Abstract
Aim: Evaluation of safety and efficacy of selective balloon-occluded transarterial chemoembolization using polyethylene glycol embolizing microspheres in patients with hepatocellular carcinoma. Materials & methods: Twenty-four consecutive patients were included in this monocentric prospective trial. Adverse events were evaluated at 24 h and 1 month. Imaging response according to modified response evaluation criteria in solid tumors was assessed at 1, 3 and 6 months. Results: The median time of follow-up was of 22.8 months (interquartile range (IQR) 17.38–26.22). Clinical grade 1/2 toxicities (0% >grade 2) were reported in 25.7% of patients, with abdominal pain being the most frequent complication (17.1%). No 30-days mortalities or liver decompensation were observed. The 1-month follow-up MRI showed an overall response rate of 74.3% Conclusion: Balloon-occluded transarterial chemoembolization was shown to be safe and effective.
Tweetable abstract
We report a new technique of chemoembolization for liver cancer that allows to selectively treat tumor nodules with fewer side effects and better tolerance. #chemoembolizationforHCC #improvinglocoregionaltreatmentinHCC #balloonmicrocatheterforTACE
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer-related deaths worldwide, with the majority being associated to cirrhosis [Citation1]. Current guidelines [Citation1,Citation2] endorse the Barcelona Clinic Liver Cancer (BCLC) classification for HCC tumor staging [Citation3]. Despite the standardized screening of high-risk populations, most patients are unfortunately diagnosed with locally advanced disease (BCLC B – intermediate stage) where palliative transarterial locoregional treatment (TA-LRT) are the only existing modalities.
TA chemoembolization (TACE) is currently recommended as first-line therapy for BCLC B disease [Citation1,Citation2]. Furthermore, TACE may also serve as ‘bridging therapy’ to liver transplant waiting-list candidates and a valuable downstaging tool to fulfill transplantation criteria [Citation4]. When contraindications to ablation, resection or hepatic transplantation exist, the stage-migration concept advocates that the next best line of therapy, in this case TACE, should be applied for early stage HCC [Citation1,Citation2,Citation5].
The first technique described in the literature was conventional TACE (cTACE) that consists in an intra-arterial injection of a lipiodol-chemotherapy suspension followed by embolization with Gelfoam particles. The development of drug-eluting microspheres (DEM) represented a major improvement in terms of tolerance and safety profile, mainly due to the fact that the cytotoxic agent is released slowly, resulting in a reduced systemic passage [Citation6–8].
Among the different commercialized microspheres, polyethylene glycol (PEG) beads have proved their efficacy and safety in the treatment of HCC patients [Citation9–11].
In order to improve the results of cTACE a new procedure called balloon-occluded TACE (B-TACE) was developed. This technique, first described in Japan, is performed using an occlusive balloon microcatheter inflated in the arterial feeders of the tumor nodules before embolization, thus inducing a drop in local blood pressure that allows a blood flow modification with a higher concentration of chemotherapy at tumor level and sparing the nontumoral parenchyma. Several reports combining B-TACE with lipiodol based chemoembolization, showed promising results in terms of safety and efficacy [Citation12–14]. Furthermore, retrospective data suggest a better tumor control of B-TACE compared with cTACE in tumors up to 4 cm in diameter [Citation12,Citation15,Citation16].
Currently, only two recent retrospective studies have evaluated B-TACE using DEMs (B-DEM-TACE) in HCC and showed positive results in terms of safety and efficacy [Citation17,Citation18].
We report here the first prospective study assessing the feasibility and safety of B-TACE using PEG embolizing microspheres loaded with 75 mg doxorubicin (B-DEM-TACE) in a selected population of HCC patients.
Materials & methods
Study design
This study was prospectively conducted in a single liver-transplantation center between April 2017 and September 2018. The study was reviewed and approved by the local ethics committee (P2017/193). Informed written consent was signed by all the patients before enrollment.
