Abstract
Asthma is a common chronic respiratory disease in which epithelial cytokines and airway inflammation play critical pathophysiological roles. Thymic stromal lymphopoietin (TSLP), an epithelial cytokine, is central in the initiation and persistence of airway inflammation in asthma. Tezepelumab is a human immunoglobulin G2λ (IgG2λ) monoclonal antibody developed for treating moderate-to-severe asthma by specifically binding to TSLP and preventing its binding to the TSLP receptor on inflammatory cells. In this narrative review, we describe the results of clinical trials that evaluated the pharmacokinetics, pharmacodynamics, efficacy and safety of tezepelumab in patients with moderate-to-severe asthma. We also introduce the ongoing clinical trials in patients with asthma as well as future trials investigating the use of tezepelumab for other indications.
Plain language summary
Asthma is a long-term disease that causes inflammation in the cells of the lung. One of the cytokines (proteins) involved in asthma is called thymic stromal lymphopoietin (TSLP). This cytokine is produced by the airway epithelium, a layer of cells covering the respiratory tract in the lungs, where it activates inflammatory cells. Tezepelumab is a new drug that blocks the activity of TSLP in the lungs and helps reduce asthma symptoms, such as coughing and breathlessness. In this article, we describe the clinical trials that investigated how well tezepelumab works, as well as its safety, in people with moderate or severe asthma. We also describe the trials of tezepelumab that are now underway in people with asthma, allergies or other inflammatory diseases.
Tweetable abstract
This review describes the pharmacokinetics, pharmacodynamics, efficacy, safety and ongoing trials of tezepelumab, a human IgG2λ monoclonal antibody designed to inhibit thymic stromal lymphopoietin produced by the airway epithelium, for treating asthma. #asthma, #lunghealth
The incidence of asthma, a common chronic respiratory disease, varies among countries and is thought to affect 1–18% of the general population. It is characterized by wheezing, shortness of breath, chest tightness, coughing and airflow limitation. These symptoms and airflow limitation, as well as their severity, vary over time [Citation1].
The understanding of asthma pathology has advanced in recent years. Factors such as exercise, exposure to allergens and irritants, weather changes and viral or bacterial respiratory infection are common asthma triggers, with airway hyper-responsiveness and chronic airway inflammation due to these stimulants being at the center of this condition. It is now known that the airway epithelium activates various airway inflammation pathways via epithelial cytokines in response to the stimuli mentioned above () [Citation2].
In allergic eosinophilic inflammation, TSLP initiates pathways involving Th2 lymphocytes, basophils, and mast cells to drive airway eosinophilia. In non-allergic eosinophilic inflammation, TSLP activates innate lymphocytes such as ILC2s that contribute to airway eosinophilia. TSLP also mediates structural mechanisms that contribute to airway remodeling, involving airway smooth muscle cells and fibroblasts.
IgE: Immunoglobulin E; IL: Interleukin; ILC2: Group 2 innate lymphoid cell; T2: Type 2; Th: T helper; TSLP: Thymic stromal lymphopoietin.
Reproduced with permission from [Citation2].
![Figure 1. Role of thymic stromal lymphopoietin in driving disease mechanisms in different asthma endotypes.In allergic eosinophilic inflammation, TSLP initiates pathways involving Th2 lymphocytes, basophils, and mast cells to drive airway eosinophilia. In non-allergic eosinophilic inflammation, TSLP activates innate lymphocytes such as ILC2s that contribute to airway eosinophilia. TSLP also mediates structural mechanisms that contribute to airway remodeling, involving airway smooth muscle cells and fibroblasts.IgE: Immunoglobulin E; IL: Interleukin; ILC2: Group 2 innate lymphoid cell; T2: Type 2; Th: T helper; TSLP: Thymic stromal lymphopoietin.Reproduced with permission from [Citation2].](/cms/asset/2c7c59b2-e608-47b9-9011-71712cf7a173/iimy_a_12338166_f0001.jpg)
Approximately 5–10% of asthma patients have severe asthma, in which asthma symptoms cannot be controlled with conventional therapies, including inhaled corticosteroids (ICSs) and long-acting β2-agonists (LABAs) [Citation3]. Such refractory cases are treated with biologics, such as an anti-immunoglobulin E (IgE) antibody, anti-IL-5 antibody, anti-IL-5Rα antibody, or anti-IL-4Rα antibody. However, some patients do not adequately respond to these biologics, which target cytokines downstream of airway inflammation [Citation1].
