Abstract
Worldwide, primary liver cancer is the fourth leading cause of cancer mortality. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. Sarcomatoid hepatocellular carcinoma (SHC) is a rare subtype of HCC with conventional HCC admixed with areas with sarcomatoid morphology. SHC is an aggressive, rapidly growing tumor with unfavorable prognosis. Pedunculated SHC is an uncommon presentation of SHC. Due to its rarity, much remains unknown about the etiopathogenesis, molecular underpinnings, and treatment of SHC. We present a case of an exophytic SHC arising in a background of cirrhosis in an older adult. A resection was performed, but the patient subsequently developed multiple additional intrahepatic metastatic lesions necessitating further treatment with chemotherapy.
Worldwide, primary liver cancer (PLC) is the sixth most common malignancy and the fourth leading cause of cancer-related mortality [Citation1]. Hepatocellular carcinoma (HCC) is the most prevalent type of PLC. Sarcomatoid hepatocellular carcinoma (SHC), also known as spindle cell HCC, is a rare subtype of HCC that makes up 0.09–0.79% of HCCs in reported clinical series [Citation2–5]. Higher rates of between 3.9 and 14.3% have been recorded in autopsy case series [Citation6,Citation7]. As underscored by its name, SHC is a primary malignant biphenotypic hepatocellular neoplasm with areas demonstrating conventional HCC and areas with sarcomatoid morphology [Citation8]. The sarcomatoid constituent usually consists of malignant spindled cells, although osteoclast-like giant cells and heterologous osteoid, chondroid and rhabdoid elements may also be seen [Citation8].
Case presentation
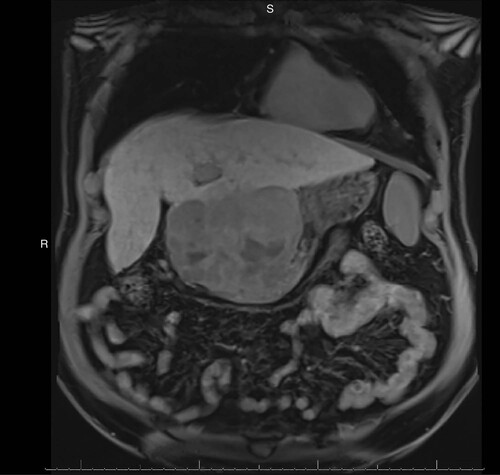
Here, we report the case of a 61-year-old obese male (BMI 31.72 kg/m2) with a history of cirrhosis, believed to be secondary to steatohepatitis, who was referred to the outpatient clinic for a liver mass detected on surveillance abdominal ultrasonography performed as part of a trauma work up for a motor vehicle accident. Follow-up CT scan and MRI confirmed a 12.3 × 9.7 × 9 cm highly heterogeneous exophytic mass in the quadrate lobe of the liver with no washout but with scattered foci showing mild late arterial enhancement (). The background liver was cirrhotic. The overall features were concerning for malignancy. The patient complained of fatigue and unintentional weight loss of 15–20 pounds over the previous 2 months. The patient’s past medical history was otherwise unremarkable. Laboratory investigations at presentation were notable for mildly elevated AFP (14 IU/ml; reference range 0–7 IU/ml), minimal elevation of aspartate transaminase (57 U/l; reference range 0–50 U/l) and normal serum level of alanine transaminase, alkaline phosphatase and total bilirubin. No viral serologies were available for review.
MRI: Magnetic resonance imaging.
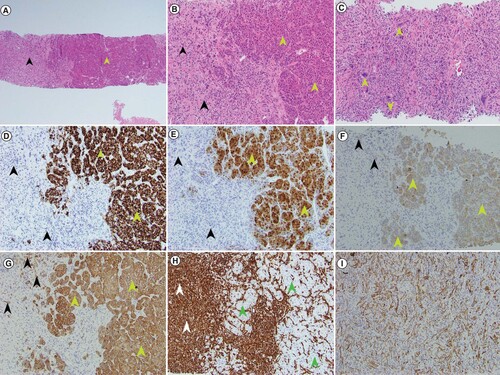
Percutaneous liver biopsy was performed shortly thereafter. Histologic examination demonstrated conventional moderately differentiated HCC along with a high-grade malignant spindle cell component with scattered multinucleated tumor giant cells, consistent with SHC (A–C). The conventional HCC component was highlighted by HepPar 1 (D), arginase 1 (E), cytokeratins CAM5.2 (F) and AE1/AE3 (G) immunohistochemical (IHC) stains; while it was negative for vimentin (H). The sarcomatoid component was diffusely positive for vimentin (H), and focally positive for cytokeratins CAM5.2 (F) and AE1/AE3 (G & I), while negative for HepPar1 and arginase 1 by IHC stains (D–E).
