Abstract
Aim: Two peptide cocktail vaccines using glypican-3, WD-repeat-containing protein up-regulated in hepatocellular carcinoma (HCC) and nei endonuclease VIII-like three epitopes were evaluated in advanced HCC in two Phase I studies. Patients & methods: Study 1 evaluated dose-limiting toxicities (DLTs) of peptides 1–3 (HLA-A24-restricted) and study 2 evaluated DLTs of peptides 1–6 (HLA-A24 or A02-restricted). Results: Overall, 18 and 14 patients were enrolled in studies 1 and 2, respectively. No DLTs were observed up to 7.1 mg of the vaccine cocktail. No complete response/partial response was observed. Stable disease was reported in nine and five patients with a disease control rate of 52.9% and 35.7% in studies 1 and 2, respectively. Conclusion: Both vaccines showed good tolerability and potential usefulness against HCC.
Clinical trial registration: JapicCTI-121933; JapicCTI-142477
Lay abstract
Two peptide cocktail vaccines (ONO-7268MX1 [HLA-A24-restricted] and ONO-7268MX2 [HLA-A24 or A02-restricted]) were derived from parts of the hepatocellular carcinoma cells to trigger the body’s immune reaction against the cancer cells. Study 1 evaluated increasing doses that would cause toxicity of peptides 1–3 and study 2 evaluated the dose of peptides 1–6. Although, no complete response or partial response was observed, stable disease was reported in nine/18 and five/14 patients with a disease control rate of 52.9% and 35.7% in studies 1 and 2, respectively. Both vaccines showed good tolerability and potential usefulness against hepatocellular carcinoma.
Hepatocellular carcinoma (HCC) is the most frequently occurring type of primary liver cancer and is among the most common incident cancers worldwide [Citation1,Citation2]. In the Asia-Pacific region, HCC is the third most common cause of cancer-related deaths [Citation3]. In Japan, HCC is reported as the fourth most common cancer in both sexes [Citation3] and HCV infection accounts for the highest proportion of HCC cases in Japan (1991 [∼70%] – 2015 [∼55%]) [Citation4].
According to an epidemiological study (1990–2006; follow-up until December 2008), among 2386 patients with HCC, extrahepatic metastases were observed in 342 (14.3%) patients. The most common extrahepatic metastasis site was the lungs (39.5%), followed by lymph nodes (34.2%), bone (25.4%) and adrenal glands (8.8%) [Citation5]. Although the prognosis of HCC with metastasis has improved since the introduction of molecular targeted drugs such as sorafenib, the development of new treatment methods to further enhance the prognosis is desired and various treatment methods are being developed [Citation6].
Among the newer treatment strategies, immunotherapeutic approaches are being evaluated to examine the role of the immune system in HCC with promising results [Citation7]. Immunotherapeutic approaches use three main strategies to improve the tumor-specific immune response: adoptive immunotherapy, using HCC-epitope immunized cells that act against the cancer cells; indirect immunological strategies, employing cytokines, immune checkpoint blockade monoclonal antibodies and cancer vaccines to enhance the immune system and indirect nonimmunological strategies, including metronomic chemotherapy, antigen-encoding mRNA strategies in HCC and oncolytic viruses [Citation7].
Three tumor antigens have been primarily identified in HCC as potential targets for immunotherapy. First is glypican-3 (GPC3), an oncofetal glycoprotein overexpressed in HCC and attached to the cell membrane by a glycophosphatidylinositol anchor. GPC3 levels have prognostic significance as a biomarker and can be used for immunoreactivity in HCC tumor cells. GPC3 is expressed in several tumor types, including lung squamous cell, ovarian and gastric carcinomas, pediatric embryonal tumors and melanomas; however, GPC3 expression is particularly high in HCC [Citation8–10]. Second is WD-repeat-containing protein upregulated in hepatocellular carcinoma (WDRPUH), identified as a novel protein with growth-promoting activity that is overexpressed in the majority of HCCs. WDRPUH encodes a predicted 620-amino acid protein containing 11 highly conserved WD40-repeat domains. WDRPUH may, thus, serve as a molecular target to treat HCCs, as its suppression retards cancer cell growth [Citation11]. The final tumor antigen is nei endonuclease VIII-like 3 (NEIL3). The mammalian NEIL3 is one of the four DNA glycosylases that recognize and remove hydantoins in the first step of the base excision repair (BER) pathway [Citation12]. DNA base lesions that accumulate during hypoxic–ischemic stress are removed by DNA glycosylases in the BER pathway to prevent mutagenesis and cytotoxicity [Citation13]. Abnormal expression of NEIL1, NEIL2 and NEIL3 in cancers can be associated with somatic mutation load [Citation14]. Using a high-throughput single-nucleotide polymorphism array, NEIL3 was shown to exhibit a high-frequency loss of heterozygosity in HCC, suggesting that it might be a potential tumor suppressor gene with a role in DNA repair [Citation15,Citation16].
Therefore, we developed two peptide cocktail vaccines (ONO-7268MX1 and ONO-7268MX2) using epitopes of GPC3, WDRPUH and NEIL3. ONO-7268MX1 is an HLA-A*24-restricted 3-epitope peptide vaccine cocktail derived from GPC3, WDRPUH and NEIL3. ONO-7268MX2 is an HLA-A*24- and HLA-A*02-restricted 6-epitope peptide vaccine cocktail derived from GPC3, WDRPUH and NEIL3. Based on the data on allele frequency released on the home page of NPO HLA Laboratory, Kyoto, Japan (as of 24 October 2012), the percentage of the Japanese population with either HLA-A*24 or HLA-A*02 or both was 85.2% [Citation17].
We conducted two Phase I, multicenter, open-label studies to evaluate the safety and efficacy of the ONO-7268MX1 and ONO-7268MX2 vaccines in patients with advanced HCC.
Materials & methods
Study design
Study 1 was a Phase I, open-label, dose-escalation, multicenter study of the ONO-7268MX1 vaccine (JapicCTI-121933; A). Briefly, the study enrolled three patients with HLA-A*24 in cohort 1 (0.9 mg once weekly [QW] group, subcutaneously). If no dose-limiting toxicities (DLTs) were observed within 4 weeks in cohort 1 (cycle 1), three additional patients were enrolled in cohort 2 (3.0 mg QW group). Similarly, if no DLTs were observed within 4 weeks in cohort 2 (cycle 1), three additional patients were enrolled in cohort 3 (7.1 mg QW for 4 weeks [cycle 1] group). Additional patients were also enrolled as replacements when patients discontinued treatment without completing the DLT evaluations.
