Abstract
Background: There is a lack of knowledge regarding the experience of patients with neuroendocrine tumors (NET) in France. Materials & methods: A patient survey that captured information on diagnosis, disease impact/management and awareness was conducted. Data of respondents from France were analyzed and compared with US data as a reference. Results: Key topics included delays in diagnosis, negative impact on quality of life, patient access to NET medical experts and treatments, and information on NET and treatments. Significant differences were observed between France and the USA regarding NET diagnosis. Conclusion: This survey highlights the considerable burden experienced by patients in France with NET and differences in patient experience between France and the USA that may result from different healthcare and social systems.
Includes only conditions mentioned by more than 5% of patients. Base: all US (n = 368 [of 758; 49%]) and French (n = 31 [of 117; 26%]) respondents who were diagnosed with other conditions prior to NET diagnosis. Question: Which of the following conditions were you initially diagnosed with before receiving a NET diagnosis?
†Statistical significance between France and the USA (blue, France > USA; red, USA > France).
NET: Neuroendocrine tumor.
US data reproduced with permission from [Citation8] Wolin E et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.
![Figure 1. Diagnoses received before a NET diagnosis.Includes only conditions mentioned by more than 5% of patients. Base: all US (n = 368 [of 758; 49%]) and French (n = 31 [of 117; 26%]) respondents who were diagnosed with other conditions prior to NET diagnosis. Question: Which of the following conditions were you initially diagnosed with before receiving a NET diagnosis?†Statistical significance between France and the USA (blue, France > USA; red, USA > France).NET: Neuroendocrine tumor.US data reproduced with permission from [Citation8] Wolin E et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.](/cms/asset/e89cc528-039b-43ae-994b-dbe65482cd7a/iije_a_12339523_f0001.jpg)
Base: All respondents in France
(n = 117) and the USA (n = 758). Question: Which of the following, if any, would have helped you have a better experience with your NET diagnosis? Bold numbers within inset table denote statistical significance between those visiting a specialist center at least once per year and those who do not.
†Statistical significance between France and the USA (blue, France > USA; red, USA > France).
HCP: Healthcare professional; NET: Neuroendocrine tumor.
US data reproduced with permission from [Citation8] Wolin et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.
![Figure 2. Desired improvements to the diagnosis of NET.Base: All respondents in France(n = 117) and the USA (n = 758). Question: Which of the following, if any, would have helped you have a better experience with your NET diagnosis? Bold numbers within inset table denote statistical significance between those visiting a specialist center at least once per year and those who do not.†Statistical significance between France and the USA (blue, France > USA; red, USA > France).HCP: Healthcare professional; NET: Neuroendocrine tumor.US data reproduced with permission from [Citation8] Wolin et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.](/cms/asset/00b2e7bf-f35f-4704-840e-2777764a0543/iije_a_12339523_f0002.jpg)
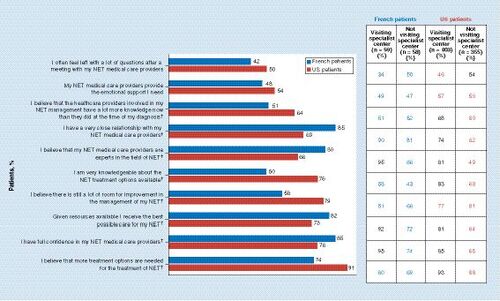
Base: all respondents in France (n = 117) and the USA (n = 758). Question: To what extent do you agree or disagree with the following statements? Top 2 box scores shown (Strongly agree/Somewhat agree). Bold numbers within inset table denote statistical significance between those visiting a specialist center at least once a year and those who do not.
* Denotes statistical significance between France and the USA (blue, France > USA; red, USA > France).
NET: Neuroendocrine tumor.
Base: all respondents in France (n = 117) and the USA (n = 758). Question: Which of the following would help with the ongoing management of your NET? Bold numbers within inset table denote statistical significance between those visiting a specialist center at least once per year and those who do not.
†Statistical significance between France and the USA (blue, France > USA; red, USA > France).
NET: Neuroendocrine tumor.
USA data reproduced with permission from [Citation8] Wolin et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.
![Figure 4. Desired improvements to the management of NET.Base: all respondents in France (n = 117) and the USA (n = 758). Question: Which of the following would help with the ongoing management of your NET? Bold numbers within inset table denote statistical significance between those visiting a specialist center at least once per year and those who do not.†Statistical significance between France and the USA (blue, France > USA; red, USA > France).NET: Neuroendocrine tumor.USA data reproduced with permission from [Citation8] Wolin et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.](/cms/asset/6e5fdbb7-0b47-4a09-8f82-1f328a78c97e/iije_a_12339523_f0004.jpg)
Base (A & B): All respondents in France (n = 117) and the USA (n = 758). Base (C): Respondents who are working full time/part time or self-employed in France (n = 32) and the USA (n = 316). Question (A): How much has each of the following areas of your life been negatively affected, if at all, by your NET? Top 2 box scores shown (A moderate amount/a lot). Question (B): Since you were diagnosed with your NET, have you had to make any of the following changes? Please select all that apply. Question (C): Has your NET impacted you at work in any of the following ways? Please select all that apply.
*Denotes statistical significance between France and the USA (blue, France > US; red, US > France).
‡Accommodations: for example, flexible work schedule, work from home, adaptive devices, opportunities for rest, etc.
NET: Neuroendocrine tumor.
US data reproduced with permission from [Citation8] Wolin et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.