Inclusion & exclusion criteria
Study participants presented inaugural HCC or newly diagnosed nodules (after LRT on different lesions), and were referred from the multidisciplinary hepatology tumor board. Diagnosis was made by noninvasive criteria for cirrhotic patients, using contrast-enhanced MRI (CE-MRI) or by pathology confirmation for noncirrhotic patients, according to European Association for the Study of the Liver/American Association for the Study of Liver Diseases guidelines. Main inclusion criteria were: unresectable HCC not eligible for curative treatments (ablative treatments or surgical resection) with at least one lesion measurable by CE-MRI, patients in the transplantation waiting list, Child-Pugh score ≤7, Eastern Cooperative Oncology Group Performance Status score ≤1, with a life expectancy ≥12 weeks. The main exclusion criteria were the presence of an infiltrative tumor, macrovascular invasion, tumor size >5 cm, >5 nodules and previous locoregional therapy in the same area or previous systemic therapy.
B-DEM-TACE procedure
Patients were admitted the day before the procedure and an intravenous perfusion was placed for a proper prehydration.
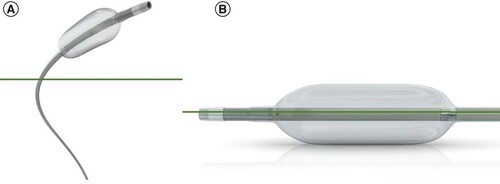
Premedication with 20 mg of dexamethasone and 8 mg of ondansetron was administered 1 h before the procedure in order to reduce the risk of postembolization syndrome (PES; i.e., abdominal pain, nausea and fever). TACE was performed through right femoral access (left if contraindicated on the right) under local anesthesia by the same interventional radiologist with 10 years of experience. A diagnostic visceral arteriography was performed, followed by a selective angiography of the common hepatic artery, in order to identity the HCC nodules needed to be treated. The performance of a diagnostic dual-phase cone-beam computed tomography was left to the discretion of the interventional radiologist. The balloon microcatheter (Occlusafe®; Terumo Europe NV, Leuven, Belgium), is composed of a catheter measuring 2.8 Fr with an occlusive balloon on the tip, and was positioned in order to opacify the tumor as selectively as possible (). Once the accurate position was reached, the occlusive balloon, made of compliant polyurethane and of 10 mm in length, was inflated, and contrast was injected again in order to ensure that the tumor was still well targeted. One 2 milliliter syringe of PEG microspheres (LifePearl®, Terumo Europe NV, 100 ± 25 or 200 ± 50 μm according to the interventional radiologists’ appreciation) loaded with 75 mg of doxorubicin was then injected. The administration was considered successful when the nodule was sufficiently filled in or when an overflow into the intrahepatic collateral pathway was observed. A nonenhanced cone-beam computed tomography was performed at the end of the procedure in order to evaluate the DEM deposition in the nodules. Patients were discharged the day after the procedure if no complications were noted. In case of suboptimal deposition, a complementary embolization was performed using nonloaded beads (Bead Block® microspheres; BTG International, London, UK, 100–300 μm). In patients with large tumors or remaining arterial feeding vessels, a second TACE session was scheduled 4 weeks later.
Study safety outcomes
The primary outcome of the study was the evaluation of safety, recorded as per procedure, early (within 24–48 h) and late (4–6 weeks) incidence of biological and clinical adverse events (AEs) according to the CIRSE classification system and Common Terminology Criteria of Adverse Events (CTCAE) version 5.0 [Citation19,Citation20]. Blood samples, including complete blood count with differential, liver function tests, serum blood urea nitrogen (BUN), creatinine, albumin and coagulations tests (international normalized ratio), were assessed at baseline, 24-h post-treatment and 1-month after the procedure. Radiological safety evaluation performed at 1 month, included the development of asymptomatic/symptomatic liver bile duct injuries such as segmental dilation or biloma formation, occurrence of portal vein narrowing, liver infarction in the nontumoral parenchyma and appearance of indirect imaging features of portal hypertension. PES was defined as the onset of fever, nausea/vomiting and pain, and was clinically evaluated during the patients’ hospital stay.
