 75–84 (2014).
- KellerRB, ElDemellawy D, QuagliaA, FinegoldM, KapurRP. Methylation status of the chromosome arm 19q MicroRNA cluster in sporadic and androgenetic-biparental mosaicism–associated hepatic mesenchymal hamartoma. Pediatr. Dev. Pathol.18(3), 218–227 (2015).
- ElDemellawy D, LeeJY, McDonellLet al. Atypical hepatic mesenchymal hamartoma: histologic appearance, immunophenotype, and molecular findings. Pediatr. Dev. Pathol.22(4), 365–369 (2019).
- ThampyR, ElsayesKM, MeniasCOet al. Imaging features of rare mesenchymal liver tumours: beyond haemangiomas. Br. J. Radiol.90(1079), 20170373 (2017).
- ChungEM, CubeR, LewisRB, ConranRM. Pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics30(3), 801–826 (2009).
- StockerJT. Hepatic tumors in children. Clin. Liver Dis.5(1), 259–281 (2001).
- WuH, FergusonW, CastroE, FinegoldM, PatelK. Pediatric mesenchymal hamartomas of the liver can show both foregut and hindgut phenotype. Pediatr. Dev. Pathol.20(6), 490–497 (2017).
- RahadianiN, StephanieM, PutraJ. Recurrent hepatic mesenchymal hamartoma with osseous metaplasia. Liver Int.38(10), 1875–1875 (2018).
- RumpP, ZeegersMPA, van EssenAJ. Tumor risk in Beckwith-Wiedemann syndrome: a review and meta-analysis. Am. J. Med. Genet. A136(1), 95–104 (2005).
- CajaibaMM, Sarita-ReyesC, ZambranoE, Reyes-MúgicaM. Mesenchymal hamartoma of the liver associated with features of Beckwith-Wiedemann syndrome and high serum alpha-fetoprotein levels. Pediatr. Dev. Pathol.10(3), 233–238 (2007).
- Abrahao-MachadoLF, de MacedoFC, DalenceCet al. Mesenchymal hamartoma of the liver in an infant with Beckwith-Wiedemann syndrome: a rare condition mimicking hepatoblastoma. ACG Case Rep. J.2(4), 258–260 (2015).
- Apellaniz-RuizM, SegniM, KettwigMet al. Mesenchymal hamartoma of the liver and DICER1 syndrome. N. Engl. J. Med.380(19), 1834–1842 (2019).
- FoulkesWD, PriestJR, DuchaineTF. DICER1: mutations, microRNAs and mechanisms. Nat. Rev. Cancer14(10), 662–672 (2014).
- VargasS, Perez-AtaydeA. Mesenchymal hamartoma of the liver and DICER1 syndrome. N. Engl. J. Med.381(6), 586–587 (2019).
- PawooN, HellerDS. Placental mesenchymal dysplasia. Arch. Pathol. Lab. Med.138(9), 1247–1249 (2014).
- AlwaidhMH, WoodhallCR, CartyHT. Mesenchymal hamartoma of the liver: a case report. Pediatr. Radiol.27(3), 247–249 (1997).
- ReedRC, BeischelL, SchoofJ, JohnsonJ, RaffML, KapurRP. Androgenetic/biparental mosaicism in an infant with hepatic mesenchymal hamartoma and placental mesenchymal dysplasia. Pediatr. Dev. Pathol.11(5), 377–383 (2008).
- BehrGG, FishmanSJ, CatyMG, KulungowskiAM, PaltielHJ, AlomariAI. Hepatic mesenchymal hamartoma and infantile hemangioma: a rare association. J. Pediatr. Surg.47(3), 448–452 (2012).
- BerteN, FilfilanA, MainardL, MansuyL, LemelleJL. Co-existing infantile hepatic hemangioma and mesenchymal hamartoma in a neonate. J. Surg. Case Rep.2018(1), rjx260 (2018).