![Figure 5. Impact of NET on quality of life (A), and changes made to daily life (B) and work life (C) as a result of NET.Base (A & B): All respondents in France (n = 117) and the USA (n = 758). Base (C): Respondents who are working full time/part time or self-employed in France (n = 32) and the USA (n = 316). Question (A): How much has each of the following areas of your life been negatively affected, if at all, by your NET? Top 2 box scores shown (A moderate amount/a lot). Question (B): Since you were diagnosed with your NET, have you had to make any of the following changes? Please select all that apply. Question (C): Has your NET impacted you at work in any of the following ways? Please select all that apply.*Denotes statistical significance between France and the USA (blue, France > US; red, US > France).‡Accommodations: for example, flexible work schedule, work from home, adaptive devices, opportunities for rest, etc.NET: Neuroendocrine tumor.US data reproduced with permission from [Citation8] Wolin et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.](/cms/asset/5e1eef8a-70df-4ee7-acd2-4c79ed74ec1d/iije_a_12339523_f0005.jpg)
Base: all respondents in France (n = 117) and the USA (n = 758). Question: Which of the following, if any, would help you living with a NET? Bold numbers within inset table denote statistical significance between those visiting a specialist center at least once a year and those who do not.
†Statistical significance between France and the USA (blue, France > USA; red, USA > France).
NET: Neuroendocrine tumor.
USA data reproduced with permission from [Citation8] Wolin et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.
![Figure 6. Desired improvements to help patients live with NET.Base: all respondents in France (n = 117) and the USA (n = 758). Question: Which of the following, if any, would help you living with a NET? Bold numbers within inset table denote statistical significance between those visiting a specialist center at least once a year and those who do not.†Statistical significance between France and the USA (blue, France > USA; red, USA > France).NET: Neuroendocrine tumor.USA data reproduced with permission from [Citation8] Wolin et al., https://journals.lww.com/pancreasjournal/pancreasjournal/fulltext/2017/05000/patient_reported_experience_of_diagnosis,.8.aspx Copyright © Wolters Kluwer Health, Inc. (2017). All rights reserved.](/cms/asset/82002bdb-de2d-4d18-86bf-495e6098d3d8/iije_a_12339523_f0006.jpg)
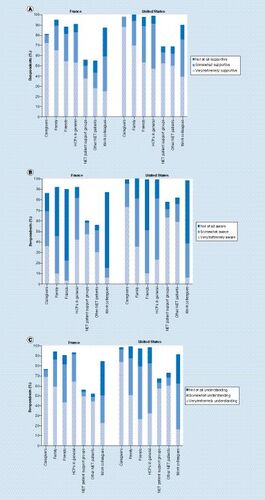
Base: all respondents in France (n = 117) and the USA (n = 758). Question (A): Which of the following best describes the level of support you receive from each of the following in helping you deal with your NET? Question (B): How would you describe awareness of NET among each of the following? Question (C): To what extent do the following people understand how having a NET affects your life?
HCP: Healthcare professionals; NET: Neuroendocrine tumor.
Neuroendocrine tumors (NET) are tumors that originate from neuroendocrine cells at any site of the body, including in the gastrointestinal (GI) tract, pancreas and lungs [Citation1]. NET are a heterogeneous group of tumors, and patient presentation is therefore clinically diverse [Citation2]. NET can be classified as functional tumors on the basis of their production of bioactive hormones or amines that cause clinical symptoms [Citation1], which can vary depending on the hormones or amines secreted by the tumor [Citation2]. A common condition caused by functional NET is carcinoid syndrome, and the classic clinical symptoms are diarrhea and flushing [Citation2]. Patients with carcinoid syndrome generally have a primary tumor originating in the small intestine, but can also occur with lung tumors, and the symptoms of carcinoid syndrome usually present after metastatic spread to the liver [Citation2]. Nonfunctional NET patients may present with mechanical difficulties owing to the tumor bulk (e.g., pain and obstruction) and the impact of metastases (usually to liver, lymph nodes and bone) [Citation2]. A large proportion of NET are diagnosed incidentally, reflecting the indolent nature of a subgroup of patients [Citation2–4].
The symptoms due to NET are often nonspecific, leading to difficulties and delays in diagnosis [Citation2]. The rarity of NET in the daily practice of healthcare professionals (HCPs) may also make diagnosis challenging [Citation5]. Because of that rarity, a potential loss of opportunity in a patient’s management could occur during the different steps to diagnosis, depending on the function and structure of the health system and its degree of expertise. A French population-based study using data obtained from 1976 to 1999 estimated the age-standardized incidence rate of digestive NET to be 0.76 per 100,000 men and 0.50 per 100,000 women [Citation6]. A prospective observational study conducted in France between 2010 and 2011 estimated the annual incidence of NET to be at least 2.06 per 100,000 persons [Citation5], indicating that the incidence may be increasing or that diagnostic techniques and/or awareness have improved. The International Neuroendocrine Cancer Alliance (INCA) currently consists of 26 independent charitable organizations and patient groups from 22 countries; it aims to be the global advocate for patients with NET. In collaboration with Novartis Pharmaceuticals Corporation, INCA conducted the first global patient survey to gather data about the experience of patients with NET in order to increase knowledge regarding NET from a patient perspective. The main goals were to increase understanding of patient needs and challenges regarding diagnosis, interactions with HCPs, knowledge and awareness of NET, information sources, and long-term management of the disease. Results of the global survey (N = 1928) have been published elsewhere [Citation7], as well as specific results from patients in the USA [Citation8]. This article presents data analyzed from patients with NET in France and provides US data as a reference for comparison.
Materials & methods
Study design & participants
Recruitment of patients for the survey was conducted primarily online via the use of website postings, emails, flyers and social media channels of the INCA member organizations/patient advocacy groups. The survey was conducted between February and May 2014.
Survey details
The survey was designed and conducted as an equal collaboration between INCA and Novartis Pharmaceuticals. The survey domains and key questions were originally developed based on discussions at the roundtable INCA meeting in March 2013 in Barcelona, Spain. Hall & Partners was commissioned to construct and professionally develop the questions; 14 NET patient health consumer groups within INCA (in France: Association des Patients porteurs de Tumeurs Endocrines Diverses [APTED]; in the USA: The Carcinoid Cancer Foundation and the Neuroendocrine Tumor Research Foundation [formerly known as the Caring for Carcinoid Foundation]) had direct input into question development, and the final questionnaire was reviewed and edited by members of INCA and Novartis between May and October 2013. For transparency, the Novartis logo was clearly displayed on all materials, and patients were notified that the INCA patient group partners or Novartis may use the data for disease awareness purposes. Hall & Partners facilitated conduction of the survey from February to May 2014 and analyzed the results. This anonymous survey was designed to be mostly completed online, with an estimated duration to completion of 25 min.