Study 1: the dosage of ONO-7268MX1 was 0.3 mg each of peptides 1–3 for cohort 1 and 1 mg each of peptides 1–3 for cohort 2. For cohort 3, the dose was 3 mg of peptide 1, 1.1 mg of peptide 2 and 3 mg of peptide 3 (the total amount of the 3 types of peptides was 0.9, 3 and 7.1 mg, respectively). Study 2: the dose of ONO-7268MX2 per administration was determined as 6 mg, which contained 1 mg each of peptides 1–6.
DLT: Dose-limiting toxicity.
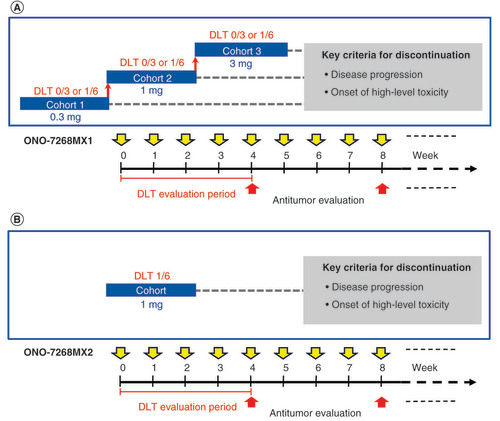
Study 2 was a Phase I, open-label, multicenter study of the ONO-7268MX2 vaccine (JapicCTI-142477; B). Briefly, six patients each with HLA-A*24 or HLA-A*02 were enrolled and observed for DLTs for 4 weeks after vaccination (cycle 1). Provision was made to enroll patients with both HLA-A*24 and HLA-A*02 as well.
All adverse events (AEs) were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0 Japanese translation Japan Clinical Oncology Group (JCOG) version [Citation18]. Treatment was discontinued if AEs that met the criteria for hematological toxicity or nonhematological toxicity (see definitions below) were observed within 4 weeks (DLT evaluation period) along with a causal relationship with the investigational vaccine.
DLT was defined as patients experiencing hematological toxicity (grade 4 decrease in the neutrophil count for ≥7 days and grade 4 decrease in the platelet count) or nonhematological toxicity (grade ≥3 AEs or alanine transaminase [ALT], aspartate transaminase [AST] and alkaline phosphatase ≥ten-times the upper limit of normal or total bilirubin increase of ≥5.0 mg/dl [study 1] or total bilirubin as a grade ≥3 AE [study 2]). The DLT evaluation phase in both studies was followed by the continuous administration phase. Each treatment cycle comprised weekly administration of the same vaccine dose for 4 weeks along with an antitumor evaluation until progression of disease (PD) or onset of high-level toxicity.
The studies were approved by the Institutional Review Boards of the National Cancer Center, Japan (Study 1: K0246, T3927; Study 2: K0370, T4075). This study was conducted in accordance with the Declaration of Helsinki and guidelines for Good Clinical Practice. Written informed consent was obtained from the patients twice, in other words, before starting the study and before moving the patients from the initial DLT evaluation phase to the continuous administration phase.
Patients
Patients aged ≥20 years with a diagnosis of histologically, cytologically or clinically confirmed HCC at the time of obtaining consent and who were willing to be hospitalized for 8 days from the day of the first vaccination were enrolled in the study. The diagnosis of clinical HCC was based on standard imaging evaluations and elevated alpha-fetoprotein (AFP) or protein induced by the absence of vitamin K or antagonist-II (PIVKA II) values compared with the individual reference range of the institution. For inclusion in the study, patients were also required to have met the following criteria: one or more measurable lesions within 14 days prior to the investigational vaccine administration, as defined in the revised Response Evaluation Criteria In Solid Tumours guidelines version 1.1 [Citation19]; unresectable HCC refractory to/intolerant of standard treatments or unresectable HCC for which no appropriate treatment was available; an expected survival of more than 3 months; ‘HLA-A*24:02’ for study 1 and ‘HLA-A*24 or HLA-A*02’ for study 2; Eastern Cooperative Oncology Group performance status of 0–1; Child-Pugh score of ≤8 points; within target clinical test results within 7 days prior to study vaccine administration (neutrophil count ≥1500/mm3, hemoglobin ≥8.5 g/dl, blood platelet count ≥5.0 × 104/mm3, AST [glutamic oxaloacetic transaminase] and ALT [glutamic-pyruvic transaminase] levels ≤5.0-times the normal upper limit of the reference range, total bilirubin ≤2.5 mg/dl and creatinine ≤2.0 mg/dl). For study inclusion, women of child-bearing potential (including those who did not have menstruation for medical reasons, such as chemical menopause) were required to practice double contraception from the date of consent to at least 120 days after the last vaccine dose, while men were required to practice double contraception from the start of vaccine administration to at least 180 days after the last vaccine dose.
Exclusion criteria were as follows: patients who had undergone treatments for HCC, such as hepatectomy, percutaneous local ablative therapy (radiofrequency ablation, percutaneous ethanol injection and percutaneous microwave coagulation therapy), transcatheter arterial (chemo) embolization and hepatic intra-arterial chemotherapy and systemic chemotherapy (excluding patients in whom sorafenib was given within 14 days prior to study drug administration) or surgical treatment with general anesthesia within 28 days prior to study drug administration. Patients who had undergone radiation therapy, including gamma-knife treatment and cyber-knife treatment, within 28 days prior to study drug administration (within 14 days for local radiation therapy for symptom relief) and who had received radiopharmaceuticals (excluding use of radiopharmaceuticals for testing and diagnosis) within 56 days prior to study drug administration were also excluded. Patients who had undergone surgical treatment with local or surface anesthesia within 14 days prior to study vaccine administration; required transplantation therapy or who had a past history of transplantation therapy (excluding autologous transplantation); had ostealgia associated with bone metastasis not stabilized with a consistent regimen of analgesics; had metastases in the brain and the meninges (except those meeting all the following criteria: no indications of cerebral edema that required any treatment on computed tomography or MRI examinations, no radiation therapy in the past 28 days and no PD observed from before the start of radiation therapy, no requirement for treatment with adrenal corticosteroids and no clinical symptoms); had received other investigational drug within 28 days prior to study vaccine administration (or 90 days for antibody preparation); had received HLA-restricted GPC3-derived peptides in the past and had a previous history of cancer immunotherapy (cancer vaccine therapy, dendritic cell therapy, activated lymphocyte therapy, etc.), had active infections that required treatment (except HBV and HCV), had congestive heart failure and other serious comorbidities and had chronic or recurrent autoimmune diseases were excluded. Those who required the use of immunosuppressants within 28 days prior to the study vaccine administration; had symptoms of hepatic encephalopathy; had ascites or pleural effusion requiring centesis; had double cancers (excluding completely resectable basal cell carcinoma, stage I squamous cell carcinoma, carcinoma in situ or superficial bladder cancer or other cancers with no metastasis/recurrence for over 5 years) and had a positive HIV-1 antibody test, HIV-2 antibody test or human T-lymphotropic virus-1 antibody test were excluded. Patients who were pregnant, breastfeeding or who may have been pregnant; had a history of serious drug hypersensitivity; continued to have adverse reactions or surgical treatment effects from previous treatments; were unable to provide written informed consent because of complication of dementia, etc. and were deemed unsuitable for the study by the principal investigator or the subinvestigator were also excluded from the study.