(A) At low magnification, the biphenotypic histology of sarcomatoid and conventional hepatocellular components is evident in the patient’s core needle biopsy specimen (green arrow head – conventional HCC component; black arrowhead – sarcomatoid component) (H&E 40× magnification). (B) Higher magnification shows widened plates of neoplastic hepatocytes (green arrowheads) and adjacent areas of spindled and bizarrely shaped sarcomatoid cells (black arrowheads) (H&E 100× magnification). (C) Higher magnification of sarcomatoid component with bizarre multinucleated giant cells (green arrowheads) (H&E 200× magnification). (D) The conventional HCC component (green arrowheads) is positive for HepPar1 IHC stain but the sarcomatoid component (black arrowheads) is negative (100× magnification). (E) The conventional HCC (green arrowheads) is positive for arginase 1 IHC stain but the sarcomatoid component (black arrowheads) is negative (100× magnification). (F) The conventional HCC (green arrowheads) is positive for cytokeratin CAM5.2 IHC with patchy positivity seen in the sarcomatoid component (black arrowheads) (100× magnification). (G) The conventional HCC is positive for cytokeratin AE1/AE3 IHC (green arrowheads) with patchy positivity seen in the sarcomatoid component (black arrowheads) (100× magnification). (H) The absence of staining for vimentin in the conventional HCC component (green arrowheads) stands in sharp contrast to the strong positivity of the sarcomatoid component (white arrowheads) (100× magnification). (I) Patchy positivity for cytokeratin AE1/AE3 present in this area of tumor only displaying sarcomatoid features is seen (100× magnification).
H&E: Hematoxylin and eosin; HCC: Hepatocellular carcinoma; IHC: Immunohistochemical.
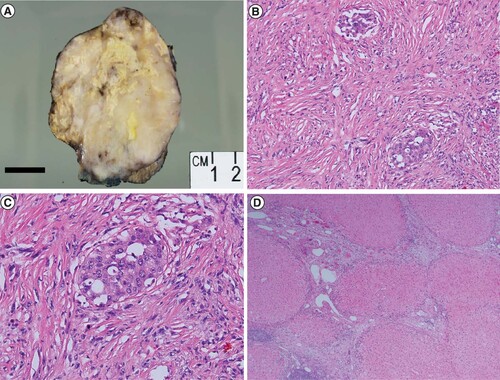
Surgical resection was performed. Intraoperatively, the tumor was found to involve segments 2 and 3 of the liver with attachment via adhesions to the stomach, omentum and pancreas. Grossly, the resection specimen showed a 10 × 8 × 7.5 cm firm, cream-colored, multinodular, variegated mass with areas of necrosis arising in a background of cirrhosis (A). Histologically, the majority of the mass demonstrated high grade malignant spindle cell component with focal areas of hepatocellular differentiation consistent with SHC as seen on the prior diagnostic biopsy (B–C). Carcinoma extended to the parenchymal margin. Small-vessel lymphovascular invasion was identified. A possible satellite tumor nodule identified on gross examination was found on microscopy to be a lymph node involved by carcinoma. The background liver was cirrhotic, but showed no evidence of steatosis or steatohepatitis (D).
(A) A gross photograph of the resection specimen shows a variegated multinodular tumor with firm cream colored areas, yellow areas of necrosis that comprise about 25% of the tumor and areas of hemorrhage. Focal background cirrhotic liver is seen at the bottom right of the image. (B) Histologic examination of the patient’s resection specimen confirmed a predominantly sarcomatoid neoplasm with focal areas of conventional hepatocellular differentiation (H&E 100× magnification). (C) Higher magnification shows neoplastic epithelioid tumor cells surrounded by malignant spindled sarcomatoid cells (H&E 200 × magnification). (D) Cirrhotic background hepatic parenchyma (H&E × 40 magnification).