Sociodemographic information, including sex, age, employment status and education level, was collected in addition to patient-reported clinical characteristics such as type, functional status and grade of NET. Furthermore, information on desired improvements, the burden of NET on daily life and work, interactions with medical teams, and sources of information on NET were also captured.
The survey was deliberately designed to be self-reporting to appropriately capture patient opinion. Question categories included initial screening; patient’s current status; diagnosis; quality of life (QoL); NET management; NET treatment; NET education; and demographics. The majority of survey questions were close-ended (i.e., participants were provided options from which to choose). The only exceptions were open-ended questions related to the collection of numerical data (e.g., age, number of visits per year to a NET specialist center, etc.). Patients were asked to rate certain parameters; responses included ‘not at all,’ ‘somewhat,’ ‘very,’ and ‘extremely’ as graded descriptors, and when asked the degree to which they (dis)agreed with a particular statement, the descriptors ‘strongly’ and ‘somewhat’ were used.
Analysis
The data were analyzed using the MERLIN (Merlinco, London, UK) survey software package in aggregate for France and the USA, as well as by subgroups, including primary site of tumor, time since diagnosis (<5 or ≥5 years), and whether the patient reported that they had visited a specialist NET treatment center within the past year (defined in the survey as a medical center that specializes in NET). Survey responses were summarized using descriptive statistics, including means, medians and percentages. Statistical differences at the 95% CI (p < 0.05) were calculated. Some responses are presented as a combination of the top two responses (e.g., somewhat agree/strongly agree; a moderate amount/a lot).
Results
Survey population
Participating countries included the USA (n = 758; 39%), Germany (n = 311; 16%), Canada (n = 164; 9%), the UK (n = 156; 8%), Oceania (n = 138; 7%), France (n = 117; 6%), Japan (n = 81; 4%) and other European countries (n = 179; 9%).
French respondents were from a wide range of regions (Supplementary Figure 1). Of participants from France, 42% heard about the survey through a patient advocacy group, 25% through a website, 6% through social media, 4% through a nurse, 2% read about it in a brochure and 21% through other means.
US respondents were widely dispersed across the country and represented 49 of the 50 states. A large number of the participants from the USA were in California (n = 87), Pennsylvania (n = 48) and Texas (n = 48). US respondents heard about the survey through a website (41%), social media (32%), a patient advocacy group (20%) or other means (6%).
Demographic & clinical characteristics
Demographics and clinical characteristics of respondents from France and the USA are shown in . Although the mean age of survey participants in France and the USA was similar (61 and 57 years, respectively), there was a significant difference in the age distribution, with a larger proportion of US than French participants in the 50–59 years age group. A higher proportion of US than French participants were in full-time employment or on medical disability, and a lower proportion of US than French participants were retired. The presence of a caregiver (defined as a close family member or friend who helps manage day-to-day activities) was reported by 62% of French and 59% of US respondents. Clinical characteristics such as grade, functional status, metastatic disease at time of diagnosis and surgical removal of the primary tumor were generally consistent between the French and US participants. However, significant differences were found in terms of the NET primary site, with a higher proportion of US participants having a GI or lung primary site, whereas a higher proportion of French than US participants had a pancreatic primary site.
NET symptoms reported by ≥25% of respondents from France included general fatigue (64%), diarrhea (47%), abdominal pain or cramping (34%), weight loss (30%) and skin reactions (26%). Among respondents from the USA, commonly reported symptoms of NET included general fatigue (61%), diarrhea (55%), abdominal pain or cramping (47%), skin reactions (47%), sweating, headaches/dizziness, nausea with/without vomiting (35%), anxiety, palpitations (33%), breathlessness/wheezing (30%), changes in blood pressure (28%) and memory loss and/or confusion (25%).
NET diagnosis
The time from first symptom onset of NET to receiving a NET diagnosis was similar between French and US respondents, at a mean of 4 years and 3 months (51.3 months) and 4 years and 11 months (59.0 months), respectively. Twenty-eight percent of French and 34% of US respondents reported ≥5 years between first symptom onset and NET diagnosis. For those individuals with a GI primary site, the mean time to diagnosis was equivalent between French and US respondents (63.6 and 61.4 months, respectively), whereas a longer mean time to diagnosis of pancreatic NET was reported for US than French respondents (53.4 and 36.3 months, respectively).
The mean number of HCPs (including all doctors, specialists and nurses) involved in the NET diagnosis was similar for French and US respondents (5.2 and 5.7 HCPs, respectively). However, the mean number of visits to HCPs to receive the NET diagnosis was significantly less for French (8.0) than US (12.7) respondents.
Twenty-six percent of French and 22% of US participants reported that they had no initial symptoms and were diagnosed with NET incidentally (during tests for another condition). NET was the first diagnosis received by significantly more French than US participants (38 vs 23%). Thirty-four percent of French respondents had been diagnosed at a NET specialist center, a significantly higher proportion than the 9% of US respondents who were diagnosed at a NET specialist center. French participants who visited a NET specialist center were more likely to receive a NET diagnosis as a first diagnosis than those who did not visit a NET specialist center (49 vs 28%), whereas there was no difference for US participants who were visitors compared with those who were nonvisitors to specialist centers (23% for both).
Twenty-six percent of French and 49% of US participants reported being diagnosed with one or more conditions before receiving a NET diagnosis. A similar proportion received one other diagnosis before the NET diagnosis (15% of French and 16% of US participants), whereas a higher proportion of US than French participants received more than one other diagnosis before eventually being diagnosed with NET (33 vs 11%). The most common diagnoses received by French participants before the NET diagnosis were anxiety/psychosomatic-type condition (35%), gastritis/other digestive disorder (32%), irritable bowel syndrome (19%) and inflammatory bowel diseases (Crohn’s disease, ulcerative colitis; 10%; ). US participants were significantly more likely than French participants to receive an initial diagnosis of irritable bowel syndrome (49%), asthma (19%), menopause (15%) or ulcer (13%).