Evaluation of tumor response & follow-up
The secondary outcome of the study was the efficacy assessment of B-DEM-TACE by the response at 4–6 weeks follow-up imaging (CE-MRI) according to modified response evaluation criteria in solid tumors (mRECIST) [Citation21]. It was categorized in four groups as follows: complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). If the evaluation suggested CR, the patients were followed every 2–3 months until a new event occurred (liver transplantation, progression, death or until analysis). In the setting of PR or SD and if the liver function allowed it, a new TACE procedure was scheduled, with or without use of a balloon-occlusive catheter. In case of PD or SD after two procedures, the treatment modalities were discussed in multidisciplinary hepatic board. Assessment of tumor response was firstly made by experienced (3–20 years) radiologists in the follow-up of HCC therapies by CE-MRI, followed by review by our expert abdominal radiologist specialized in oncology. Overall response (OR) rate was defined as the percentage of treated nodules that reached CR or PR. For nodules that underwent more than one B-DEM-TACE, OR was evaluate after the last treatment. Best OR (BOR) was defined as the best response recorded from the start of the study treatment until the disease progression/recurrence.
Statistical methods
The statistical analysis was performed using the statistical software package, SPSS version 25 (IBM, NY, USA).
Descriptive statistics are reported as means and standard deviations for continuous variables and were compared by Student’s t-test or general linear models for repeated values. Frequencies and percentages were used for categoric variables that were compared thereafter by nonparametric tests. We used ANOVA for repeated measurements for multiple laboratory values. The Bonferroni correction was applied in order to adjust comparison between several dependent values. Statistical significance was defined as p-value < 0.05. Overall survival (OS) is reported using Kaplan–Meier method, with means ± standard errors and medians with 95% CIs of times to event. Censoring was performed at the date of cutoff, and additionally to liver transplantation date.
Results
Demographics & baseline characteristics
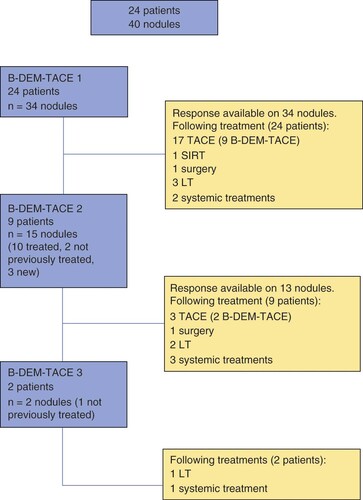
Baseline characteristics of included patients and TACE procedures are listed in . A total of 24 consecutive patients underwent 35 procedures for 40 HCC nodules (). No patient was discharged from the study during screening. Most of the participants were males (95.8%), with a mean age of 66 ± 10.1 years. Three patients had already received a previous treatment on other nodules: two of them had undergone surgery and one had received TACE on a different nodule with CR. All patients presented cirrhosis, 95.8% were CHILD A, with a mean Model for End-Stage Liver Disease score of 10.1 ± 3.3, and alcohol-related liver disease was the major cause of liver impairment (66.7%). The majority of patients were BCLC A (62.5%), they were equally distributed between unifocal and multifocal disease, had a median of 1 nodule (range between 1–3) with a mean size of 32.7 ± 11.8 mm. Regarding transplantation requirements, 75% of patients fulfilled the Milan criteria and 83.3% the University of California San Francisco criteria [Citation22,Citation23].
Table 1. Baseline and transarterial chemoembolization characteristics.
Available responses are shown in the right side of the image.
B-DEM-TACE: Balloon-occluded transarterial chemoembolization using drug-eluting microspheres; LT: Liver transplantation; SIRT: Selective internal radiation therapy.
TACE characteristics
Technical success was achieved in all 24 patients. Most of them underwent a single procedure, nine of them had a second one and two had a third TACE mainly on the same nodules (). The majority of treatments reached a proper selectivity (34.3% superselective and 57.1% selective, more specifically at most multisegmental artery). The PEG microspheres (LifePearl) employed for the procedures were all loaded with doxorubicin and were either 100 μm (54.3%) or 200 μm (45.7%) in diameter. Only two cases needed Bead Block use in order to complete embolization (100–300 μm). The mean dose of doxorubicin per procedure was 73.5.2 ± 16.5 mg. In 29 out of 35 procedures, patients received one vial of PEG loaded with 75 mg of doxorubicin. In the other five procedures, patients received less than one vial of doxorubicin loaded microspheres. One patient, that underwent three procedures, received two vials during the first B-DEM-TACE.