- BejaranoPA, SerranoMF, CasillasJet al. Concurrent infantile hemangioendothelioma and mesenchymal hamartoma in a developmentally arrested liver of an infant requiring hepatic transplantation. Pediatr. Dev. Pathol.6(6), 552–557 (2003).
- TuckerSM, CooperK, BrownschidleS, WilcoxR. Embryonal (undifferentiated) sarcoma of the liver with peripheral angiosarcoma differentiation arising in a mesenchymal hamartoma in an adult patient. Int. J. Surg. Pathol.20(3), 297–300 (2012).
- LiQ, WangJ, SunY, CuiY, HaoX. Hepatic angiosarcoma arising in an adult mesenchymal hamartoma. Int. Semin. Surg. Oncol.4, 3 (2007).
- KulkarniMP, AgasheSR, SinghRV, SulhyanKR. Hepatic angiosarcoma arising in an adult mesenchymal hamartoma. Indian J. Pathol. Microbiol.53(2), 322–324 (2010).
- LauwersGY, GrantLD, DonnellyWHet al. Hepatic undifferentiated (embryonal) sarcoma arising in a mesenchymal hamartoma. Am. J. Surg. Pathol.21(10), 1248–1254 (1997).
- de ChadarévianJP, PawelBR, FaerberEN, WeintraubWH. Undifferentiated (embryonal) sarcoma arising in conjunction with mesenchymal hamartoma of the liver. Mod. Pathol.7(4), 490–493 (1994).
- O’SullivanMJ, SwansonPE, KnollJ, TaboadaEM, DehnerLP. Undifferentiated embryonal sarcoma with unusual features arising within mesenchymal hamartoma of the liver: report of a case and review of the literature. Pediatr. Dev. Pathol.4(5), 482–489 (2001).
- PutraJ, OrnvoldK. Undifferentiated embryonal sarcoma of the liver: a concise review. Arch. Pathol. Lab. Med.139(2), 269–273 (2015).
- ArrunateguiAM, CaicedoLA, ThomasLSet al. Giant mesenchymal hamartoma in pediatric patients: a new indication for liver transplantation. J. Pediatr. Surg. Case Rep.21, 1–3 (2017).
- PanET, YoeliD, KuehtMLet al. Liver transplantation as definitive treatment of an unresectable mesenchymal hamartoma in a child with Beckwith–Wiedemann Syndrome. J. Surg. Case Rep.2017(8), rjx167 (2017).
- TsaoK, HiroseS, SydorakRet al. Fetal therapy for giant hepatic cysts. J. Pediatr. Surg.37(10), E31 (2002).
- AnilG, FortierM, LowY. Cystic hepatic mesenchymal hamartoma: the role of radiology in diagnosis and perioperative management. Br. J. Radiol.84(1001), e091–e094 (2011).
- ShiY, RojasY, ZhangWet al. Characteristics and outcomes in children with undifferentiated embryonal sarcoma of the liver: a report from the National Cancer Database. Pediatr. Blood Cancer.64(4), e26272 (2017).
- WeinbergAG, FinegoldMJ. Primary hepatic tumors of childhood. Hum. Pathol.14(6), 512–537 (1983).
- NgK, MogulDB. Pediatric liver tumors. Clin. Liver Dis.22(4), 753–772 (2018).
- StockerJT, IshakKG. Undifferentiated (embryonal) sarcoma of the liver. Report of 31 cases. Cancer42(1), 336–348 (1978).
- WuZ, WeiY, CaiZ, ZhouY. Long-term survival outcomes of undifferentiated embryonal sarcoma of the liver: a pooled analysis of 308 patients. ANZ J. Surg. doi: 10.1111/ans.15684 (2020) (Epub ahead of print).
- EstebanSMG, EmilioCGU, EmmanuelABF, OscarSJ, PaulinaCE, AngelMM. Undifferentiated embryonal sarcoma of the liver in adult patient: a report of two cases. Ann. Hepato-Biliary-Pancreat. Surg.22(3), 269–273 (2018).