Only about half of French and US respondents felt that they received adequate information about NET and had their questions answered after receiving their diagnosis (50 and 55%, respectively for French; 45 and 57%, respectively for USA). Many respondents reported feeling shocked, scared or uncertain at the time of diagnosis. French respondents who visited specialist centers were less likely than nonvisitors to have felt scared (44 vs 67%) or isolated (15 vs 31%) at diagnosis. The majority of French respondents reported that they received comprehensive medical care after diagnosis (82%) but a similar proportion reported that they believe there is a great deal of room for improvement in the process of diagnosing NET (83%).
Desired improvements to the diagnosis of NET are shown in . A large proportion of NET respondents from France wanted clearer information on the long-term impact of NET, better direction on where to find useful resources on NET, and immediate access to NET patient support groups. In most instances, a significantly larger percentage of respondents from the USA than from France reported that they desired improvements in the diagnosis of NET. A larger percentage of French than US respondents reported that everything went to their satisfaction.
NET management
Fifty percent of respondents in France and 53% in the USA reported attending a NET specialist center at least once a year for the ongoing management of their NET. Among those who visited a NET specialist center, the mean number of visits per year was 5.0 (range 0–18) among French respondents and 3.6 (range 0–30) among US respondents. Among both French and US respondents, a mean of approximately 3 HCPs were involved in ongoing management. There were significant differences in the HCPs commonly involved in the NET management team for France compared with US participants: general practitioner (GP)/primary care physician (PCP)/internist reported by 70% of French and 53% of US participants, GI specialist reported by 56% of French and 34% of US respondents, oncologist/hematologist reported by 50% of French and 87% of US respondents, nuclear medicine specialist reported by 29% of French and 20% of US respondents, physician assistant reported by 3% of French and 15% of US respondents. No appreciable difference was found between France and the USA for the involvement of surgeons (41 and 37%), endocrinologists (27 and 22%), nurse/nurse practitioners (34 and 27%), pulmonologists/lung specialists (5 and 11%) and nutritionists (7 and 8%). Nurses, GP/PCPs and GI specialists were seen significantly more often in France than the USA for ongoing NET management. On average, nurses were seen monthly in France and every 3 months in the USA, GP/PCPs were seen every 3 months in France and every 5 months in the USA, and GI specialists every 6 months in France and every 9 months in the USA. Oncologists/hematologists were reported to be seen on average approximately every 4 months by both French and US respondents.
Median distance traveled by respondents to see their main NET medical provider was 58 km in France and 48 km in the USA. Thirty percent of French and 27% of US respondents traveled 1–20 km to their main NET medical provider, 17% of French and 23% of US respondents traveled 21–50 km, 10% of French and 13% of US respondents traveled 51–100 km, 16% of French and 10% of US respondents traveled 101–200 km and 23% of French and 22% of US respondents traveled >200 km. Six percent of French and 3% of US respondents traveled outside of France and the USA, respectively, for treatment or testing.
The mean number of tests for disease monitoring in a 1-year period was reported to be 5.1 in France and 5.5 in the USA. Sixty percent of French and 68% of US respondents had tests up to five-times per year, 14% of both French and US respondents had tests six–ten-times per year, and 9% of French and 16% of US respondents had tests more than ten-times per year. The most frequent tests used for disease monitoring among French and US respondents are shown in . Visiting a NET specialist center in France did not significantly change the likelihood of tests being performed. A higher percentage of respondents in the USA than in France reported use of imaging, chromogranin A, urinary and serum 5-HIAA, and endoscopic procedures for monitoring of their disease ().
Respondents discussed the following topics with their NET medical providers: results of ongoing tests (80% of French and 85% of US respondents), changes in physical health (72% of French and 77% of US respondents), changes in symptoms (72% of French and 79% of US respondents), overall well-being (64% of French and 70% of US respondents), new treatment options available (46% of French and 44% of US respondents), new research developments in the NET field (29% of French and 34% of US respondents) and emotional/mental health (24% of French and 28% of US respondents). Among French respondents, visiting a specialist center increased the likelihood of discussing changes in physical health (81% of visitors vs 62% of nonvisitors). Among US respondents, those who visited a specialist center had an increased likelihood of discussing the results of ongoing tests compared with those who did not visit a specialist center (89 vs 79%), changes in symptoms (83 vs 76%), changes in physical health (82 vs 72%), overall well-being (75 vs 65%), new treatment options (55 vs 32%) and new research developments (44 vs 23%).
In terms of making decisions on care, survey participants were questioned regarding the level of input that their NET medical care providers had in guiding their decision making. A higher proportion of respondents from France than from the USA fully relied on their NET medical care providers to guide their decisions (46 vs 23%). Thirty-two percent of French respondents liked to work in partnership with their NET medical care providers to make decisions and 15% preferred to make the decisions (with some input from NET medical care providers). Among US respondents, 12% preferred to make decisions and 61% partnered with their medical providers to make decisions.
Regarding the quality of currently available NET treatments, 43% of French and 52% of US respondents reported that the treatments were good or very good and 30% of French and 34% of US respondents felt that current treatments were poor or very poor. French patients visiting specialist centers at least once a year rated the overall quality of available NET treatments higher than nonvisitors (56 vs 29%). However, many respondents reported that they did not have access to treatments such as peptide receptor radionuclide therapy (PRRT; ). There was no significant difference in access to PRRT or receipt of PRRT between respondents from France and those from the USA. A significantly larger percentage of US than French respondents received surgery and interventional radiology and were placed on observation, whereas a larger percentage of French than US respondents received chemotherapy. Visiting a specialist center in France did not appear to significantly change access to treatments, whereas visiting a specialist center in the USA increased access to surgery, drug therapy other than chemotherapy and interventional radiology. Access to PRRT at specialist centers in France and the USA was equivalent (20% of French and 21% of US respondents reported access), as was the rate of current or past receipt of PRRT (12 and 11%, respectively). Among those who did not visit a specialist center, the rate of current or past receipt of PRRT was 9% in France and 8% in the USA. Fifty percent of respondents in France and 37% of respondents in the USA reported that they had participated in a clinical trial for NET treatment.