Tezepelumab was developed as a biologic with a unique mechanism of action that involves inhibiting the action of thymic stromal lymphopoietin (TSLP). This epithelial cytokine activates a wide range of immune cells involved in airway inflammation in asthma () [Citation2]. In this review article, we summarize the characteristics of tezepelumab, including its mechanism of action, pharmacokinetics, pharmacodynamics, efficacy, and safety. We also discuss its potential as a treatment option for patients with severe uncontrolled asthma poorly controlled with other biologics.
Mechanism of action
TSLP is produced by airway epithelial cells following the inhalation of allergens, viruses, fungi or bacteria that irritate the airway epithelium. TSLP activates several inflammatory cells, such as dendritic cells, group 2 innate lymphoid cells (ILC2s) and mast cells, which promotes type 2 inflammation by increasing type 2 cytokine production () [Citation2,Citation4–6]. It was further reported that TSLP induces neutrophilic inflammation under specific in vitro conditions, indicating it is also involved in non-type 2 inflammation [Citation7]. Furthermore, it has been confirmed that TSLP increases collagen production from fibroblasts and induces the growth of airway smooth muscle, indicating that it affects airway remodeling [Citation2]. Thus, we know that TSLP is involved in the induction and perpetuation of airway inflammation and that it widely activates immune cells and inflammatory pathways related to the pathology of type 2 and non-type 2 asthma [Citation2,Citation4].
Reportedly, there is increased expression of TSLP in the airway tissue of asthma patients compared with that of healthy adults. Additionally, the increase in TSLP correlates with symptom severity, respiratory function, airway obstruction, airway hyperresponsiveness, airway remodeling and steroid resistance [Citation2,Citation4].
Tezepelumab is a human immunoglobulin G2λ (IgG2λ) monoclonal antibody designed to specifically bind to TSLP and inhibit it from binding to the TSLP (IL-7Rα heterodimeric) receptor [Citation8]. In vitro studies showed that tezepelumab inhibited TSLP and suppressed the activation of TSLP receptor-bearing cells, thus confirming its mechanism of action. When tezepelumab was added to TSLP receptor-bearing cells, it inhibited TSLP-induced phosphorylation of STAT5 and cell proliferation. Furthermore, tezepelumab suppressed the production of CCL17 from human dendritic cells induced by TSLP [Citation9].
Pharmacokinetics & pharmacodynamics
The pharmacokinetics of tezepelumab were investigated in two phase I ascending-dose trials in healthy adults [Citation10]. After a single subcutaneous dose of tezepelumab 210 mg, the median time to maximum blood concentration (tmax) was 93.65 h, the mean half-life was 25.7 days, and the bioavailability was 81%. In the multiple-dose trial in which tezepelumab 210 mg was subcutaneously administered three times (every 4 weeks), the median tmax after the last dose was 167 h. After administration of tezepelumab 210 mg every 4 weeks, the mean serum trough concentration increased over time, approaching a steady state by Week 12 [Citation11].
In the phase IIb PATHWAY trial, subcutaneous administration of tezepelumab 210 mg once every 4 weeks was predicted to achieve approximately 90% of the peak pharmacologic action for suppressing asthma exacerbations and a decrease in fractional exhaled nitric oxide (FeNO) based on the pharmacokinetic–pharmacodynamic model. Additionally, this was selected as the dosage regimen for a phase III trial [Citation12].
In the phase III NAVIGATOR trial, antidrug antibodies were detected in 4.9% of patients in the tezepelumab group and 8.3% in the placebo group. These values were low and were not considered to affect the efficacy and safety of tezepelumab [Citation13].
The pharmacodynamic effect of tezepelumab in a clinical setting was first investigated in a phase I trial in which adult patients with mild allergic asthma received three intravenous doses of placebo or tezepelumab (700 mg, once every 4 weeks). The decreases in forced expiratory volume in 1 s (FEV1) during early and late asthmatic responses after allergen inhalation were suppressed in the tezepelumab group compared with the placebo group. In addition, tezepelumab significantly reduced blood and sputum eosinophil counts and suppressed the elevation of FeNO after allergen inhalation [Citation8].