Dosage & administration
Peptides 1–3 (ONO-7268-01, ONO-7268-02 and ONO-7268-03) were evaluated in study 1, while peptides 1–6 (peptides 1–3, ONO-7268-04, ONO-7268-05 and ONO-7268-06) were evaluated in study 2. Each peptide comprised a sequence of nine amino acids from the epitopes of GPC3, WDRPUH and NEIL3 antigen proteins.
In study 1, patients in cohort 1 were vaccinated with peptides 1–3 at a dose of 0.3 mg each (subcutaneously QW), followed by cohort 2 with peptides 1–3 at a dose of 1.0 mg each (subcutaneously QW) and cohort 3 at a dose of 3.0 mg of peptide 1, 1.1 mg of peptide 2 and 3.0 mg of peptide 3 (subcutaneously QW).
The vaccine for study 1 was prepared in saline at concentrations of 0.6 mg/ml each of peptides 1–3 in cohort 1; 2.0 mg/ml each of peptides 1–3 in cohort 2; and 6.0 mg/ml of peptide 1, 2.2 mg/ml of peptide 2 and 6.0 mg/ml of peptide 3 in cohort 3. The emulsion was prepared by mixing an equal volume of adjuvant Montanide ISA 51 VG solution (SEPPIC, Paris, France). The final concentration of peptides 1–3 after preparation of the emulsion solution was 0.3 mg/ml each for cohort 1; 1.0 mg/ml each for cohort 2 and 3.0 mg/ml of peptide 1, 1.1 mg/ml of peptide 2 and 3.0 mg/ml of peptide 3 for cohort 3. Each administration dose was 1.0 ml at any one site of either the left and right axillary regions or the left and right groin regions (total four sites). Of note, the administration site was changed for each administration.
Rationale for the dosing regimen of ONO-7268MX1: We determined the dose of ONO-7268MX1 based on the results of preclinical studies, properties of peptides contained in ONO-7268MX1 and results of clinical research studies. In the 4-week, repeated-dose toxicity study of ONO-7268MX1 in rats and dogs (weekly intermittent subcutaneous administration), no adverse effects due to ONO-7268MX1 were observed and the no-observed-adverse-effect level (NOAEL) of ONO-7268MX1 in rats and dogs was 5 and 2.5 mg/kg, respectively, which were the maximum doses that could be administered. However, there was a limit to the concentration at which each peptide contained in ONO-7268MX1 could be dissolved. Accordingly, the maximum concentration of each peptide after preparation of the emulsion solution was 3 mg/ml. One ml of the emulsion solution was equivalent to 0.05 mg/kg of each peptide assuming the body weight was 60 kg and was considered to provide a safety margin of ≥100-fold and ≥50-fold of the NOAEL of ONO-7268MX1 in rats and dogs obtained in the preclinical study, respectively. Therefore, we considered it possible to set the maximum dose of ONO-7268MX1 at the dose containing up to 3 mg of each peptide (data on file). Furthermore, generally in peptide vaccines, it is considered that there are no animal species having the HLA molecule to which the peptide binds and the T-cell receptor recognizing its complex whose structures are identical to those of humans. Therefore, it is believed important to set the initial dose and dosing schedule in humans based on the experience of administration in humans as well as the results of preclinical studies [Citation20,Citation21]. In view of this, we deemed it appropriate to set the maximum dose of ONO-7268MX1 at the dose containing up to 3 mg of each peptide for the following reasons: in the clinical research study using peptide ONO-7268-01 contained in ONO-7268MX1 (examined dose: 0.3, 1, 3, 10 and 30 mg/body) [Citation22], no serious toxicity was observed at doses up to 30 mg in patients with HCC and there are no reports of significant toxicity for many previous cancer peptide vaccine therapies [Citation20] (although the therapies did not contain peptides included in ONO-7268MX1), where the clinical dose commonly used was 0.1–10 mg. However, it was difficult to manufacture a formulation containing 3 mg of each peptide. Accordingly, after considering the formulation that could be manufactured, the maximum dose of ONO-7268MX1 was determined to be the dose including 3 mg of ONO-7268-01, 1.1 mg of ONO-7268-02 and 3 mg of ONO-7268-03 (Data on file).
The initial dose of ONO-7268MX1 was set at the dose containing 0.3 mg of each peptide for the following reasons: in a clinical research study using peptide ONO-7268-01 contained in ONO-7268MX1 (examined dose: 0.3, 1, 3, 10 and 30 mg/body) [Citation22], cytotoxic T lymphocyte (CTL) was induced from 0.3 mg and CTL was induced at 0.1 or 0.3 mg for previously studied cancer peptide vaccine therapies [Citation20], although they did not contain peptides included in ONO-7268MX1.
The patients in cohort 2 (study 2) were vaccinated with six peptides at a dose of 1.0 mg each (subcutaneously QW).
For study 2, a similar preparation of 1.0 mg/ml peptide for each of the six peptides was prepared. The dose of ONO-7268MX2 per administration was determined as 6 mg, which contained 1.0 mg each of peptides 1–6. Each administration cycle comprised four subcutaneous QW injections for 4 weeks.