- BeksacK, MammadovR, CiftciT, GunerG, AkyolA, KaynarogluV. Undifferentiated embryonal sarcoma of the liver in an adult patient. Cureus10(7), e3037 (2018).
- EllisIO, CottonRE. Primary malignant mesenchymal tumour of the liver in an elderly female. Histopathology7(1), 113–121 (1983).
- HorowitzME, EtcubanasE, WebberBLet al. Hepatic undifferentiated (embryonal) sarcoma and rhabdomyosarcoma in children. Results of therapy. Cancer59(3), 396–402 (1987).
- ShiM, XuH, SangsterGP, GuX. Pulmonary metastases from an undifferentiated embryonal sarcoma of the liver: a case report and review. Case Rep. Oncol. Med.2018, 7840865 (2018).
- BisognoG, PilzT, PerilongoGet al. Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer94(1), 252–257 (2002).
- HuX, ChenH, JinMet al. Molecular cytogenetic characterization of undifferentiated embryonal sarcoma of the liver: a case report and literature review. Mol. Cytogenet.5, 26 (2012).
- SoweryRD, JensenC, MorrisonKB, HorsmanDE, SorensenPH, WebberEM. Comparative genomic hybridization detects multiple chromosomal amplifications and deletions in undifferentiated embryonal sarcoma of the liver. Cancer Genet. Cytogenet.126(2), 128–133 (2001).
- RajaramV, KnezevichS, BoveKE, PerryA, PfeiferJD. DNA sequence of the translocation breakpoints in undifferentiated embryonal sarcoma arising in mesenchymal hamartoma of the liver harboring the t(11;19)(q11;q13.4) translocation. Genes. Chromosomes Cancer.46(5), 508–513 (2007).
- MathewsJ, DuncavageEJ, PfeiferJD. Characterization of translocations in mesenchymal hamartoma and undifferentiated embryonal sarcoma of the liver. Exp. Mol. Pathol.95(3), 319–324 (2013).
- BegueretH, TrouetteH, VielhPet al. Hepatic undifferentiated embryonal sarcoma: malignant evolution of mesenchymal hamartoma? Study of one case with immunohistochemical and flow cytometric emphasis. J. Hepatol.34(1), 178–179 (2001).
- LepreuxS, RebouissouS, LeBail Bet al. Mutation of TP53 gene is involved in carcinogenesis of hepatic undifferentiated (embryonal) sarcoma of the adult, in contrast with Wnt or telomerase pathways: an immunohistochemical study of three cases with genomic relation in two cases. J. Hepatol.42(3), 424–429 (2005).
- KimJH, SioCA, ParkH, KimH, ShinHD, JungK. Undifferentiated embryonal sarcoma of the liver in a child: a whole exome sequencing analysis. Dig. Liver Dis.49(8), 944–946 (2017).
- RosPR, OlmstedWW, DachmanAH, GoodmanZD, IshakKG, HartmanDS. Undifferentiated (embryonal) sarcoma of the liver: radiologic-pathologic correlation. Radiology161(1), 141–145 (1986).
- QiuLL, YuRS, ChenY, ZhangQ. Sarcomas of abdominal organs: computed tomography and magnetic resonance imaging findings. Semin. Ultrasound CT MRI.32(5), 405–421 (2011).
- GaborF, Franchi-AbellaS, MerliL, AdamsbaumC, ParienteD. Imaging features of undifferentiated embryonal sarcoma of the liver: a series of 15 children. Pediatr. Radiol.46(12), 1694–1704 (2016).
- YoonJY, LeeJM, KimDYet al. A case of embryonal sarcoma of the liver mimicking a hydatid cyst in an adult. Gut and Liver4(2), 245–249 (2010).
- YuRS, ChenY, JiangB, WangLH, XuXF. Primary hepatic sarcomas: CT findings. Eur. Radiol.18(10), 2196 (2008).