The clinical pharmacodynamics of tezepelumab have been further investigated in global studies (), including the 52-week phase IIb PATHWAY trial in adults with severe uncontrolled asthma [Citation14], the 28-week Phase II CASCADE trial in adults with uncontrolled moderate-to-severe asthma [Citation15], and the 52-week phase III NAVIGATOR trial of adults and adolescents with severe uncontrolled asthma [Citation13]. Compared with placebo, administration of tezepelumab was associated with reductions in a range of type 2 inflammatory biomarkers and clinical markers, such as airway submucosal and blood eosinophil counts, FeNO, total serum IgE, IL-5, IL-13, periostin and thymus activation regulated chemokine () [Citation15,Citation16]. Furthermore, in the NAVIGATOR trial, the changes in the blood eosinophil counts and FeNO were apparent within 2 weeks of starting tezepelumab, and in the follow-up, long-term DESTINATION extension, the changes were sustained to Week 104, along with a gradual decline in total serum IgE levels until Week 104 [Citation17].
(A) Blood eosinophil count, (B) FeNO, (C) IgE, (D) IL-5, (E) IL-13, (F) periostin, (G) TARC.
FeNO: Fractional exhaled nitric oxide; IgE: Immunoglobulin E; IL: Interleukin; MAD: Median absolute deviation; Q4W: Every 4 weeks; TARC: Thymus and activation-regulated chemokine.
Reprinted from [Citation16] under an open access license (http://creativecommons.org/licenses/by-nc/4.0/).
![Figure 2. Median percentage change from baseline in biomarker levels over 52 weeks. (A) Blood eosinophil count, (B) FeNO, (C) IgE, (D) IL-5, (E) IL-13, (F) periostin, (G) TARC.FeNO: Fractional exhaled nitric oxide; IgE: Immunoglobulin E; IL: Interleukin; MAD: Median absolute deviation; Q4W: Every 4 weeks; TARC: Thymus and activation-regulated chemokine.Reprinted from [Citation16] under an open access license (http://creativecommons.org/licenses/by-nc/4.0/).](/cms/asset/d20098d0-3a3b-4d19-af50-f2fbf395a8f7/iimy_a_12338166_f0002.jpg)
Table 1. Overview of key clinical trials for tezepelumab in patients with asthma.
The effect of tezepelumab on airway hyperresponsiveness was investigated in the Phase II CASCADE trial. Tezepelumab improved airway hyperresponsiveness induced by mannitol stimulation compared with placebo, measured in terms of the least-squares mean (LSM) change in mannitol PD15 from baseline to end of treatment (197.4 vs 58.6 mg; the difference in LSM change: 138.8 mg, 95% confidence interval [CI] 14.2–263.3; p = 0.030) [Citation15].
Overall, these studies confirmed the pharmacodynamic effects of tezepelumab on inhibiting TSLP and improving the underlying pathological conditions in asthma.
Efficacy
The main clinical studies that were conducted to investigate the efficacy and safety of tezepelumab in patients with asthma are summarized in .
In the Phase III NAVIGATOR trial, tezepelumab was associated with a significant reduction in the annual asthma exacerbation rate (AAER) versus placebo at Week 52 (rate ratio [RR] 0.44, 95% CI: 0.37–0.53; p < 0.001), together with improvements in prebronchodilator FEV1 (difference vs placebo 0.13 L, 95% CI: 0.08–0.18; p < 0.001), Asthma Control Questionnaire-6 (ACQ-6; difference vs placebo -0.33, 95% CI: -0.46 to -0.20; p < 0.001), and Asthma Quality of Life Questionnaire (AQLQ; difference vs placebo 0.34, 95% CI: 0.20–0.47; p < 0.001). Notably, improvements in prebronchodilator FEV1 were confirmed at 2 weeks post-tezepelumab initiation () [Citation13].
Bars indicate 95% confidence intervals.
FEV1: Forced expiratory volume in 1 s.
Reprinted with permission from [Citation13].