Rationale for the dosing regimen of ONO-7268MX2: We determined the dose of ONO-7268MX2 based on the properties of peptides contained in ONO-7268MX2 and the results of preclinical studies and clinical research studies. In the 4-week, repeated-dose toxicity study of ONO-7268MX2 in rats (weekly intermittent subcutaneous administration), no adverse effects due to ONO-7268MX2 were observed and the NOAEL of ONO-7268MX2 in rats was 2 mg/kg, which was the maximum dose that could be administered. However, there was a limit to the concentration at which each peptide contained in ONO-7268MX2 could be dissolved. Accordingly, the maximum concentration of each peptide after preparation of the emulsion solution was 1 mg/ml. When 1 ml of the emulsion solution was given, up to 1 mg of each peptide in ONO-7268MX2 could be administered. When this dose was converted to a dose per body weight based on the human body weight of 60 kg (mg/kg), it had a safety margin of 120-fold of the NOAEL of ONO-7268MX2 in rats (2 mg/kg) obtained in the preclinical study (Data on file). Based on the clinical research study using peptides (ONO-7268-01 and ONO-7268-04) contained in ONO-7268MX2 [Citation22] and reports for previous cancer peptide vaccine therapies [Citation20] (although they did not contain peptides included in ONO-7268MX2) as described in the rationale for the dosage of ONO-7268MX1, we considered it possible to set the dose of ONO-7268MX2 containing 1 mg of each peptide. Moreover, in clinical research studies using peptides with the same amino acid sequence as ONO-7268-01 or ONO-7268-04 contained in ONO-7268MX2 (examined dose: 0.3, 1, 3, 10 and 30 mg/body) [Citation22], not only CTL was induced but contribution to life expectancy was suggested at doses of 1 mg or more. Based on the above, the dose of ONO-7268MX2 in this study was set at the dose containing 1 mg of each peptide, which was determined based on the maximum solubility concentration of each peptide. The dosing schedule of ONO-7268MX1 and ONO-7268MX2 was decided to be QW, which had been commonly used in clinical research studies of cancer peptide vaccine therapies conducted in Japan at the time of planning each Phase I study. For the route of administration of ONO-7268MX1 and ONO-7268MX2, though subcutaneous and intradermal injections [Citation23–26] are used in cancer peptide vaccine therapies because of abundant antigen-presenting cells in the epidermis that capture the administered cancer peptide and induce CTL specific to the cancer peptides in the body, we selected subcutaneous injection because it is a simple procedure.
In studies 1 and 2, patients who did not develop DLTs during the first 4-week cycles were transitioned to the continuous administration phase. Other discontinuation criteria were according to the investigator’s discretion based on AE status regardless of the presence/absence of a causal relationship with the vaccine, according to the investigator’s discretion for any other reason, or PD (clinically evident exacerbation).
Prohibited concomitant drugs & combination therapy
Treatments that were prohibited during the DLT evaluation phase and the continuous administration phase were as follows: immunosuppressants and adrenal corticosteroids (external use and local administration of adrenal corticosteroids for diseases other than autoimmune diseases were permitted; additionally, temporary use of adrenal corticosteroids for the prevention of contrast agent allergy or hypersensitivity due to allergens was permitted); chemotherapy for malignant tumors (including hormonal therapy, gene therapy, treatment with biologics); radiation therapy; treatment with radiopharmaceuticals (excluding the use of radiopharmaceuticals for testing and diagnosis); surgical treatment or immunotherapy (tumor vaccine, cytokine, etc.); granulocyte colony-stimulating factor drug formulation (prohibited only during the DLT evaluation phase) and all other investigational drugs.
End points
The primary end point was safety and tolerability assessed throughout the study period until treatment discontinuation. DLTs were assessed during the first 4 weeks of treatment initiation for each study cohort. Secondary end points included efficacy (complete response [CR], partial response [PR], stable disease [SD] and disease control rate [CR + PR + SD]) and pharmacological effects (CTL response), which were evaluated for each cycle in the continuous treatment phase after the DLT evaluation phase. A post hoc exploratory analysis of progression-free survival (PFS) in patients with a CTL response 2 or 3 and 0 or 1 was also performed. CTL responses were evaluated using the standard enzyme-linked immunosorbent spot (ELISPOT) assay with the human interferon-gamma ELISPOT PLUS kit (Mabtech, Nacka Strand, Sweden). The CTL response positivity was classified into 4 grades (−, +, ++ and +++) depending on the amount of peptide-specific spots and invariability of peptide-specific spots at different responder/stimulator ratios. In both studies, CTL responses were evaluated at cycle 1, cycle 2 and cycle 3 and every 3 cycles thereafter.
The evaluation was continued until the end of vaccination due to disease progression, the onset of any intolerable AEs or the investigator’s discretion for any other reason. A case was judged to be positive when the algorithm indicated +, ++ or +++ at any of the evaluation cycles [Citation27]. Frequencies of Tregs and myeloid-derived suppressor cells (MDSCs) in peripheral blood; serum concentrations of IL-6, IL-10, IL-13 and TGF-β and plasma concentrations of kynurenine were also measured as biomarkers associated with the suppression of the antitumor immune response. In study 2, peptide-induced whole blood gene expression analysis was also examined.
Statistical analysis
The safety analysis set (SAS) comprised all patients who received at least one dose of the investigational vaccine. The DLT evaluation set comprised those patients who were included in the SAS and had appropriately completed the DLT evaluation phase and met any of the following criteria: received the investigational vaccine three or more times during the DLT evaluation period (cycle 1) and were not discontinued for reasons other than the development of a DLT and had a DLT during the DLT evaluation period (cycle 1).
The full analysis set comprised those patients included in the SAS and the largest set of patients eligible for the efficacy analysis who met any of the following three criteria: not a case of noncompliance with the Good Clinical Practice guidelines; advanced HCC, with no standard or adequate therapeutic options; and available efficacy evaluation or biomarker evaluation after vaccination.
Results
Patient disposition
Overall, 18 Japanese patients (cohort 1, three patients; cohort 2, eight patients; and cohort 3, seven patients) were enrolled in study 1. As a result of the prespecified DLT evaluation in the initial three patients in each cohort, the safety and tolerability of up to 7.1 mg of the vaccine cocktail in cohort 3 were confirmed; therefore, it was decided to add three patients in both cohort 2 and cohort 3 to further evaluate the safety, tolerability, efficacy and pharmacological effect. However, since two patients in cohort 2 discontinued the study without completing the DLT evaluation, two further patients were additionally enrolled in cohort 2 (total eight). Of the three patients in cohort 3, one discontinued without completing the DLT evaluation; therefore, one further patient was additionally enrolled in cohort 3 (total seven). Overall, no DLTs were observed in any patient in the three cohorts who completed the DLT evaluation.
Overall, 14 patients (HLA-A*24 cohort, seven patients; HLA-A*02 cohort, six patients; both HLA-A*24 and HLA-A*02 cohort, one patient) were enrolled in study 2. The single patient with both HLA-A*24 and HLA-A*02 was evaluated under the HLA-A*02 cohort.
Treatment discontinuation
In study 1, all patients discontinued the clinical trial due to PD/clinically evident exacerbations (PD based on Response Evaluation Criteria In Solid Tumours version 1.1 and PD based on clinical symptoms) assessed by radiological imaging or due to clinically obvious exacerbations (e.g., apparent exacerbation of symptoms).
In study 2, all patients discontinued the clinical trial due to PD/clinically evident exacerbations assessed by radiological imaging or due to clinically obvious exacerbations except one. This patient with HLA-A*02 discontinued the clinical trial because the investigator considered it difficult for the patient to continue the trial due to the occurrence of a grade 2 AE (interstitial lung disease [ILD]).