Postoperatively, the patient did well, however, 10 weeks thereafter, surveillance abdominal CT scan demonstrated multiple small (0.5 to 2 cm) metastatic lesions in the left lobe of the liver. Additional imaging did not reveal metastatic disease elsewhere. Adjuvant chemotherapy was initiated with gemcitabine and cisplatin.
Discussion
Like conventional HCCs, patients with SHC are predominantly male, with a median age at diagnosis between 58 and 62 years [Citation2–4]. Patients most commonly present with right upper quadrant pain [Citation9]. As in our case, clinical diagnosis can be more challenging because serum levels of α-fetoprotein, liver enzymes and bilirubin tend to be lower than is usually encountered in conventional HCC [Citation4]. Imaging findings also tend to be varied and not characteristic of conventional HCC [Citation10]. At the time of diagnosis, imaging commonly shows a high rate of vascular invasion and invasion of adjacent organs [Citation11]. But at least one other study has shown no significant difference in the incidence of radiologically detected major portal vein invasion at the time of diagnosis between SHC and nonsarcomatoid PLCs [Citation4].
Secondary sarcomatous transformation of conventional HCC has long been recognized in patients treated with nonsurgical modalities like transarterial chemoembolization [Citation7], ethanol injection therapy [Citation12] and radiofrequency ablation [Citation13]. But for the most part, there does not seem to be a difference in etiology between de novo SHC and conventional HCC. Liao et al. found no significant differences in demographics, etiologic factors and other baseline characteristics between patients with SHC and matched controls with conventional HCC. In particular, there were no significant differences in the rates of hepatitis B and hepatitis C monoinfection, hepatitis B and C coinfection, non-B-non-C hepatitis, alcoholism, metabolic syndrome and cirrhosis [Citation4]. In up to 22.5% of cases, an underlying etiology was not found [Citation4].
Uncertainty surrounds the histogenesis of SHC and other hepatic mixed epithelial-mesenchymal/sarcomatoid tumors. Some studies have suggested an origin of the sarcomatoid element from the epithelial neoplasm via metaplastic [Citation14,Citation15] or neoplastic [Citation16] transformation. In addition to a common gain of chromosome 6p in both components, the sarcomatoid component has been shown to harbor fewer chromosomal changes than the epithelial component. This has led to the theory that both components originate from a common precursor with greater tumor progression in the epithelial component [Citation17]. Murata et al. identified mutation of the TP53 gene in both the epithelial and sarcomatoid cells of a combined hepatocellular-cholangiocarcinoma with sarcomatoid features [Citation16]. Fayyazi et al. proposed clonal expansion of totipotent stem cells with divergent transformation along both epithelial and mesenchymal pathways [Citation18]. Secondary sarcomatoid change in nonsurgically treated HCC has also given rise to the hypothesis that SHC arises from selection pressure for sarcomatoid clones exerted by treatment by way of chronic cellular injury [Citation19].
Compared with conventional HCC, patients with SHC, as in our case, tend to have tumors of larger size [Citation2–4,Citation11,Citation20], more frequent occurrence of tumor necrosis [Citation10,Citation21], more advanced stage [Citation3] and higher grade, with higher rates of positive lymph nodes [Citation11,Citation20]. The conventional HCC component is more often poorly differentiated or undifferentiated [Citation2–4]. The sarcomatoid component stains positively for vimentin and cytokeratins [Citation8] and negatively for mesenchymal markers like SMA, caldesmon, desmin, DOG-1 and CD34 [Citation4,Citation22]. But while the epithelial component stains similar to conventional HCC of the same grade, hepatocellular markers like HepPar1 and arginase 1, are negative in the sarcomatoid areas. A positive reaction for vimentin is often seen in the sarcomatoid component, but not in the epithelial component [Citation23].
SHC must be differentiated from primary hepatic sarcoma. The diagnosis of sarcoma is based on a combination of the characteristic morphology and IHC profile, and the absence of epithelial differentiation. The terms SHC and hepatic carcinosarcomas are often used synonymously [Citation17]. However, despite their close morphologic resemblance, a distinction can be made. In carcinosarcoma, the spindle cells are negative for keratin while the converse holds in SHC [Citation24]. SHC should also be distinguished from collision tumors with separate primary foci of HCC and sarcoma. Imaging and thorough sampling on gross examination will reveal independent tumors. The absence of intimate commingling of dissimilar tumors on histology and distinct immunoprofiles in the separate tumors is diagnostic.