![Figure 3. Change from baseline to Week 52 in prebronchodilator FEV1.Bars indicate 95% confidence intervals.FEV1: Forced expiratory volume in 1 s.Reprinted with permission from [Citation13].](/cms/asset/1872796d-faa1-46e9-b3ba-495758a8be0a/iimy_a_12338166_f0003.jpg)
The efficacy of tezepelumab was also investigated in subgroups of patients divided by baseline blood eosinophil count [Citation13]. In patients with a baseline blood eosinophil count of ≥300 cells/μl, tezepelumab suppressed the AAER by 70% versus placebo (RR 0.30, 95% CI: 0.22–0.40), which was accompanied by improvements in prebronchodilator FEV1 (difference vs placebo 0.23 L, 95% CI: 0.15–0.31), ACQ-6 (difference vs placebo -0.50, 95% CI: -0.69 to -0.31), and AQLQ (difference vs placebo 0.51, 95% CI: 0.30–0.71). Tezepelumab was also associated with a suppression of AAER by 41% in patients with a baseline blood eosinophil count of <300 cells/μl (RR 0.59, 95% CI: 0.46–0.75) and by 39% in patients with a baseline blood eosinophil count of <150 cells/μl (RR 0.61, 95% CI: 0.42–0.88). Thus, although the suppression in AAER was greater in patients with a high baseline eosinophil count, the results demonstrate the effectiveness of tezepelumab in patients with a low baseline eosinophil count. Moreover, in a pooled analysis of the Phase IIb PATHWAY and phase III NAVIGATOR trials, tezepelumab showed clinically meaningful effects on suppressing AAER, regardless of baseline blood eosinophil count, FeNO level, and perennial antigen positive/negative status () [Citation11]. These efficacy findings, including improved lung function, asthma control, and health-related quality of life, were maintained over 104 weeks in DESTINATION, a long-term extension trial [Citation17].
Time at risk was defined as the total duration of time in which a new exacerbation could occur (i.e., the total follow-up time minus time during exacerbation and 7 days after).
aAllergic status as defined by a serum immunoglobulin E result specific to any perennial aeroallergen in the fluoroenzyme immunoassay panel.
CI: Confidence interval; FeNO: Fractional exhaled nitric oxide; ppb: Parts per billion; Q4W: Every 4 weeks.
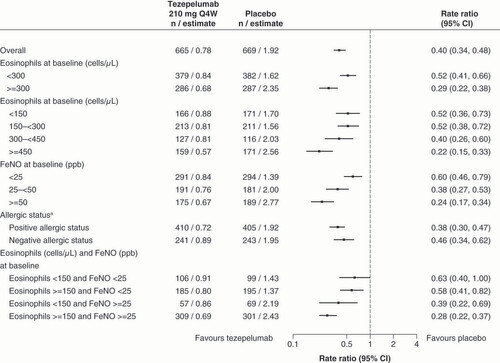
In other subanalyses of the PATHWAY trial, tezepelumab suppressed the AAER versus placebo in each season with RRs of 0.36 (95% CI: 0.17–0.78), 0.20 (95% CI: 0.07–0.59), 0.18 (95% CI: 0.06–0.52), and 0.33 (95% CI: 0.14–0.79) in winter, spring, summer, and autumn, respectively [Citation18]. Moreover, tezepelumab suppressed the AAER in patients with nasal polyps (RR 0.25, 95% CI: 0.07–0.85) and without nasal polyps (RR 0.27, 95% CI: 0.14–0.53) [Citation19].
The efficacy of tezepelumab on asthma exacerbations was also confirmed in Japanese patients with severe asthma enrolled in the NAVIGATOR trial [Citation20], and in the phase III NOZOMI trial performed in Japan [Citation21]. In Japanese patients with severe asthma enrolled in the NAVIGATOR trial, tezepelumab suppressed the AAER by 51% versus placebo (RR 0.49, 95% CI: 0.25–0.99), a similar reduction to that observed in the full analysis population [Citation20]. In the single-arm NOZOMI trial, we found that the AAER decreased from 1.3/patient-year for the year before starting treatment to 0.11/patient-year after 52 weeks of treatment with tezepelumab, a notable reduction considering the trial was conducted during the COVID-19 pandemic (June 2019–June 2021) [Citation21].
Indirect comparisons of AAER between tezepelumab and other biologics were made in a systematic review of randomized controlled trials, which included the phase III NAVIGATOR trial and phase IIb PATHWAY trial. The analyses revealed that tezepelumab suppressed the AAER by 63% versus placebo (RR 0.37, 95% CI: 0.23–0.57). The RRs of AAER for each biologic versus tezepelumab were similar among the biologics, with no significant differences. However, the ranking of the AAER showed that the surface under the cumulative ranking curve (SUCRA) was greatest for tezepelumab (84%) among the biologics assessed [Citation22]. Furthermore, in a meta-analysis that compared tezepelumab with other biologics, the SUCRA for AAER in tezepelumab-treated patients was 79.9%, 75.6%, 85.0% and 80.5% in patients with a baseline blood eosinophil count of <150, ≥150, <300 and ≥300 cells/μl, respectively. These values were greater for tezepelumab than those for the other biologics in each subgroup [Citation23]. Additionally, a recent Bayesian network meta-analysis found that tezepelumab and dupilumab were associated with greater improvements in both exacerbation rates and lung function compared with other biologics, further supporting the above results [Citation24]. Based on these analyses, tezepelumab demonstrated the strongest inhibitory effects on asthma exacerbation among the biologics examined in the systematic review.