- FarajW, MukherjiD, ElMajzoub N, ShamseddineA, ShamseddineA, KhalifeM. Primary undifferentiated embryonal sarcoma of the liver mistaken for hydatid disease. World J. Surg. Oncol.8, 58 (2010).
- BuetowPC, BuckJL, Pantongrag-BrownLet al. Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology203(3), 779–783 (1997).
- QiuLL, YuRS, ChenY, ZhangQ. Sarcomas of abdominal organs: computed tomography and magnetic resonance imaging findings. Semin. Ultrasound CT MR32(5), 405–421 (2011).
- RosPR, OlmstedWW, DachmanAH, GoodmanZD, IshakKG, HartmanDS. Undifferentiated (embryonal) sarcoma of the liver: radiologic-pathologic correlation. Radiology161(1), 141–145 (1986).
- BuetowPC, BuckJL, Pantongrag-BrownLet al. Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology203(3), 779–783 (1997).
- ZhengJM, TaoX, XuAM, ChenXF, WuMC, ZhangSH. Primary and recurrent embryonal sarcoma of the liver: clinicopathological and immunohistochemical analysis. Histopathology51(2), 195–203 (2007).
- KianiB, FerrellLD, QualmanS, FrankelWL. Immunohistochemical analysis of embryonal sarcoma of the liver. Appl. Immunohistochem. Mol. Morphol.14(2), 193–197 (2006).
- WeiZG, TangLF, ChenZM, TangHF, LiMJ. Childhood undifferentiated embryonal liver sarcoma: clinical features and immunohistochemistry analysis. J. Pediatr. Surg.43(10), 1912–1919 (2008).
- Pérez-GómezRM, Soria-CéspedesD, deLeón-BojorgeB, Ortiz-HidalgoC. Diffuse membranous immunoreactivity of CD56 and paranuclear dot-like staining pattern of cytokeratins AE1/3, CAM5.2, and OSCAR in undifferentiated (embryonal) sarcoma of the liver. Appl. Immunohistochem. Mol. Morphol.18(2), 195–198 (2010).
- ZhangH, LeiL, ZuppanCW, RazaAS. Undifferentiated embryonal sarcoma of the liver with an unusual presentation: case report and review of the literature. J. Gastrointest. Oncol.7(Suppl. 1), S100–S106 (2016).
- LevyM, TrivediA, ZhangJet al. Expression of glypican-3 in undifferentiated embryonal sarcoma and mesenchymal hamartoma of the liver. Hum. Pathol.43(5), 695–701 (2012).
- LiaoSH, SuTH, JengYMet al. Clinical manifestations and outcomes of patients with sarcomatoid hepatocellular carcinoma. Hepatology69(1), 209–221 (2019).
- OhsieSJ, SarantopoulosGP, CochranAJ, BinderSW. Immunohistochemical characteristics of melanoma. J. Cutan. Pathol.35(5), 433–444 (2008).
- MohamedA, GonzalezRS, LawsonD, WangJ, CohenC. SOX10 expression in malignant melanoma, carcinoma, and normal tissues. Appl. Immunohistochem. Mol. Morphol.21(6), 506–510 (2013).
- LopesLF, WestRB, BacchiLM, vande Rijn M, BacchiCE. DOG1 for the diagnosis of gastrointestinal stromal tumor (GIST): comparison between 2 different antibodies. Appl. Immunohistochem. Mol. Morphol.18(4), 333–337 (2010).
- AgaimyA, VassosN, CronerRS, StrobelD, LellM. Hepatic angiomyolipoma: a series of six cases with emphasis on pathological-radiological correlations and unusual variants diagnosed by core needle biopsy. Int. J. Clin. Exp. Pathol.5(6), 512–521 (2012).
- TechavichitP, MasandPM, HimesRWet al. Undifferentiated embryonal sarcoma of the liver (UESL): a single-center experience and review of the literature. J. Pediatr. Hematol. Oncol.38(4), 261–268 (2016).