Due to the rarity of this neoplasm, there is currently no consensus on the management of SHC. Various treatment modalities and combinations thereof have been utilized, including surgical resection, adjuvant and neoadjuvant systemic therapy (chemotherapy, hormone therapy and immunotherapy), ablation, radiotherapy, thermotherapy [Citation9] and transplantation [Citation3]. In multivariate analysis, chemotherapy has been found to be associated with improved overall survival [Citation3].
Stage-for-stage, patients with SHC have comparatively worse outcomes than patients with conventional HCC. SHC is associated with shorter recurrence-free and overall survival and higher rates of extrahepatic metastasis [Citation4,Citation21]. Liao et al. found estimated 1-, 3- and 5-year overall survival rates of 45, 17.5 and 10.0% for SHC compared with 75.0, 48.1 and 28.1% for conventional HCC [Citation4]. Other studies have shown even worse outcomes. Zakka et al. found SHC with the worst 5-year overall survival (9.6%) of all variants of HCC [Citation3]. Wu et al. found a 5-year overall survival of 5.7% for SHC versus 30.1% for conventional HCC [Citation2]. In the study by Li et al., 1-year overall survival for SHC was 22%, and no patients were alive at 5 years [Citation11]. Median overall survival for treated SHC ranges from 4.7 [Citation3] to 8.8 months [Citation5]; the median survival for untreated disease is 5.4 months [Citation5].
Independent risk factors for overall survival include vascular invasion, nonradical surgical resection [Citation11], untreated disease [Citation5], tumor, node, and metastasis (TNM) stage and local invasion [Citation25]. The more frequent occurrence of positive surgical margins in SHC [Citation11,Citation15] also contributes to poorer outcomes [Citation3]. Due to ease of complete resection, outcomes for pedunculated lesions might be better [Citation26].
Conclusion
SHC is a rare, aggressive variant of hepatocellular carcinoma with rapid tumor growth and disease progression. Thus, this neoplasm portends a poorer prognosis compared with conventional HCC.
Future perspective
The lack of a clear understanding of the etiopathogenesis of SHC highlights the need for further studies using experimental, molecular, ultrastructural, IHC and other techniques. Thus, a multicenter collaborative effort is needed to pool cases for larger clinicopathologic studies of this rare entity. These will be important steps to help bridge the development of more effective therapies for this very aggressive lesion.
Sarcomatoid hepatocellular carcinoma (SHC) is a rare, aggressive subtype of hepatocellular carcinoma (HCC) with a poor prognosis.
The clinical diagnosis of SHC is challenging because of nonspecific imaging findings, along with lower levels of liver function tests and serum tumor markers than encountered with conventional HCC.
The diagnosis of SHC is made by histopathologic analysis which demonstrates a component of conventional HCC admixed with areas of spindled and pleomorphic sarcomatoid morphology.
By immunohistochemistry, the conventional HCC component stains positive with typical HCC markers including HepPar1 and arginase 1, along with cytokeratins; while the sarcomatoid component is negative for HepPar1 and arginase 1 with positivity for vimentin and cytokeratins.
The etiopathogenesis of SHC remains unsettled with competing metaplastic and neoplastic theories.
Current therapy relies primarily on complete surgical resection.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- BrayF, FerlayJ, SoerjomataramI, SiegelRL, TorreLA, JemalA. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.68(6), 394–424 (2018).
- WuL, TsilimigrasDI, FarooqAet al.Management and outcomes among patients with sarcomatoid hepatocellular carcinoma: a population-based analysis. Cancer125(21), 3767–3775 (2019).
- ZakkaK, JiangR, AleseOBet al.Clinical outcomes of rare hepatocellular carcinoma variants compared to pure hepatocellular carcinoma. J. Hepatocell. Carcinoma6, 119–129 (2019).
- LiaoSH, SuTH, JengYMet al.Clinical manifestations and outcomes of patients with sarcomatoid hepatocellular carcinoma. Hepatology69(1), 209–221 (2019).
- KanA, GuoRP. The prognosis of subsequent surgical treatment in patients with sarcomatoid carcinoma in the liver: a retrospective study. Int. J. Surg.55, 145–151 (2018).
- KakiozeS, KojiroM, NakashimaT. Hepatocellular carcinoma with sarcomatous change clinicopathologic and lmmunohistochemical studies of 14 autopsy cases. Cancer59(2), 310–316 (1987).