The oral corticosteroid-sparing effect of tezepelumab was evaluated in the global phase III SOURCE trial in patients with severe asthma who continuously required oral corticosteroid (OCS) in addition to ongoing treatment with an ICS and LABA [Citation25]. The proportion of patients with a baseline blood eosinophil count <300 cells/μl was 65%. A significant difference was not found between the tezepelumab and placebo groups in terms of the percent reduction in the OCS dose at Week 48 relative to baseline (odds ratio [OR] 1.28, 95% CI: 0.69–2.35; p = 0.43). Many patients in both treatment groups achieved a ≥90% reduction in OCS dose (54.1% for tezepelumab vs 46.1% for placebo), demonstrating a large placebo effect. Two important factors that likely contributed to the considerable placebo response include the long duration of the OCS reduction phase and the possibility of multiple down-titration attempts, which were both unique features of the SOURCE trial design. Nevertheless, when patients were divided according to baseline blood eosinophil count, tezepelumab was associated with a greater percent reduction in the OCS dose among patients with an eosinophil count of ≥150 cells/μl (OR 2.58, 95% CI: 1.16–5.75). Additionally, 54% of patients in the tezepelumab group were able to discontinue OCS administration by 48 weeks versus 46% in the placebo group. The OCS discontinuation rate for tezepelumab was similar to the values reported for other biologics (dupilumab: 48% [Citation26]; benralizumab: 52% [Citation27]; mepolizumab: 14% [Citation28]).
The efficacy of tezepelumab in other diseases has also been evaluated. One trial examined whether tezepelumab induced resistance against allergen stimulation when administered in combination with allergen immunotherapy in patients with rhinitis symptoms caused by cat allergy [Citation29]. Administration of subcutaneous allergen immunotherapy (SCIT) plus intravenous tezepelumab for 52 weeks significantly improved the peak nasal symptoms, with effects that persisted to Week 104, compared with SCIT alone. In addition, the transcriptomic analysis of the nasal epithelium at Week 104 revealed a persistent reduction in the expression of genes related to type 2 inflammation in patients treated with SCIT and tezepelumab.
Safety
The safety and tolerability of tezepelumab have been investigated both in animal models and in clinical trials of patients with moderate-to-severe asthma.
Multiple studies of cynomolgus monkeys treated with tezepelumab (intravenous and/or subcutaneous) for up to 6 months [Citation30] revealed no evidence of proliferative or preneoplastic lesions, no harmful reproductive or developmental toxicities, and no harmful effects on the mother, fetus, or offspring in pregnant monkeys. Furthermore, there were no harmful effects of tezepelumab over 6.5 months in any of the offspring.
In the phase III NAVIGATOR trial [Citation13], adverse events (AEs) occurred in 77.1% of patients in the tezepelumab group and 80.8% in the placebo group; serious AEs occurred in 9.8 and 13.7% of patients, respectively. Thus, the overall frequencies of AEs and serious AEs were comparable in both groups. The most common AEs were nasopharyngitis (tezepelumab vs placebo: 21.4 vs 21.5%), upper respiratory tract infection (11.2 vs 16.4%), headache (8.1 vs 8.5%), and asthma (5.1 vs 11.1%). Similar outcomes were reported in other international clinical trials [Citation10,Citation15,Citation25].
The safety and tolerability of tezepelumab were also assessed in Japanese patients enrolled in the NAVIGATOR [Citation20] and NOZOMI trials [Citation21]. Among Japanese patients enrolled in the NAVIGATOR trial, AEs occurred in 86.2% of patients in the tezepelumab group and 87.2% in the placebo group, consistent with the overall population. No new safety signals were identified in the Japanese subgroup [Citation20]. In the NOZOMI trial, in which safety was the primary endpoint, AEs and serious AEs were observed in 60.0% and 6.2% of patients, respectively [Citation21]. Overall, the results of these trials provide evidence for the safety and tolerability of tezepelumab in Japanese patients. The safety profile of tezepelumab found in these studies was consistent with that observed over 104 weeks in the DESTINATION trial [Citation17].
Regarding AEs of special interest, one case of Guillain–Barré syndrome related to tezepelumab was reported in a patient who experienced a viral infection in the PATHWAY trial [Citation14]. However, similar events were not reported in subsequent trials.