Safety & complications
AEs were subdivided in three categories: clinical, biological and radiological. No AEs occurred during the procedures.
Nine clinical AEs were reported at 24–48 h after TACE (early AEs) (25.7%), the majority being abdominal pain (6/35 procedures, 17.1%) followed by the onset of low-grade fever (2/35 procedures, 5.7%) and vomiting (1/35 procedures, 2.9%). All of these complications were grade 1–2 according to CTCAE version 5. Ascites grade 1 (diagnosed by MRI, not clinically significant) developed in four patients that received one B-DEM-TACE.
Early (24–48 h) and late (1 month) biochemical, all grade 1 according to CTCAE version 5, are outlined in . At 24–48-h postprocedure, a grade 1 increase in aspartate aminotransferase (AST) levels was reported in 31.3% of procedures, alanine aminotransferase (ALT) in 9.4%, bilirubin in 6.3%, international normalized ratio in 31.3% and albumin decrease in 10%, respectively. depicts the evolution of biological parameters at the different time points after the B-DEM-TACE.
Table 2. Early and late laboratory toxicities (grade 1 according to Common Terminology Criteria of Adverse Events version 5).
Table 3. Evolution of biological parameters at different time points after the balloon-occluded transarterial chemoembolization using drug-eluting microspheres 1 (mean ± standard deviation, variables with normal distribution).
In terms of radiological complications at 1 month, CE-MRI showed the development of two asymptomatic bilomas (2/34 procedures evaluated, 5.9%). The first patient presented a PES after B-DEM-TACE. He was proposed for resection after left portal vein embolization, but unfortunately the MRI performed before the planned surgery revealed a progression in the form of a multifocal disease. The second patient presented no early AEs, and had a PR on the treated nodules, unfortunately new lesions were discovered at the 1-month control, and therefore, a treatment with sorafenib was initiated. No portal vein narrowing or indirect imaging features of portal hypertension were reported.
All patients were able to receive sequential treatment after a maximum of three B-DEM-TACE procedures, due to the preservation of a good liver function. Nine of them (37.5%) underwent other TACE procedures without the balloon microcatheter, one received selective internal radiation therapy (SIRT) (4.2%), two had surgery (8.3%), six patients were transplanted (25%) and six were proposed for systemic therapies (i.e., sorafenib, immunotherapy) (25%), but one patient refused treatment.
Radiological tumor response
In total we performed 35 B-DEM-TACEs on 40 nodules. The 1-month follow-up imaging post-treatment by CE-MRI was available for 39 nodules (one patient delayed assessment due to orthopedic immobilization) and showed an OR per nodule of 74.3% (48.7% CR and 25.6% PR) and a BOR of 70.9% (41.7% CR and 29.2% PR) ( & ).
Table 4. Modified response evaluation criteria in solid tumors-best overall response.
Table 5. Modified response evaluation criteria in solid tumors-response on 1-month follow-up per nodule.
Nevertheless, when assessing response by procedure, including nontreated nodules, 17.6% (6/34) of progressions were reported due to the onset of new nodules in the nontreated areas.
At 3-months assessment most reported were SD (5/14 patients, 35.7%) and PD (6/14 patients, 42.9%). Six-months assessments were available for eight patients, most of them presented a PD (62.5%).
Resection & liver transplantation
TACE was considered as neoadjuvant therapy in 16 patients (76.7%), with four patients (16.7%) proposed for surgery and 12 patients (50%) for liver transplantation, either by downstaging or bridging-to-transplantation. Nevertheless, only two patients underwent surgery, and eight benefited from liver transplantation (six of them after one to three B-DEM-TACE procedures followed by two patients after an additional DEM-TACE with a traditional catheter). None of the resected patients had portal vein embolization before surgery. Three out of four patients that did not fulfill the Milan criteria at diagnosis were successfully downstaged and transplanted. None of the transplanted patients presented recurrence.