Patient demographics & baseline characteristics
In both studies, the patients were predominantly male, with a median age range of 53 to 70.5 years ().
Table 1. Baseline characteristics and demographics of patients enrolled in the ONO-7268MX1 study and the ONO-7268MX2 study.
Number of study vaccine doses
In study 1, the median (range) number of vaccine doses was 23 (20–71) in cohort 1 (0.9 mg group), 12 (2–27) in cohort 2 (3.0 mg group) and 8 (3–16) in cohort 3 (7.1 mg group). Similarly, in study 2, the median (range) number of vaccine doses was 8 (2–35) in patients with HLA-A*24 or HLA-A*02, 8 (2–28) in patients with HLA-A*24 and 8 (4–35) in patients with HLA-A*02.
Number of 4-week vaccination cycles
In study 1, the median (range) number of 4-week vaccination cycles was 6 (5–18) in cohort 1 (0.9 mg group), 3 (1–7) in cohort 2 (3.0 mg group) and 2 (1–4) in cohort 3 (7.1 mg group). Similarly, in study 2, the median (range) number of 4-week vaccination cycles was 2 (1–9) in patients with HLA-A*24 or HLA-A*02, 2 (1–7) in patients with HLA-A*24 and 2 (1–9) in patients with HLA-A*02.
Safety
No DLTs were observed in both studies in the initial DLT evaluation phase. The only serious adverse drug reaction (ADR) in these studies was grade 2 ILD in one patient in study 2. The most common study vaccine-related AEs were grade 1/2 injection-site reactions (erythema, induration and pain) observed in eight/18 (44.4%) patients in study 1 and 11/14 (78.6%) patients in study 2 (). A decrease in platelet count was observed in 7% of patients in the ONO-7268MX2 study only and no increase in ALT, AST or alkaline phosphatase levels was observed in either study. The incidence/frequency of grade ≥3 AEs categorized by System Organ Class in studies 1 and 2 are presented in online Supplementary Tables 1 & 2, respectively.
Table 2. Safety assessed as frequency of adverse drug reactions occurring in >10% of total patients in the ONO-7268MX1 study or the ONO-7268MX2 study.
Efficacy & immunological evaluation
No CR or PR was observed in both studies. SD was observed in nine and five patients in studies 1 and 2, respectively. In study 1, the median (range) PFS was 2.8 (0.5–16.8) months. In study 2, the median (range) PFS was 1.9 (0.4–8.3) months. In studies 1 and 2, the median duration of SD (the median PFS for confirmed SD patients) was 3.7 and 5.7 months, respectively.
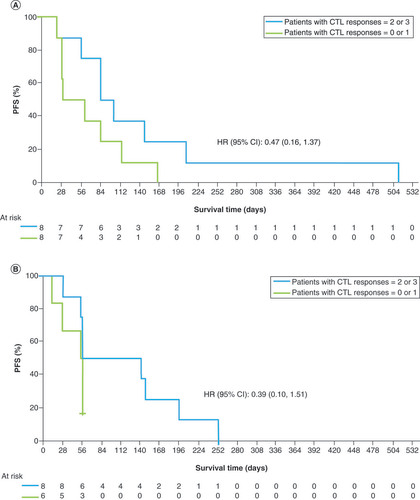
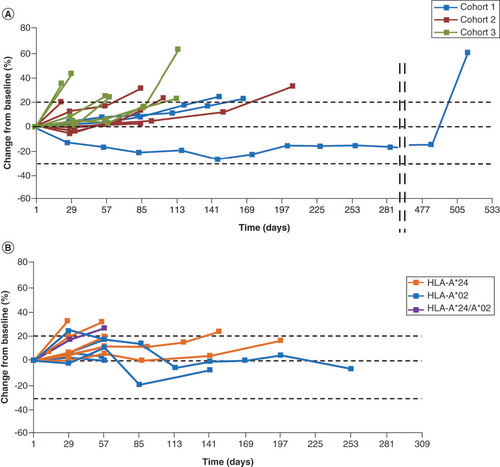
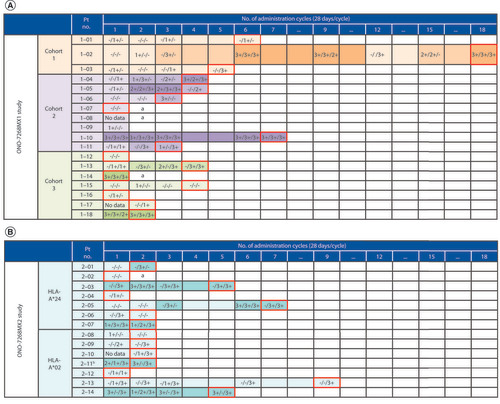
In studies 1 and 2, the disease control rate (CR + PR + SD) was 52.9% and 35.7% (ONO-7268MX1, n = 10; ONO-7268MX2, n = 5), respectively. Some patients showed long-term SD (>6 weeks) and tumor regression (). Peptide-specific CTL responses (1+–3+) were observed in both studies with the 3- and 6-epitope peptide dose range of 0.3–3 mg (). The exploratory analysis showed a tendency for prolonged PFS in patients when a greater number (2 or 3 vs 0 or 1) of CTL types were induced (). There was no apparent change in any of the biomarkers (frequencies of Tregs and MDSCs in peripheral blood; serum concentrations of IL-6, IL-10, IL-13 and TGF-β and plasma concentrations of kynurenine) regardless of the period of disease control (online Supplementary Figures 1–3). Peptide-induced whole blood gene expression analysis did not show any clear immune reaction to any of the peptides (online Supplementary Figure 4).
Cohort 1 (n = 3); cohort 2 (n = 8); cohort 3 (n = 7).
Cells with red borders indicate the cycle of discontinuation. Data are represented as GPC3/WDRPUH/NEIL3: measured at the time of cycle completion. Result of evaluation: negative (−), positive (+; 1+–3+) and blank (no data). The indicators (−, +, ++ and +++) represent the amount of peptide-specific spots and invariability of peptide-specific spots at different responder/stimulator ratios.
aProgression of disease discontinued in the midst of the first cycle.
bPatients with both HLA-A*24 and HLA-A*02.
CTL: Cytotoxic T lymphocyte; GPC3: Glypican-3; NEIL3: Nei endonuclease VIII-like 3; No.: Number; Pt: Patient; WDRPUH: WD-repeat-containing protein up-regulated in hepatocellular carcinoma.
Discussion
ONO-7268MX1 and ONO-7268MX2 showed good tolerability as no DLTs were observed in the initial DLT evaluation phase in both studies. The most common study vaccine-related AEs were grade 1/2 injection-site reactions (erythema, induration and pain). The only serious ADR in these studies was grade 2 ILD reported in one patient in study 2. No CR or PR was observed in both studies, whereas SD was observed in nine and five patients in studies 1 and 2, respectively. While the median duration of SD was 3.7 months in study 1 and 5.7 months in study 2, some patients did show long-term SD (>6 weeks) and tumor regression.