- KojiroM, SugiharaS, KakizoeS, NakashimaO, KiyomatsuK. Hepatocellular carcinoma with sarcomatous change: a special reference to the relationship with anticancer therapy. Cancer Chemother. Pharmacol.23(1), S4–S8 (1989).
- ShafizadehN, KakarS. Hepatocellular carcinoma: histologic subtypes. Surg. Pathol. Clin.6(2), 367–384 (2013).
- MaQ, JiangL, BondaS, LuoD, ZhangW. A rare case of hepatic sarcomatoid carcinoma: exceeding expectations in a stage IV primary hepatic sarcomatoid carcinoma patient. Int. J. Clin. Exp. Pathol.12, 378–383 (2019).
- ShiD, MaL, ZhaoDet al.Imaging and clinical features of primary hepatic sarcomatous carcinoma. Cancer Imaging18(1), 36 (2018).
- LiZ, WuX, BiXet al.Clinicopathological features and surgical outcomes of four rare subtypes of primary liver carcinoma. Chin. J. Cancer Res.30(3), 364–372 (2018).
- KomadaN, YamagataM, KomuraKet al.Hepatocellular carcinoma with sarcomatous change arising in primary biliary cirrhosis. J. Gastroenterol.32(1), 95–101 (1997).
- KodaM, MaedaY, MatsunagaY, MimuraK, MurawakiY, HorieY. Hepatocellular carcinoma with sarcomatous change arising after radiofrequency ablation for well-differentiated hepatocellular carcinoma. Hepatol. Res.27(2), 163–167 (2003).
- DahmHH. Immunohistochemical evaluation of a sarcomatoid hepatocellular carcinoma with osteoclastlike giant cells. Diagn. Pathol.10, 40 (2015).
- NomuraK, AizawaS, UshigomeS. Carcinosarcoma of the liver: report of an autopsy case with review of the literature and histogenetic consideration. Archives of Pathology & Laboratory Medicine124, 888–890 (2000).
- MurataM, MiyoshiY, IwaoKet al.Combined hepatocellular/cholangiocellular carcinoma with sarcomatoid features: genetic analysis for histogenesis. Hepatol. Res.21(3), 220–227 (2001).
- SchaeferIM, SchweyerS, KuhlgatzJ. Chromosomal imbalances in primary hepatic carcinosarcoma. Hum. Pathol.43(8), 1328–1333 (2012).
- FayyaziA, NolteW, OestmannJW, SattlerB, RamadoriG, RadzunHJ. Carcinosarcoma of the liver. Histopathology32, 385–387 (1998).
- TandanS, SunK, GaglioPJ. A rare finding on liver explant. Clin. Gastroenterol. Hepatol.12(9), e85–86 (2014).
- JerniganPL, WimaK, HansemanDJet al.Natural history and treatment trends in hepatocellular carcinoma subtypes: insights from a national cancer registry. J. Surg. Oncol.112(8), 872–876 (2015).
- KooHR, ParkM-S, KimM-Jet al.Radiological and clinical features of sarcomatoid hepatocellular carcinoma in 11 cases. J. Comput. Assist. Tomogr.32(5), 745–749 (2008).
- GiunchiF, VasuriF, BaldinP, RosiniF, CortiB, D’errico-GrigioniA. Primary liver sarcomatous carcinoma: report of two cases and review of the literature. Pathol. Res. Pract.209(4), 249–254 (2013).
- MaedaT, AdachiE, KajiyamaK, TakenakaK, SugimachiK, TsuneyoshiM. Spindle cell hepatocellular carcinoma. A clinicopathologic and lmmunohistochemical analysis of 15 cases. Cancer77, 51–57 (1996).
- TorbensonMS. Morphologic Subtypes of hepatocellular carcinoma. Gastroenterol. Clin. North Am.46(2), 365–391 (2017).
- LuJ, ZhangJ, XiongXZet al.Primary hepatic sarcomatoid carcinoma: clinical features and prognosis of 28 resected cases. J. Cancer Res. Clin. Oncol.140(6), 1027–1035 (2014).
- ZhangJ, NiJ, ZhengB. Giant pedunculated sarcomatoid hepatocellular carcinoma: a case report. The Chinese-German Journal of Clinical Oncology10(11), 669–671 (2011).