Follow-up & survival
Median time of follow-up was 22.8 months (IQR: 17.38–26.22). No patients were lost to follow-up. We recorded six deaths (25%), all being cancer or cirrhosis related.
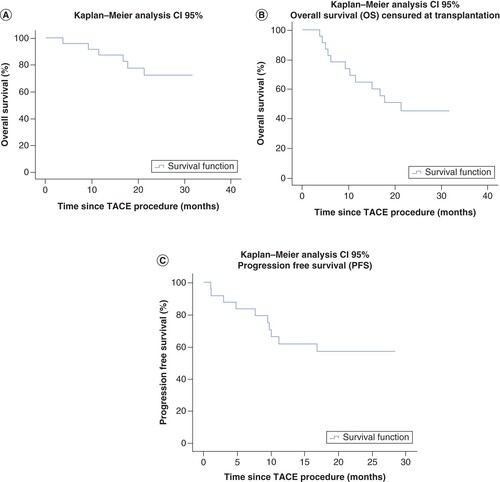
OS was 75% (23.1–30.2, 95% CI), with a mean time to event of 26.7 ± 1.8 months whereas progression-free survival was 50% (15.1–23.8, 95% CI) with a mean time to progression of 19.4 ± 2.2 months. Median time to event could not be estimated due to the low number of events.
Transplantation-free survival, defined as survival censored at liver transplantation, was of 50% (15.5–24.9, 95% CI) with a mean time to event of 20.6 ± 2.4 months (median time to event not estimable because of the low number of events).
The Kaplan–Meier curves of OS, progression-free survival and transplantation-free survival are depicted in .
Discussion
To our knowledge, this study is the first prospective evaluation of B-TACE using PEG embolizing microspheres loaded with doxorubicin (B-DEM-TACE) for a specific population of HCC patients, and exhibits its technically feasibility, with a 100% rate of technical success. These results are in accordance with the first publications of B-TACE and the retrospective trials with B-DEM-TACE [Citation12,Citation16,Citation18,Citation24]. Furthermore, this technique has been proven to have a high safety rate, with few clinical, biological or radiological AEs. In our series we observed fewer complications than expected allowing the possibility of a sequential treatment when needed.
Regarding DEM-TACE using a regular catheter, this technique was associated with better tolerance and fewer complications than cTACE, particularly regarding systemic effects (i.e., alopecia) [Citation6–8]. The most frequent AEs described with DEM-TACE in the literature are pain, nausea and fever, also known as PES that was observed in 15–86.5% of patients [Citation25]. No severe general drug-related AEs were described with PEG embolics in the previous publications, with a recent retrospective study showing a rate of PES in 6% of patients [Citation9–11]. The use of a microballoon catheter is hoped to further decrease toxicity and increase efficacy of TACE due to several phenomena. First of all, a complete occlusion of the feeding artery by the inflated balloon prevents backflow and thus sparing an already fragile parenchyma from DEM agents. Second, a drop in arterial pressure was observed at the distal tip of the catheter allowing the blood flow to be redirected toward the hypervascular HCC nodules. Furthermore, a maintenance of the arterial flow in the targeted nodule has also been observed and explained by the opening of small collaterals with a redistribution of flow to the low-resistance tissues when the balloon was inflated, such as the HCC nodules, resulting in a more selective treatment with preservation of nontumoral parenchyma [Citation17,Citation26].