Both vaccines achieved long-term disease control or tumor regression in some patients in whom peptide-specific CTL responses were induced. Peptide-specific CTL responses (1+–3+) were observed in both studies with the 3- or 6-epitope peptide dose range of 0.3 to 3 mg. In studies 1 and 2, the median PFS was 2.8 and 1.9 months, respectively. A longer PFS was observed with a higher number of types of peptide-specific CTL responses (2 or 3 vs 1 or 0) induced by the epitope peptides contained in ONO-7268MX1 and ONO-7268MX2. This suggests that enhanced efficacy of these vaccines could be expected in patients in whom CTLs can be induced efficiently by peptide administration. Conversely, some of the patients in whom CTLs were induced did not show an antitumor effect and this observation needs further exploration. In these studies, frequencies of Tregs and MDSCs in peripheral blood; serum concentrations of IL-6, IL-10 and IL-13; and plasma concentrations of kynurenine, an IDO1-mediated tryptophan metabolite, were measured as biomarkers associated with the suppression of the antitumor immune response. However, there was no apparent change in any of the biomarkers regardless of the period of disease control (see online Supplementary Figures 1–3). In study 2, the possibility of evaluating peptide-specific CTL responses by a method different from the ELISPOT method was also examined. The method and the results are shown in online Supplementary Figure 4. As a result, no clear immune reaction to any of the peptides was observed.
The postulated mechanism of action for the vaccines was that the 3- and 6-epitope peptides from the three HCC antigens (GPC3, WDRPUH and NEIL3) in the cocktail vaccines would induce an antigen-specific CTL response with the help of antigen-presenting cells. These CTLs would, in turn, act as HCC-epitope immunized cells that recognize and destroy HCC cells expressing the three antigens. Although antigen-presenting cells play some role in CTL activation as a mechanism of action of these vaccines, the administration of any of the nine epitope peptides would not lead to antigen presentation on HLA through normal antigen processing by the antigen-presenting cells. There is a possibility that CTL could be activated by the patient’s own epitope antigen on HLA and that the antigen-presenting cells would have to compete with or be replaced by the peptide vaccine utilizing antigen-presenting cells.
It can be postulated that, in order to increase the efficacy of ONO-7268MX1 or ONO-7628MX2, novel drugs targeting all three antigens on HCC may be used in combination. This will require further understanding of the relationship between the target antigens. Emerging evidence does show an intratumoral reciprocal (inverse) expression of monocarboxylate transporter 4 (MCT4) and GPC3 as the most frequent pattern in MCT4+/GPC3+ HCCs; increased MCT4 expression was related to decreased GPC3 immunoreactivity and vice versa [Citation28].
However, in cancer patients, it is also proposed that tumor cells modulate immune cell population including Tregs, MDSCs and tumor-associated macrophages or express immunosuppressive molecules, such as programmed cell death-ligand 1, which suppress antitumor immune responses of the CD8+ T cells via programmed cell death-1, in the tumor microenvironment [Citation29–32]. Therefore, it is postulated that the efficacy of ONO-7268MX1 and ONO-7268MX2 could be enhanced by combination therapy with immunomodulatory agents or immune checkpoint inhibitor drugs (e.g., antiprogrammed cell death-1 antibody).
Overall, ONO-7268MX1 and ONO-7268MX2 showed good tolerability; both vaccines induced CTL responses that might be associated with antitumor effects and long-term disease control in some patients.
Although direct comparisons cannot be made with other therapeutic strategies for HCC, the efficacy of ONO-7268MX1 and ONO-7268MX2 was inferior to that of nivolumab (Checkmate 040 trial) [Citation33], an immune checkpoint inhibitor, or that of cabozantinib [Citation34], a tyrosine kinase inhibitor recently approved by the FDA.
There continues to be an unmet need in the HCC treatment landscape. According to the Barcelona Clinic Liver Cancer algorithm, for patients with advanced-stage (C) HCC with preserved liver function, first-line systemic therapy includes sorafenib and lenvatinib (estimated survival time 11–13 months) and second-line therapy includes regorafenib, cabozantinib and ramucirumab (estimated survival time 8–10 months) [Citation6]. Among the newer treatment options, the National Comprehensive Cancer Network guideline recommends atezolizumab combined with bevacizumab as the preferred first-line systemic therapy for patients with unresectable or metastatic HCC [Citation35,Citation36]. In addition, biologic agents have shown poor efficacy in HCC. Although angiogenic pathways and the cytokine cascade may become possible targets for HCC treatment, the evaluation of major epigenetic (e.g., DNA methylation, miRNA and histone deacetylase inhibitors) and genetic processes involved in HCC carcinogenesis, including the tumor microenvironment, could be the future. Molecular and epigenetic research on DNA methylation processes and miRNA activity are being evaluated in several studies [Citation37]. In parallel, vaccines, which have been the first therapeutic strategy in immuno-oncology, cannot be overlooked. Alongside investigations on conventional peptide-based vaccines, investigations using new modalities, such as pulsed dendritic cells and DNA-based vaccines, are also in progress [Citation7].
Conclusion
ONO-7268MX1 and ONO-7268MX2 showed good tolerability for the treatment of patients with HCC. Although no CR or PR was observed, SD was observed in nine and five patients in studies 1 and 2, respectively; some patients also showed long-term disease control. Both vaccines induced CTL responses; this may be related to their antitumor effect because an increase in PFS was observed with a higher number of types of peptide-specific CTL responses. These results imply the potential usefulness of these vaccines for the treatment of HCC.
Two Phase I studies were conducted to evaluate the safety and efficacy of two peptide cocktail vaccines (ONO-7268MX1 and ONO-7268MX2) using three tumor antigens (glypican-3, WD-repeat-containing protein up-regulated in hepatocellular carcinoma and nei endonuclease VIII-like 3) epitopes in patients with advanced hepatocellular carcinoma.
Patients had to have HLA-A*24 for study 1 and HLA-A*24 or HLA-A*02 for study 2.
No dose-limiting toxicities were observed in both studies in the initial dose-limiting toxicity evaluation phase.
No complete response or partial response was observed in both studies.
Stable disease (SD) was observed in nine/18 and five/14 patients in studies 1 and 2, respectively.
In studies 1 and 2, the median duration of SD (the median progression-free survival for confirmed SD patients) was 3.7 and 5.7 months, respectively.