In the NAVIGATOR trial, infection was reported as a serious AE in 2.5% of patients in the tezepelumab group and 2.4% of patients in the placebo group, and malignant tumor was reported in 0.9% of patients in both groups [Citation17]. These results suggest that tezepelumab does not increase the risk of infection or malignant tumors.
In the DESTINATION trial [Citation17], a higher number of serious cardiac disorders was reported in the tezepelumab group than in the placebo group. However, there were no consistent patterns regarding the cause or timing of these cardiac-related AEs. All patients who experienced such events had at least one baseline cardiovascular risk factor, heart disease, or other confounding factors that presumably influenced these events. The incidence of adjudicated major adverse cardiac events was 0.65 and 0.46 per 100 patient-years in the tezepelumab and placebo groups, respectively (difference 0.19/100 patient-years, 95% CI: -0.58–0.85) [Citation17]. The incidence rate of cardiac AEs in patients treated with tezepelumab in the DESTINATION trial was similar to other biologics for the treatment of severe asthma [Citation31–34]. Regarding infection risk, the DESTINATION trial also revealed that the incidence rates of any severe infection or infestation were 2.39/100 patient-years in patients who received tezepelumab versus 2.38/100 patient-years in those who received placebo [Citation17]. Finally, the VECTOR trial (NCT05062759) is expected to provide information regarding the effects of tezepelumab on influenza vaccine antibody production.
Approval status
Tezepelumab was first approved in the USA on 17 December 2021 as an add-on maintenance treatment for severe asthma in patients aged ≥12 years [Citation35]. By the end of 2022, tezepelumab had been approved in Japan, the EU, Canada, and Switzerland, while the United Arab Emirates, Mexico, Iceland, Norway, Israel and Brazil approved the use of tezepelumab by the end of January 2023 [Citation30,Citation36].
Conclusion
The efficacy and safety of tezepelumab for severe uncontrolled asthma were confirmed in multiple clinical trials conducted internationally and in Japan. Tezepelumab suppresses asthma exacerbations in patients with a variety of asthma phenotypes by inhibiting TSLP, an epithelial cytokine that acts as a source of airway inflammation, and by suppressing the activation of a wide range of immune cells.
The results of several ongoing clinical trials will provide further evidence regarding the clinical efficacy and safety of tezepelumab in patients with asthma. In particular, the SUNRISE (NCT05398263) and WAYFINDER (NCT05274815) trials will further investigate the OCS-sparing effect of tezepelumab. Beyond these trials, it will be important to accumulate data on the efficacy and safety of tezepelumab in real-world clinical practice; that is, from post-marketing surveillance.
The present review is limited by the fact that it is based solely on published information, and any as-yet unpublished findings cannot be reflected. Furthermore, a systematic review methodology was not applied to the literature search.
Considering that tezepelumab improves airway hyperresponsiveness as well as a wide range of biomarkers, we may look to achieve asthma remission, the ultimate goal of treatment advocated by Menzies-Gow A, et al. [Citation37]. To help realize this goal, early intervention trials are needed to establish a treatment strategy that identifies ideal timing for treatment initiation according to each therapeutic agent as well as effective dosages.
Because of the pathophysiological role of TSLP, it is an important signaling molecule not only in asthma but also in a range of inflammatory disorders [Citation2,Citation4]. Therefore, tezepelumab is expected to be useful for treatment of various diseases. Several trials are ongoing in patients with inflammatory disorders, including the WAYPOINT trial (NCT04851964) in patients with chronic sinusitis with nasal polyps, the COURSE trial (NCT04039113) in patients with moderate-to-severe chronic obstructive pulmonary disease, and the INCEPTION trial (NCT04833855) in patients with spontaneous urticaria. The results of these trials will guide appropriate use of tezepelumab for a variety of indications.
Introduction
Asthma is a common chronic respiratory disease that affects up to 18% of the general population and is characterized by wheezing, shortness of breath, chest tightness, coughing and airflow limitation.
The pathophysiology of asthma includes airway inflammation, mediated by epithelial cytokines, one of which is thymic stromal lymphopoietin (TSLP).
Mechanism of action
Tezepelumab is a human immunoglobulin G2λ (IgG2λ) monoclonal antibody that binds to TSLP and inhibits its binding to the TSLP receptor, suppressing the inflammatory activities mediated by TSLP.
Pharmacokinetics & pharmacodynamics
Pharmacokinetic studies showed that administering tezepelumab at a dose of 210 mg every 4 weeks was predicted to achieve approximately 90% of the peak pharmacologic action.