Recent publications that have evaluated the use of a balloon microcatheter with either cTACE or DEM-TACE show no significant difference in clinical symptoms compared with regular catheters [Citation12,Citation18]. There is currently no direct comparison between B-DEM-TACE and B-TACE. Nevertheless, DEM-TACE already presents a better tolerance and fewer complications and the use of a microballoon catheter is expected to further improve the toxicity profile. In our series the most frequent aspect of PES was abdominal pain (6/35 procedures, 17.1%) followed by the onset of low-grade fever (2/35 procedures, 5.7%) and vomiting (1/35 patients, 2.9%). These findings are consistent with the incidence described in recent literature that reported rates of abdominal pain evaluated between 20.8 and 36.7% and fever from 4.2 to 44.9% [Citation12,Citation17]. Nausea/vomiting was however much lower than described so far and evaluated between 16.3 and 20.8%. Furthermore, clinical AEs reported in our series were grade 1–2, no complications ≥grade 3. Preliminary results from the PARIS Registry, a multicenter, prospective, single-arm study, using standard (more than 90% of cases) or balloon microcatheter with safety as primary end point, that included 187 TACE procedures, presented at CIRSE 2019, showed a toxicity rate of ≥grade 3 clinical toxicities at 20.6%, with 21 patients presenting serious AEs. A subanalysis group regarding their balloon microcatheter population is awaited.
Regarding laboratory findings, only grade 1 toxicities were seen in our series, with increase serum AST levels being the most important, emerging after 31.3% (10/32) of procedures, nevertheless these variations showed no clinical impact.
Imaging at 1 month, performed by CE-MRI showed only two cases of biloma/liver infarct that did not impair further systemic treatment for these patients. Guiu et al. showed that DEB-TACE is independently associated with biloma/liver infarct (OR = 9.78), but with a lower incidence of bile duct injury reported in cirrhotic versus noncirrhotic patients [Citation27]. In the preliminary results of the PARIS Registry liver/biliary toxicities were described in 18.1% of procedures using doxorubicin, including eight bilomas. Our low rate of biliary complications combined with the preservation of a good liver function led to a sequential treatment for most of our patients.
This technique enables to improve the selectivity of TACE, which is an added value for the treatment of HCC particularly in cirrhotic patients, who are most likely to present an impaired liver function. This is emphasized by the fact that nine patients (37.5%) received a second treatment and two of them (22.2%) went on to a third treatment without worsening of the liver function. Furthermore, all 24 patients were candidates for multimodal treatment after B-DEM-TACE due to the lack of hepatic injury. Currently, a key point of locoregional therapy is the preservation of liver function, ever since the development of various systemic therapies that improve OS of these patients.
Literature regarding the therapeutic effects of B-DEM-TACE is scarce, with most of existing studies reporting outcomes of B-TACE compared with cTACE and different chemotherapeutics, mainly platin derivates. The use of a microballoon catheter significantly improved tumor control compared with cTACE, and OR rates varied between 56.3% reported by Minami et al. and 93.9% assessed by Arai et al. [Citation12,Citation24]. Ogawa et al. reached a CR of 49.2% compared with 27% for cTACE [Citation16]. Goldman et al. included in their series B-TACE and B-DEM-TACE with an OR of more than 90% (60% CR and 33.3% PR). The only study presenting results from an exclusive B-DEM-TACE cohort, coming from Lucatelli et al., reached an OR on per patient analysis of 90.9% at 1 month evaluation (31.8% CR and 59.1% PR) [Citation17]. So far, no comparison was made between DEM-TACE and B-DEM-TACE. In our cohort we reached a lower OR of 74.3% (48.7% CR, 25.6% PR) in a per treated nodule analysis and a BOR rate of 70.9% according to mRECIST, despite a similar mean diameter of nodules, but with a higher number of nodules. Nevertheless, the anthracycline dosage injected in our series was less important, with most of the patients receiving one vial of DEM loaded with doxorubicin.
Our study presents several limitations, more precisely this is a single-center study with a small number of participants, without randomization or matched control group (either regular catheter DEM-TACE or B-TACE). The survival analysis is also limited by the small sample size and the limited period of follow-up available, nevertheless this was not the main focus of the study. Furthermore, a bias in per patient response might occur due to the inclusion of three patients that had already undergone DEM-TACE on other nodules located in a different segment. Further prospective, randomized studies are needed to define the right place for B-DEM-TACE.