Seventy-two percent of respondents in France felt that NET medical providers always functioned as a well-coordinated team, and this belief was more prevalent among those visiting NET specialist centers (76% of visitors vs 67% of nonvisitors). In contrast, 43% of respondents in the USA felt that NET medical providers always functioned as a well-coordinated team (51% of visitors vs 34% of nonvisitors). Significant differences were found between respondents from France and the USA in terms of their perception of the current medical management of their NET (). French respondents attending a specialist center were more likely to believe that their medical providers were experts in the field of NET, that they are given the best possible care available and to have full confidence in their medical care provider.
Desired improvements in the management of NET are shown in . A significantly larger percentage of US than French respondents desired improvements in six of the seven assessed categories of the management of NET. Having more treatments made available in France than are currently available in other countries was cited as a desired improvement by the highest percentage of French respondents, whereas having a wider range of treatment options was cited most commonly by US respondents.
Quality of life
Seventy-seven percent of French and 73% of US respondents reported that their NET had a large or moderate negative impact on their QoL in general. Among French respondents, patients with GI NET were more likely than those with pancreatic NET to experience at least a moderately negative impact on their QoL (89 vs 67%). Among US respondents, patients with GI NET and pancreatic NET did not differ in their reporting of a negative impact on their QoL (73 and 74%, respectively).
French respondents reported a negative impact on different aspects of their daily lives as a result of their NET, especially overall energy levels, emotional health and ability to engage in leisure activities (A). A negative impact on relationships with friends and family was reported by approximately a third of French respondents, as well as a negative impact on finances. Although the negative impact on different aspects of life was fairly consistent between French and US respondents, the negative financial impact was significantly larger among US than French respondents. A negative impact on attitude toward daily life and the ability to travel were reported by a significantly higher percentage of French than US respondents.
French respondents reported having made several lifestyle changes because of their NET, in particular, dietary changes and changes in physical activity, and traveling for treatment (B). A significantly larger percentage of US than French respondents reported having to make dietary changes, increase spending on travel to attend medical appointments, cut back on social life and leisure purchases, and increase spending on nutritional products.
Among French and US respondents who were not employed at the time of the survey, 79% (15/19 and 151/192, respectively) reported that they had to stop working as a direct result of their NET. Among those who had retired in France (n = 59), 12% reported having to retire earlier than planned, 14% had to stop working altogether for a period of time and 17% had to take days off from work. Among those who had retired in the USA (n = 209), 31% reported having to retire earlier than planned, 11% had to stop working altogether for a period of time and 14% had to take days off from work.
Among those French and US respondents currently working, over half reported that they missed days of work because of their NET (C). A significant difference was observed between French and US respondents with regard to having to stop working altogether for a period of time and needing to seek disability benefits.
Many French and US respondents reported that their NET made them feel concerned (50 and 60%), anxious/worried (49 and 45%) and uncertain (51 and 45%). The most common feelings reported by French respondents were worrying about the uncertainty of the future (62 vs 57% of US respondents), finding they were not able to participate in activities that they used to enjoy (45 vs 45% of US respondents) and anxiety/stress (43 vs 58% of US respondents).
Desired improvements to help patients live with their disease are shown in . The most common desired improvements to help with living with NET reported by French respondents were greater awareness of the NET ‘in general’ to make it easier for the patient to be more open about the disease, and a better understanding of the steps that can be taken to help manage their treatment-related and disease-related symptoms. Differences between French and US respondents were significant regarding the desire to have access to a NET medical team, better access to NET-specific medical treatments, and to have a more knowledgeable health team.
NET awareness/understanding/support
Although French respondents were generally open about their NET with family and friends, approximately a third of respondents reported not disclosing their condition to their neighbors or acquaintances, and 25% reported not telling their coworkers. The main reasons provided for choosing not to disclose their condition were: wanting privacy (from neighbors: 55%, from acquaintances: 44%, from coworkers: 55%), and not wanting to be viewed differently (from neighbors: 29%, from acquaintances: 32%, from coworkers: 38%). Among US respondents, a large percentage did not disclose their condition to acquaintances (53%) and neighbors (41%), and a smaller percentage did not tell their coworkers (11%). The main reasons for not disclosing their condition to acquaintances and neighbors were because they wanted privacy (42 and 50%, respectively) and because they knew little about NET and found it hard to explain (54 and 40%, respectively).
NET patients in France and the USA generally reported feeling supported by many different people, including HCPs in general, caregivers, family and friends (A). Both French and US respondents generally considered work colleagues to be the least supportive. French respondents felt that there was a high awareness of NET among caregivers and HCPs in general (B). Both French and US respondents reported that friends and work colleagues demonstrated the lowest awareness about NET. French and US respondents felt that caregivers had the highest understanding of how NET affects a patient’s life, and many respondents (both French and US) reported that their work colleagues were not at all understanding (C).
NET educational resources
Only 29% of French respondents felt very (23%) or extremely (6%) knowledgeable about their NET. Sixty-one percent felt somewhat knowledgeable, and 7% felt not at all knowledgeable. In contrast, 49% of US respondents felt very or extremely knowledgeable. In terms of sources of information on NET, most French respondents (65%) received information from NET websites; French patients diagnosed within the last 5 years were more likely to use NET websites (73 vs 55%). The most common websites used were www.apted.fr (69%) and www.renaten.org (53%). The websites www.apted.fr and www.renaten.org were considered very or extremely useful by 73 and 71% of French respondents, respectively. French respondents found many other information sources very or extremely useful, including World NET Cancer Awareness Day, in-person meetings/patient advocacy groups, NET conferences or events, and brochures provided by patient advocacy groups or HCPs. Information about clinical trials and how to manage side effects of treatment were noted as the greatest unmet need in terms of patient resources for French respondents.