In studies 1 and 2, the disease control rate (complete response + partial response + SD) was 52.9% and 35.7% (ONO-7268MX1, n = 10; ONO-7268MX2, n = 5), respectively.
The exploratory analysis showed a tendency for prolonged progression-free survival in patients when a greater number (2 or 3 vs 0 or 1) of cytotoxic T lymphocyte types were induced.
Author Contributions
J Furuse, T Okusaka, M Ikeda and K Gemmoto contributed in conception and design and development of methodology; T Okusaka, M Ikeda, S Kondo, H Ueno, C Morizane, I Ohno and S Mitsunaga contributed in acquisition of data; J Furuse, T Okusaka, M Ikeda, Y Ushida, H Suna and K Gemmoto assisted in analysis and interpretation of data; M Ikeda, T Okusaka, S Mitsunaga, I Ohno, C Morizane, H Ueno, S Kondo, K Gemmoto, Y Ushida, H Suna and J Furuse contributed in writing, review and/or revision of the manuscript; Administrative, technical or material support: not applicable; study supervision was provided by J Furuse; other: not applicable.
All authors are accountable for all aspects of the work and provide their final approval of the manuscript.
Ethical conduct of research
The studies were approved by the Institutional Review Boards of the National Cancer Center, Japan (Study 1: K0246, T3927; Study 2: K0370, T4075). This study was conducted in accordance with the Declaration of Helsinki and guidelines for Good Clinical Practice. Written informed consent was obtained from the patients twice, i.e., before starting the study and before moving the patients from the initial dose-limiting toxicity evaluation phase to the continuous administration phase.
Supplemental Document
Download Zip (3.6 MB)Acknowledgments
The authors express sincere gratitude toward all patients and their families who participated in both the ONO-7268MX1 study and the ONO-7268MX2 study.
We are also thankful to the clinical study teams that participated in these studies. The peptides evaluated in the ONO-7268MX1 study and the ONO-7268MX2 study were originally developed by OncoTherapy Science, Inc. We are grateful to members of the Data and Safety Monitoring Board, including Osamu Yokosuka (Japan Community Health care Organization Funabashi Central Hospital) and Hiroshi Ishii (Chiba Cancer Center) for their support. We also express sincere gratitude toward Tetsuya Nakatsura of the National Cancer Center. These studies were funded by Ono Pharmaceutical Co., Ltd., Osaka, Japan.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/imt-2020-0278
Financial & competing interests disclosure
Both the ONO-7268MX1 study and the ONO-7268MX2 study were funded by Ono Pharmaceutical Co., Ltd, Osaka, Japan.
M Ikeda reports personal fees and other (advisory board, research funding) from Ono Pharmaceutical; personal fees (honoraria, advisory board) from Teijin Pharma; personal fees and other (honoraria, advisory board, research funding) from Bristol-Myers Squibb, Bayer Yakuhin, Eli Lilly Japan and Eisai; personal fees and other (advisory board, research funding) from Chugai pharmaceutical; personal fees and other (honoraria, research funding) from MSD; personal fees (honoraria) from Sumitomo Dainippon Pharma, EA Pharma and Gilead; and research funding from Takeda Pharmaceutical, AstraZeneca and Merck Serono during the conduct of the study. M Ikeda also reports personal fees and other (board membership, research funding) from ASLAN Pharmaceuticals; personal fees and other (honoraria, advisory board, research funding) from Daiichi Sankyo and Novartis; personal fees and other (advisory board, research funding) from Kyowa Kirin and NanoCarrier; personal fees and other (honoraria, research funding) from Yakult Honsha and Taiho Pharmaceutical; personal fees (advisory board) from Shire; research funding from Baxalta and J-Pharma and personal fees (honoraria) from Nobelpharma, Mylan, NIHON SERVIER, Astellas Pharma and Otsuka Pharmaceutical outside the submitted work.
T Okusaka reports research grants and personal fees (honoraria) from Ono Pharmaceutical and Bristol-Myers Squibb during the conduct of the study. T Okusaka also reports research grants and personal fees (honoraria) from MSD, Eli Lilly Japan, Pfizer Japan Inc, Bayer Yakuhin, Chugai Pharmaceutical, Yakult Honsha and Eisai; research grants and personal fees (advisory role) from Sumitomo Dainippon Pharma and Zeria Pharmaceutical; research grants and personal fees (advisory role, honoraria) from Taiho Pharmaceutical and Bristol-Myers Squibb; research grants from Baxter, Kyowa Kirin and NanoCarrier; research grants and personal fees (advisory role, honoraria) from Daiichi Sankyo and personal fees (honoraria) from EA Pharma, FUJIFILM RI Pharma, Teijin Pharma, Shire, AbbVie, Takeda Pharmaceutical, Meiji Seika, Mundipharma, NIHON SERVIER, Nippon Shinyaku and Celgene outside the submitted work.
C Morizane reports grants from Ono Pharmaceutical and Bristol-Myers Squibb during the conduct of the study. C Morizane also reports personal fees from Novartis, Teijin Pharma, Taiho Pharmaceutical and MSD; grants and personal fees from Yakult Honsha and Eisai and grants from J-Pharma, AstraZeneca and Merck biopharma outside the submitted work.
I Ohno, S Mitsunaga, S Kondo, H Ueno and J Furuse report grants from Ono Pharmaceutical and Bristol-Myers Squibb during the conduct of the study.
S Kondo has received research funding from ASLAN Pharmaceuticals, AstraZeneca, Bayer Yakuhin, Eli Lilly Japan, MSD and Pfizer Japan Inc outside the submitted work.
J Furuse also reports grants from Ono Pharmaceutical, MSD, Sumitomo Dainippon Pharma, J-Pharma, Yakult Honsha, AstraZeneca, Daiichi Sankyo, Eisai, Bayer Yakuhin, Pfizer Japan Inc, NanoCarrier, Kyowa Kirin, Taiho Pharmaceutical, Chugai pharmaceutical, Sanofi, Takeda Pharmaceutical, Mochida Pharmaceutical, Astellas Pharma and Eli Lilly Japan and personal fees from Eisai, Bayer Yakuhin, Taiho Pharmaceutical, Ono Pharmaceutical, Novartis, Yakult Honsha, Teijin Pharma, Shionogi, EA Pharma, Eli Lilly Japan, Takeda Pharmaceutical, Chugai pharmaceutical, Mochida Pharmaceutical, NIHON SERVIER, Sanofi, Fujifilm Toyama Chemical, Nobelpharma, Pfizer Japan Inc, Sawai Pharmaceutical, Daiichi Sankyo, Sumitomo Dainippon Pharma, Merck Serono, Nippon Kayaku, MSD, Shire and Kyowa Kirin outside the submitted work.