Conclusion
Despite these limitations, B-DEM-TACE has proven to be an effective treatment for HCC patients with a very good safety profile. In our series we have observed fewer AEs than previously described with other catheters, suggesting an added value of this technique in terms of liver function preservation. If future trials will confirm these observations, B-DEM-TACE may become a valuable tool for intra-arterial LRTs mostly for frail patients.
Future perspective
TA-LRT are constantly evolving. The newly developed B-DEM-TACE has proven to be an effective treatment for HCC patients with a very good safety profile and preservation of the liver function. A prospective randomized study is expected with a larger sample size and more advanced disease in terms of nodule size. Furthermore, a registry of patients already treated by B-DEM-TACE has been started in order to confirm these observations and to define the best candidates for this treatment.
Hepatocellular carcinoma (HCC) accounts for 90% of primary liver cancers and represents a growing health problem worldwide.
Transarterial chemoembolization (TACE) is recommenced as first-line treatment for patients with locally advanced HCC, it may also serve as ‘bridging therapy’ to liver transplantation candidates while on the waiting list and, it can represent a downstaging tool to transplantation criteria.
TACE is constantly evolving and a newly developed technique using an occlusive balloon microcatheter inflated in the arterial feeders of the tumor nodules before embolization has shown promising results in terms of safety and efficacy.
We present the first prospective evaluation of balloon-occluded TACE using polyethylene glycol embolizing microspheres loaded with doxorubicin for a specific population of HCC patients.
Technical success was achieved in all patients, with a low number of early clinical complications (25.7%), all grades 1–2 according to Common Terminology Criteria of Adverse Events version 5.
Imaging at 1 month, performed by contrast-enhanced MRI showed only two cases of biloma/liver infarct that did not impair further systemic treatment for these patients.
All patients were able to receive sequential treatment after a maximum of three balloon-occluded TACE using drug-eluting microspheres procedures, due to the preservation of a good liver function.
The 1-month follow-up imaging post-treatment by contrast-enhanced MRI was available for 39 nodules and showed an overall response per nodule of 74.3% (48.7% CR and 25.6% PR) and a best overall response of 70.9% (41.7% CR and 29.2% PR)
In our series we have observed fewer adverse events than previously described with other catheters, suggesting an added value of this technique in terms of liver function preservation.
Author contributions
A-M Bucalau contributed in the investigation and writing of original draft. I Tancredi and R Leveque performed the B-DEM-TACE procedures. M Pezzullo and S Picchia contributed in the imaging validation. J-L Van Laethem contributed in drafting methodology. G Verset, I Tacredi and R Leveque contributed in conceptualization and supervision of the study and manuscript.
Financial & competing interests disclosure
A-M Bucalau, M Pezzullo, S Picchia and J-L Van Laethem have no conflicts to declare. I Tancredi is a consultant for Terumo Europe NV and BTG International. R Leveque is a consultant for BTG International. G Verset is a consultant for Terumo Europe NV and BTG International. This is an investigator-initiated study supported by a grant from Terumo Europe NV. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Approval was obtained from Erasmus Hospital Ethics Committee P2017/193.
Permission to reproduce material
Occlusafe® device figures reproduced with the approval from Terumo Europe NV.
Additional information
Funding
References
- GallePR, FornerA, LlovetJMet al.EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol.69(1), 182–236 (2018).
- HeimbachJK, KulikLM, FinnRSet al.AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology67(1), 358–380 (2018).
- FornerA, ReigM, BruixJ. Hepatocellular carcinoma. Lancet391(10127), 1301–1314 (2018).
- SanMiguel C, MuffakK, TrigueroJet al.Role of transarterial chemoembolization to downstage hepatocellular carcinoma within the Milan criteria. Transplant. Proc.47(9), 2631–2633 (2015).
- PomfretEA, WashburnK, WaldCet al.Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl.16(3), 262–278 (2010).
- GaoS, YangZ, ZhengZet al.Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology60(124), 813–820 (2013).
- LammerJ, MalagariK, VoglTet al.Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION v study. Cardiovasc. Intervent. Radiol.33(1), 41–52 (2010).