Discussion
The findings of this survey of patients with NET residing in France provide valuable information on the patient perspective of living with NET and highlight key areas for the improvement of diagnosis and management of the disease in the French healthcare system. The survey also provides insight into the differences in the patient experience based on whether the patient resides in France or the USA. Although the healthcare and social systems and the processes for accessing innovation are clearly very different between the USA and France, comparisons between the French and US participants’ responses to survey questions yield crucial information to improve the management of NET in France.
The mean number of visits to HCPs before receiving a NET diagnosis was significantly less for French than US participants, a higher proportion of French than US participants received their diagnosis at a NET specialist center and NET was the first diagnosis received by a significantly greater proportion of French than US participants. This appeared to be reflected in the greater desire for improvements in the diagnosis of NET among US than French participants. Overall, the misdiagnoses and delay to diagnosis underscore the need for improved physician education on the diagnosis of NET, which is a goal of RENATEN, a French national network consisting of 17 healthcare centers specializing in the management of patients with NET. The RENATEN specialist centers bring together practitioners from different disciplines in multidisciplinary team meetings for the management of NET. The differences in access to specialist healthcare between French and US patients with NET may account for these findings; the USA does not have a system of NET specialist centers comparable with that of France (the French Group of Endocrine Tumors and RENATEN networks) or Europe (ENETS Centers of Excellence [with 4 ENETS-accredited centers in France] and EURACAN [with 2 centers for NET in France]). Furthermore, to our knowledge, many US states may not even have one NET specialist, and most NET specialists in the USA will assess patients only after they have received a diagnosis based on a biopsy specimen. In France, the French Group of Endocrine Tumors (GTE; Groupe d’étude des tumeurs endocrines) and the three national networks supported by the French Cancer National Institute each contribute to the optimization of care of patients with NET: TENpath, TENgen and RENATEN. TENpath provides expertise for the anatomo-pathologic diagnosis of NET and was created by the French National Institute of Cancer in 2010; by 2014, 22 expert centers were participating in TENpath. TENgen is a set of laboratories with expertise in the molecular diagnosis of inherited NET.
Although a larger proportion of French than US participants reported feeling that everything went to their satisfaction for their NET diagnosis, the majority of French participants did not feel this way, and French participants reported a number of desired improvements in the diagnosis of NET. Key desired improvements for the time of diagnosis identified among French participants were improved information on the long-term impact of the disease, advice on where to find useful information on NET and immediate access to NET patient support groups (e.g., APTED, AFNEM and Le Sourire de Sabrina).
The most commonly desired improvement to help French respondents live with their NET was improved awareness of NET in general to make it easier for the patient to be more open about the disease with other people. Better understanding of the steps that patients can take to help manage disease- and treatment-related symptoms was a common desired improvement among respondents from both France and the USA, along with the availability of more treatments for NET in their country (i.e., treatments that are currently available in other countries). At the time of the survey, access to PRRT in the USA and France was restricted to clinical trials [Citation8]; French recruiting centers were limited to locations in Paris, Lyon, Toulouse, Nantes and Marseille. Although awareness of PRRT was higher among respondents from the USA compared with respondents from France, access to PRRT was similar in both countries, and one in ten respondents from both countries reported that they either had received or were currently receiving PRRT. The patient-reported rate of current or past use of PRRT (10%) in this survey was higher than previously reported by physicians during 2010–2011, which was 5.6% in France and 5.4% in the USA [Citation9] and in a survey of French patients conducted during 2014–2015, 4% reported use of PRRT [Citation10]. Notably, a higher proportion of respondents from the USA reported that they had been placed on observation (i.e., watch and wait) or were currently on observation compared with French respondents to this survey. The wait-and-watch approach is considered acceptable for asymptomatic patients with slowly progressive, low-volume disease as long as patients are closely observed [Citation11], and this strategy appears to be adopted more often in the USA than in France, although this could be partially explained by a lower proportion of respondents in the USA having pancreatic primary tumors.
Consistent with previous studies that demonstrated a lower QoL in patients with NET compared with the general population in the USA [Citation12], Sweden [Citation13] and Norway [Citation14], 77% of French respondents reported that their NET had a large or moderate negative impact on their QoL in general. It is to be expected that QoL is reduced for a patient who has a chronic progressive disease with symptoms, especially one without curative treatment. Similar observations are noticed in all patients with a chronic progressive disease with symptoms. In terms of the negative impact of NET on work life, approximately half of respondents from the USA and France reported that they have had to take days off from work. Furthermore, 50% of French respondents reported that they had to stop working altogether for a period of time and disability benefits were sought by 16% of French respondents. Differences in the social care system between France and the USA may explain the lower proportion of US respondents who stop working and seek disability benefits after being diagnosed with NET. Alternatively, another explanation may be the variation in demographics of survey respondents between France and the USA.
A higher percentage of US respondents reported that they had increased spending on travel to/from medical appointments (60% of US and 27% of French respondents); however, the median distance to the main NET medical provider was similar (58 km in France and 48 km in the USA). This may reflect differences in the transport system between the countries or what respondents interpreted as their NET medical provider, which could have been their referring physician.