K Gemmoto, H Suna and Y Ushida are employees of Ono Pharmaceutical.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by Annirudha Chillar, MD, PhD, of Cactus Life Sciences (part of Cactus Communications), and funded by Ono Pharmaceutical Co., Ltd.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data: JapicCTI-121933; JapicCTI-142477. The data will not be made publicly available.
Additional information
Funding
References
- Singal AG , LamperticoP , NahonP. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J. Hepatol.72(2), 250–261 (2020).
- Siegel RL , MillerKD , JemalA. Cancer statistics, 2020. CA Cancer J. Clin.70(1), 7–30 (2020).
- Zhu RX , SetoWK , LaiCL , YuenMF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver.10(3), 332–339 (2016).
- Tateishi R , UchinoK , FujiwaraNet al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J. Gastroenterol.54(4), 367–376 (2019).
- Uchino K , TateishiR , ShiinaSet al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer117(19), 4475–4483 (2011).
- Villanueva A . Hepatocellular carcinoma. N. Engl. J. Med.380, 1450–1462 (2019).
- Longo V , GnoniA , CasadeiGardini Aet al. Immunotherapeutic approaches for hepatocellular carcinoma. Oncotarget8(20), 33897–33910 (2017).
- Nakatsura T , YoshitakeY , SenjuSet al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem. Biophys. Res. Commun.306(1), 16–25 (2003).
- Nishida T , KataokaH. Glypican 3-targeted therapy in hepatocellular carcinoma. Cancers (Basel)11(9), 1339 (2019).
- Jing JS , YeW , JiangYKet al. The value of GPC3 and GP73 in clinical diagnosis of hepatocellular carcinoma. Clin. Lab.63(11), 1903–1909 (2017).
- Silva FP , HamamotoR , NakamuraY , FurukawaY. WDRPUH, a novel WD-repeat-containing protein, is highly expressed in human hepatocellular carcinoma and involved in cell proliferation. Neoplasia7(4), 348–355 (2005).
- Rolseth V , KrokeideSZ , KunkeDet al. Loss of Neil3, the major DNA glycosylase activity for removal of hydantoins in single stranded DNA, reduces cellular proliferation and sensitizes cells to genotoxic stress. Biochim. Biophys. Acta1833(5), 1157–1164 (2013).
- Sejersted Y , HildrestrandGA , KunkeDet al. Endonuclease VIII-like 3 (Neil3) DNA glycosylase promotes neurogenesis induced by hypoxia-ischemia. Proc. Natl Acad. Sci. USA108(46), 18802–18807 (2011).
- Shinmura K , KatoH , KawanishiYet al. Abnormal expressions of DNA glycosylase genes NEIL1, NEIL2, and NEIL3 are associated with somatic mutation loads in human cancer. Oxid. Med. Cell. Longev.2016, 1546392 (2016).
- Liu M , DoubliéS , WallaceSS. Neil3, the final frontier for the DNA glycosylases that recognize oxidative damage. Mutat. Res.743–744, 4–11 (2013).
- Zhang H , MaH , WangQet al. Analysis of loss of heterozygosity on chromosome 4q in hepatocellular carcinoma using high-throughput SNP array. Oncol. Rep.23(2), 445–455 (2010).
- HLA Laboratory . HLA affinity in Japanese population 2012. (2012). http://hla.or.jp/med/frequency_search/en/allele/
- Japan Clinical Oncology Group . National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0 Japanese translation Japan Clinical Oncology Group (JCOG) version. www.jcog.jp/doctor/tool/ctcaev4.html
- Eisenhauer EA , TherasseP , BogaertsJet al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer45(2), 228–247 (2009).
- Yamaguchi Y , YamaueH , OkusakaTet al. The Committee of Guidance for Peptide Vaccines for the Treatment of Cancer, The Japanese Society for Biological Therapy. Guidance for peptide vaccines for the treatment of cancer. Cancer Sci.105(7), 924–931 (2014).
- U. S. Department of Health and Human Services,Food and Drug Administration, Center for Biologics Evaluation and Research . Guidance for industry clinical considerations for therapeutic cancer vaccines. (2011). www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-considerations-therapeutic-cancer-vaccines
- Sawada Y , YoshikawaT , NobuokaDet al. Phase I trial of glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunological evidence and potential for improving overall survival. Clin. Cancer Res.18(13), 3686–3896 (2012).
- Slingluff CL Jr , YamshchikovG , NeeseP , GalavottiH , EasthamS , EngelhardVH. Phase I trial of a melanoma vaccine with gp100 (280–288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin. Cancer Res.7(10), 3012–3024 (2001).
- Lienard D , RimoldiD , MarchandMet al. Ex vivo detectable activation of Melan-A-specific T cells correlating with inflammatory skin reactions in melanoma patients vaccinated with peptides in IFA. Cancer Immun.4, 4 (2004).
- Odunsi K , QianF , MatsuzakiJ , Mhawech-FaucegliaP , AndrewsC , HoffmanEW. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc. Natl Acad. Sci. USA104(31), 12837–13842 (2007).
- Oka Y , TsuboiA , TaguchiTet al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc. Natl Acad. Sci. USA101(38), 13885–13890 (2004).
- Kono K , IinumaH , AkutsuYet al. Multicenter, Phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens. J. Transl. Med.10, 141 (2012).
- Yorita K , OhnoA , NishidaT , KondoK , OhtomoT , KataokaH. Intratumoral reciprocal expression of monocarboxylate transporter 4 and glypican-3 in hepatocellular carcinomas. BMC Res. Notes.12(1), 741 (2019).
- Nowicki TS , Hu-LieskovanS , RibasA. Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J.24(1), 47–53 (2018).
- Togashi Y , ShitaraK , NishikawaH. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat. Rev. Clin. Oncol.16(6), 356–371 (2019).
- Lindau D , GielenP , KroesenM , WesselingP , AdemaGJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology138(2), 105–115 (2013).
- Chanmee T , OntongP , KonnoK , ItanoN. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel)6(3), 1670–1690 (2014).
- El-Khoueiry AB , SangroB , YauTet al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase I/II dose escalation and expansion trial. Lancet389(10088), 2492–2502 (2017).
- Abou-Alfa GK , MeyerT , ChengALet al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med.379(1), 54–63 (2018).
- Finn RS , QinS , IkedaMet al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med.382(20), 1894–1905 (2020).
- Benson B , D’AngelicaMI , AbbottDEet al. NCCN guidelines (Hepatobiliary Cancers). www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- Gnoni A , SantiniD , ScartozziMet al. Hepatocellular carcinoma treatment over sorafenib: epigenetics, microRNAs and microenvironment. Is there a light at the end of the tunnel? Expert Opin. Ther. Targets 19(12), 1623–1635 (2015).