Clinical trials, such as PATHWAY, CASCADE, and NAVIGATOR, have confirmed that tezepelumab improves type 2 inflammatory biomarkers, airway submucosal and blood eosinophil count, cytokine levels, fractional exhaled nitric oxide, and forced expiratory volume in 1 second in asthma patients, confirming its pharmacodynamic effects.
Efficacy
The NAVIGATOR and PATHWAY trials demonstrated that tezepelumab suppressed the annualized asthma exacerbation rate (AAER) compared with placebo. This effect was also observed regardless of the baseline biomarker level.
Tezepelumab also demonstrated effectiveness in reducing the AAER in a subset of Japanese patients enrolled in the NAVIGATOR trial, as well as the NOZOMI trial conducted in Japan.
In the SOURCE trial, tezepelumab was associated with a reduction in the oral corticosteroid dose among patients with a baseline blood eosinophil count of ≥150 cells/μL.
Safety
Regarding safety, no harmful effects of tezepelumab were observed in a range of toxicity studies in cynomolgus monkeys.
Results of clinical trials, including in Japanese patients, indicate that tezepelumab is well tolerated and has a low risk of serious adverse events.
Approval status
Based on the clinical trials, tezepelumab has thus far been approved in the USA, the EU, Japan, Canada, Switzerland, the United Arab Emirates, Mexico, Iceland, Norway, Israel and Brazil.
Conclusion
Several ongoing trials will provide more insight into the use of tezepelumab in asthma and in other potential indications, including chronic obstructive pulmonary disease, chronic sinusitis with nasal polyps and spontaneous urticaria.
Author contributions
M Shinkai and T Yabuta developed the concept for the review article and conducted the literature search and analysis. Both authors participated in the visualization of the article, writing of the original draft, and revisions and editing of the final version of the manuscript.
Financial & competing interests disclosure
M Shinkai received honoraria for lectures and funding for medical writing from AstraZeneca and has participated as the primary investigator of the NOZOMI and NAVIGATOR studies. T Yabuta is an employee of AstraZeneca. Funding was provided by AstraZeneca K.K. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors thank Keyra Martinez Dunn of Edanz, Japan, and Nicholas D. Smith (EMC K.K.) for providing medical writing support, which was funded by AstraZeneca, Japan, through EMC K.K., Japan, in accordance with Good Publication Practice guidelines (www.ismpp.org/gpp-2022).
Additional information
Funding
References
- Global Initiative for Asthma . Global strategy for asthma management and prevention (2022 update). Available from: https://ginasthma.org/reports/ (Accessed: March10, 2023).
- Gauvreau GM , SehmiR , AmbroseCS , GriffithsJM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin. Ther. Targets24(8), 777–792 (2020).
- Chung KF , WenzelSE , BrozekJLet al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J.43(2), 343–373 (2014).
- Corren J , ZieglerSF. TSLP: from allergy to cancer. Nat. Immunol.20(12), 1603–1609 (2019).
- Nolasco S , PelaiaC , SciosciaGet al. Tezepelumab for asthma. Drugs Today (Barc)58(12), 591–603 (2022).
- Pelaia C , PelaiaG , CrimiCet al. Tezepelumab: A potential new biological therapy for severe refractory asthma. Int. J. Mol. Sci.22(9), 4369 (2021).
- Tanaka J , WatanabeN , KidoMet al. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin. Exp. Allergy39(1), 89–100 (2009).
- Gauvreau GM , O’ByrnePM , BouletLPet al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med.370(22), 2102–2110 (2014).
- Verstraete K , PeelmanF , BraunHet al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun.8, 14937 (2017).
- Parnes JR , SullivanJT , ChenL , DiasC. Pharmacokinetics, safety, and tolerability of tezepelumab (AMG 157) in healthy and atopic dermatitis adult subjects. Clin. Pharmacol. Ther.106(2), 441–449 (2019).
- TEZSPIRE® (tezepelumab, AstraZeneca), EPAR product information, last updated 20 February 2023. www.ema.europa.eu/en/documents/product-information/tezspire-epar-product-information_en.pdf (Accessed: March10, 2023).
- Ly N , ZhengY , GriffithsJMet al. Pharmacokinetic and pharmacodynamic modeling of tezepelumab to guide phase 3 dose selection for patients with severe asthma. J. Clin. Pharmacol.61(7), 901–912 (2021).
- Menzies-Gow A , CorrenJ , BourdinAet al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N. Engl. J. Med.384(19), 1800–1809 (2021).