- GolfieriR, GiampalmaE, RenzulliMet al.Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer111(2), 255–264 (2014).
- AlibertiC, CarandinaR, SartiDet al.Hepatic arterial infusion of polyethylene glycol drug-eluting beads for primary and metastatic liver cancer therapy. Anticancer Res.36(7), 3515–3521 (2016).
- AlibertiC, CarandinaR, SartiDet al.Chemoembolization adopting polyethylene glycol drug-eluting embolics loaded with doxorubicin for the treatment of hepatocellular carcinoma. AJR Am. J. Roentgenol.209(2), 430–434 (2017).
- Veloso GomesF, OliveiraJA, CorreiaMTet al.Chemoembolization of hepatocellular carcinoma with drug-eluting polyethylene glycol embolic agents: single-center retrospective analysis in 302 patients. J. Vasc. Interv. Radiol.29(6), 841–849 (2018).
- AraiH, AbeT, TakayamaHet al.Safety and efficacy of balloon-occluded transcatheter arterial chemoembolization using miriplatin for hepatocellular carcinoma. Hepatol. Res.45(6), 663–666 (2015).
- MaruyamaM, YoshizakoT, NakamuraT, NakamuraM, YoshidaR, KitagakiH. Initial experience with balloon-occluded trans-catheter arterial chemoembolization (B-TACE) for hepatocellular carcinoma. Cardiovasc. Intervent. Radiol.39(3), 359–366 (2016).
- HatanakaT, AraiH, KakizakiS. Balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma. World J. Hepatol.10(7), 485–495 (2018).
- IrieT, KuramochiM, KamoshidaT, TakahashiN. Selective balloon-occluded transarterial chemoembolization for patients with one or two hepatocellular carcinoma nodules: retrospective comparison with conventional super-selective TACE. Hepatol. Res.46(2), 209–214 (2016).
- OgawaM, TakayasuK, HirayamaMet al.Efficacy of a microballoon catheter in transarterial chemoembolization of hepatocellular carcinoma using miriplatin, a lipophilic anticancer drug: short-term results. Hepatol. Res.46(3), E60–E69 (2016).
- LucatelliP, GinnaniCorradini L, DeRubeis Get al.Balloon-occluded transcatheter arterial chemoembolization (b-TACE) for hepatocellular carcinoma performed with polyethylene-glycol epirubicin-loaded drug-eluting embolics: safety and preliminary results. Cardiovasc. Intervent. Radiol.42(6), 853–862 (2019).
- GoldmanDT, SinghM, PatelRSet al.Balloon-occluded transarterial chemoembolization for the treatment of hepatocellular carcinoma: a single-center US preliminary experience. J. Vasc. Interv. Radiol.30(3), 342–346 (2019).
- FilippiadisDK, BinkertC, PellerinO, HoffmannRT, KrajinaA, PereiraPL. Cirse quality assurance document and standards for classification of complications: the Cirse classification system. Cardiovasc. Intervent. Radiol.40(8), 1141–1146 (2017).
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE).v.5.0 (2017). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- LencioniR, LlovetJM. Modified recist (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis.30(1), 52–60 (2010).
- MazzaferroV, RegaliaE, DociRet al.Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med.334(11), 693–699 (1996).
- YaoFY, FerrellL, BassNMet al.Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology33(6), 1394–1403 (2001).
- MinamiY, MinamiT, ChishinaHet al.Balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma: a single-center experience. Oncology89(Suppl. 2), 27–32 (2015).
- MalagariK, PomoniM, SpyridopoulosTNet al.Safety profile of sequential transcatheter chemoembolization with DC bead™: results of 237 hepatocellular carcinoma (HCC) patients. Cardiovasc. Intervent. Radiol.34(4), 774–785 (2011).
- AramburuJ, AntónR, RivasAet al.Numerical zero-dimensional hepatic artery hemodynamics model for balloon-occluded transarterial chemoembolization. Int. J. Numer. Method Biomed. Eng.34(7), e2983 (2018).
- GuiuB, DeschampsF, AhoSet al.Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J. Hepatol.56(3), 609–617 (2012).