Fifty-nine percent of respondents from the USA reported a negative impact of their NET in terms of finances, whereas this was noted by only 32% of French respondents. The difference in the impact on finances may be owing to the higher cost of medical therapy for NET in the USA compared with that in France [Citation15,Citation16], as well as to other differences in the healthcare systems. In 2000, the WHO measured the overall performance of health systems for 191 countries and found France to be among the top three countries, whereas the USA was ranked at number 37 [Citation17]. A recent Global Burden of Disease study quantified personal healthcare access and quality for 195 countries based on mortality rates from 32 causes that should not be fatal in the presence of effective medical care (e.g., epilepsy, appendicitis, measles and tetanus) and found both France and the USA to have high healthcare access and quality indexes in 2015 (87.9 and 81.3 [of 100], respectively) [Citation18] and 2016 (91.7 and 88.7, respectively) [Citation19]. However, an analysis of 11 countries in 2017 by The Commonwealth Fund found France and the USA, along with Canada, to rank among the lowest in overall healthcare system performance [Citation20]. The 2017 Commonwealth Fund performance analysis examined 5 domains of the healthcare system: care process, access, administrative efficiency, equity and healthcare outcomes [Citation20]. Both the USA and France had the poorest performance in terms of equity and administrative efficiency domains, and the USA had the poorest performance in the healthcare outcomes domain and the affordability subdomain, despite the fact that the USA had the highest healthcare spending as a percentage of gross domestic product (16.6%) [Citation20]. In this respect, the US system provides an example of high healthcare spending that does not translate into improved care for all patients [Citation21]. The US healthcare system is primarily based on financing by private health insurance (63.9% in 2012), with public health insurance provided by the government (Medicare) for only the most vulnerable populations, such as the elderly and disabled [Citation22]. However, with Medicare and private health insurance in the USA, the complexities in reimbursement systems and additional co-payments/out-of-pocket payments may limit patient access to treatment [Citation22]. In France, by contrast, basic universal public healthcare coverage is available [Citation23]; all medical procedures at both private and public hospitals are fully covered by the National Health Insurance (NHI) and therefore there is no substantial out-of-pocket cost for cancer patients seeking treatment [Citation22].
One important problem identified in this survey appears to be access to clinical trials in France. However, from our perspective, the apparent problems with access to clinical trials in France may be attributed to misperceptions, with several possible explanations (for example, the potential difficulty in obtaining information about ongoing studies focused on NET). In France, the GTE network website has begun to present the main clinical trial summaries on its website with the aim of improving access to this information. A special effort is also underway concerning equality of patient access between the French territories (e.g., from overseas departments vs metropolitan ones) in accordance with the French third national cancer plan.
Survey limitations
A patient-reported design for this survey was employed without independent verification, leading to potential recall bias. QoL was evaluated using a multiple-choice questionnaire and did not utilize standardized, validated QoL assessments. Recruitment was conducted primarily through patient advocacy groups and online sources, which may have resulted in a potentially biased sample that is not fully representative of the heterogeneous NET patient population. Respondents were more likely to be highly engaged and motivated care seekers and/or those with a poorer prognosis (>50% had metastatic disease at the time of diagnosis, with a mean time since diagnosis of 5–6 years). The attendance of a NET specialist center was reported by patients and was not independently verified according to strict criteria. The sample of respondents from France may be considered small and unbalanced with respect to the US population from a statistical perspective, and there were clear differences in the demographics and clinical characteristics of the French and US populations. However, considering the large population differences of each country and that this is a rare tumor type, and acknowledging that healthcare systems vary significantly from country to country, examination of the survey results from the French population allowed isolation of the unique challenges and experiences of patients with NET from France, with the US data serving as a useful reference point.
Overall conclusion
This survey highlights the considerable burden experienced by patients living with a NET in France and identifies the need for improved awareness of this condition among HCPs to facilitate a faster diagnosis and referral for treatment. Although many patients have access to NET specialist centers and participation in clinical trials, improved access for all French patients to innovation and information is of key importance.
Table 1. Patient-reported demographics and clinical characteristics.
Table 2. Tests received for ongoing NET management.
Table 3. Availability of NET treatments.
Summary points
The findings of this survey highlight the significant physical, emotional, social and economic impact of neuroendocrine tumors (NET) among French patients.
A feeling of isolation is common among patients with NET because this cancer is quite different from more commonly diagnosed cancers.
There is a need for greater awareness and education about NET in France.
Greater awareness/education about NET would allow patients to have improved quality of life and potentially improved outcomes.
Need to educate nonspecialist physicians to recognize the disease.
Author contributions
G Goldstein helped create and review all survey questions, promoted and shared the survey with patients with NET throughout the USA, and was involved in drafting and review of the manuscript. All other authors made substantial contributions to the conception of survey questions or the acquisition, analysis, or interpretation of data, and participated in drafting and critically revising the work. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Ethical conduct of research
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this survey.
Supplemental Figure 1
Download MS Word (264.8 KB)Acknowledgments
We thank the patients who participated in this survey and our patient advocacy partners throughout the world who contributed to this project. We especially thank the French Networks RENATEN and TENpath, and The Unicorn Foundation (Australia), vzw NET & MEN Kanker (Belgium), Association of Cancer Patients and Friends (APOZ, Bulgaria), Carcinoid-Neuroendocrine Tumour Society (CNETS, Canada), APTED (France), Netzwerk Neuroendokrine Tumoren (Germany), PanCAN (Japan), Unicorn Foundation NZ (New Zealand), CarciNor (Norway), Carcinoid & Neuroendocrine Tumor Society (CNETS, Singapore), NET Patient Foundation and The Association for Multiple Endocrine Neoplasia Disorders (AMEND, UK), and The Carcinoid Cancer Foundation and the Neuroendocrine Tumor Research Foundation (formerly known as the Caring for Carcinoid Foundation) (USA). Hall & Partners, a research organization, fielded the survey and analyzed the results.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2144/000112170
Financial & competing interests disclosure
Supported by Novartis Pharmaceuticals and conducted as an equal collaboration between the International Neuroendocrine Cancer Alliance and Novartis Pharmaceuticals. Financial support for medical writing assistance was provided by Novartis Pharmaceuticals Corporation. C Lombard-Bohas reports no conflicts of interest in this work. C Do Cao reports receiving honoraria for advisory boards from Novartis, Ipsen and Pfizer. J-P Metges reports serving on advisory boards for Novartis and Sandoz and as a speaker for Novartis, Sandoz and Ipsen. P Ruszniewski reports serving as a scientific advisor to Advanced Accelerator Applications, Novartis, Ipsen and ITM. D Smith reports participation in advisory boards for Ipsen, Advanced Accelerator Applications and Novartis. R Guimbaud reports participation in the advisory board for AstraZeneca and attending meetings/congresses for Roche, P. Fabre, Amgen, BMS, Servier and Novartis. C Lepage reports participation in advisory boards for Novartis and Advanced Accelerator Applications and serving as a speaker for Bayer, Amgen, Novartis and Advanced Accelerator Applications. R Hollander has no conflicts of interest to report. G Goldstein reports that the Carcinoid Cancer Foundation has received grants from Advanced Accelerator Applications, Ipsen, Lexicon Pharmaceuticals and Novartis. E Wolin reports participation in advisory boards for Progenics, Advanced Accelerator Applications and Lexicon. A Santos is an employee of Novartis. E Baudin reports personal financial interests, including serving on expert boards for Ipsen, Novartis, Advanced Accelerator Applications, Pfizer, and Hutchinson Pharma and drug supply for Pfizer and Advanced Accelerator Applications; institutional financial interests include research grants from Novartis and HRA and serving as a principal investigator for Ipsen; non-financial interests include serving as a past president of the French Group of Endocrine Tumors (GTE), a coordinator of the Neuroendocrine and French Adrenal Cancer Networks, and on the advisory board of ENSAT and ENETS networks. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by R Gordon, of ApotheCom (Yardley, PA, USA). Financial support for medical writing assistance was provided by Novartis Pharmaceuticals Corporation.
References
- RindiG, KlimstraDS, Abedi-ArdekaniB et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 31(12), 1770–1786 (2018).
- VinikAI, ChayaC. Clinical presentation and diagnosis of neuroendocrine tumors. Hematol. Oncol. Clin. North Am. 30(1), 21–48 (2016).
- CaplinME, BaudinE, FerollaP et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. 26(8), 1604–1620 (2015).
- TuragaKK, KvolsLK. Recent progress in the understanding, diagnosis, and treatment of gastroenteropancreatic neuroendocrine tumors. CA Cancer J. Clin. 61(2), 113–132 (2011).
- ScoazecJY, CouvelardA, MongesG et al. Professional practices and diagnostic issues in neuroendocrine tumour pathology: results of a prospective one-year survey among French pathologists (the PRONET Study). Neuroendocrinology 105(1), 67–76 (2017).
- LepageC, BouvierAM, PhelipJM et al. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut 53(4), 549–553 (2004).
- SinghS, GranbergD, WolinE et al. Patient-reported burden of a neuroendocrine tumor (NET) diagnosis: results from the first global survey of patients with NETs. J. Glob. Oncol. 3(1), 43–53 (2017).
- WolinEM, LeydenJ, GoldsteinG et al. Patient-reported experience of diagnosis, management, and burden of neuroendocrine tumors: results from a large patient survey in the United States. Pancreas 46(5), 639–647 (2017).
- CascianoR, WangX, SternL et al. International practice patterns and resource utilization in the treatment of neuroendocrine tumors. Pancreas 42(2), 339–347 (2013).
- PlanteA, BaudinE, DoCao C et al. Patient-reported tolerance in treatments approved in neuroendocrine tumors: a national survey from the French Group of Endocrine Tumors. Clin. Res. Hepatol. Gastroenterol. 42(2), 153–159 (2018).
- StrosbergJR, HalfdanarsonTR, BellizziAM et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 46(6), 707–714 (2017).
- BeaumontJL, CellaD, PhanAT et al. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 41(3), 461–466 (2012).
- FrojdC, LarssonG, LampicC, von EssenL. Health related quality of life and psychosocial function among patients with carcinoid tumours. A longitudinal, prospective, and comparative study. Health Qual. Life Outcomes 5, 18 (2007).
- HauglandT, VatnMH, VeenstraM et al. Health related quality of life in patients with neuroendocrine tumors compared with the general Norwegian population. Qual. Life Res. 18(6), 719–726 (2009).
- ChuangCC, BhurkeS, ChenSY et al. Clinical characteristics, treatment patterns, and economic burden in patients treated for neuroendocrine tumors in the United States: a retrospective cohort study. J. Med. Econ. 18(2), 126–136 (2015).
- MartyR, RozeS, KurthH. Decision-tree model for health economic comparison of two long-acting somatostatin receptor ligand devices in France, Germany, and the UK. Med. Devices (Auckl). 5, 39–44 (2012).
- TandonA, MurrayCJL, LauerJA, EvansDB. Measuring overall health system performance for 191 countries. WHO, Geneva, Switzerland (2000). GPE Discussion Paper Series: No. 30.
- GBD2015 Healthcare Access and Quality Collaborators. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet 390(10091), 231–266 (2017).
- GBD2016 Healthcare Access and Quality Collaborators.Measuring performance on the healthcare access and quality index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet 391(10136), 2236–2271 (2018).
- SchneiderEC, SarnakDO, SquiresD et al. Mirror, Mirror 2017: international comparison reflects flaws and opportunities for better U.S. Healthcare. The Commonwealth Fund, Washington DC, USA (2017). https://interactives.commonwealthfund.org/2017/july/mirror-mirror/
- Global Burden of Disease Health Financing Collaborator Network. Trends in future health financing and coverage: collabfuture health spending and universal health coverage in 188 countries, 2016–40. Lancet 391(10131), 1783–1798 (2018).
- BenjaminL, ButhionV, Vidal-TrecanG, BriotP. Impact of the healthcare payment system on patient access to oral anticancer drugs: an illustration from the French and United States contexts. BMC Health Serv. Res. 14, 274 (2014).
- ChevreulK, BergBringham K, Durand-ZaleskiI, Hernandez-QuevedoC. France. Health system review. Health Syst. Trans. 17(3), 251 (2015).