- Corren J , ParnesJR , WangLet al. Tezepelumab in adults with uncontrolled asthma. N. Engl. J. Med.377(10), 936–946 (2017).
- Diver S , KhalfaouiL , EmsonCet al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med.9(11), 1299–1312 (2021).
- Corren J , PhamTH , GarciaGil Eet al. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy77(6), 1786–1796 (2022).
- Menzies-Gow A , WechslerME , BrightlingCEet al. Long-term safety and efficacy of tezepelumab in people with severe, uncontrolled asthma (DESTINATION): a randomised, placebo-controlled extension study. Lancet Respir. Med.11(5), 425–438 (2023) [ published correction appears in Lancet Respir. Med. 11(3), e25 (2023)].
- Corren J , KarpeforsM , HellqvistÅ , ParnesJR , ColiceG. Tezepelumab reduces exacerbations across all seasons in patients with severe, uncontrolled asthma: a post hoc analysis of the PATHWAY phase 2b study. J. Asthma Allergy14, 1–11 (2021).
- Emson C , CorrenJ , SałapaK , HellqvistÅ , ParnesJR , ColiceG. Efficacy of tezepelumab in patients with severe, uncontrolled asthma with and without Nasal Polyposis: a Post Hoc Analysis of the Phase 2b PATHWAY Study. J. Asthma Allergy14, 91–99 (2021).
- Ishizuka T , Menzies-GowA , OkadaHet al. Efficacy and safety of tezepelumab in patients recruited in Japan who participated in the phase 3 NAVIGATOR study. Allergol. Int.72(1), 82–88 (2023).
- Shinkai M , EbisawaM , FukushimaYet al. One-year safety and tolerability of tezepelumab in Japanese patients with severe uncontrolled asthma: results of the NOZOMI study. J. Asthma60(3), 616–624 (2023).
- Menzies-Gow A , SteenkampJ , SinghSet al. Tezepelumab compared with other biologics for the treatment of severe asthma: a systematic review and indirect treatment comparison. J. Med. Econ.25(1), 679–690 (2022).
- Ando K , FukudaY , TanakaA , SagaraH. Comparative Efficacy and Safety of Tezepelumab and Other Biologics in Patients with Inadequately Controlled Asthma According to Thresholds of Type 2 Inflammatory Biomarkers: A Systematic Review and Network Meta-Analysis. Cells11(5), 819 (2022).
- Nopsopon T , LassiterG , ChenMLet al. Comparative efficacy of tezepelumab to mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: A Bayesian network meta-analysis. J. Allergy Clin. Immunol.151(3), 747–755 (2023).
- Wechsler ME , Menzies-GowA , BrightlingCEet al. Evaluation of the oral corticosteroid-sparing effect of tezepelumab in adults with oral corticosteroid-dependent asthma (SOURCE): a randomised, placebo-controlled, phase 3 study. Lancet Respir. Med.10(7), 650–660 (2022).
- Rabe KF , NairP , BrusselleGet al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med.378(26), 2475–2485 (2018).
- Nair P , WenzelS , RabeKFet al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N. Engl. J. Med.376(25), 2448–2458 (2017).
- Bel EH , WenzelSE , ThompsonPJet al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med.371(13), 1189–1197 (2014).
- Corren J , LarsonD , AltmanMCet al. Effects of combination treatment with tezepelumab and allergen immunotherapy on nasal responses to allergen: a randomized controlled trial. J. Allergy Clin. Immunol.151(1), 192–201 (2023).
- TEZSPIRE® (tezepelumab, AstraZeneca), EPAR public assessment report, published 21 September 2022. www.ema.europa.eu/documents/assessment-report/tezspire-epar-public-assessment-report_en.pdf (Accessed: March10, 2023).
- Panettieri RA Jr , SjöbringU , PéterffyAet al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir. Med.6(7), 511–525 (2018).
- Iribarren C , RahmaouiA , LongAAet al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: results from EXCELS, a prospective cohort study in moderate to severe asthma. J. Allergy Clin. Immunol.139(5), 1489–1495; e5 (2017).
- DUPIXENT® (dupilumab) injection, for subcutaneous use. Initial U.S. Approval: 2017 highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761055s007lbl.pdf (Accessed: March10, 2023).
- Fala L . Nucala (Mepolizumab): First IL-5 antagonist monoclonal antibody FDA approved for maintenance treatment of patients with severe asthma. Am. Health Drug Benefits9(Spec Feature), 106–110 (2016).
- TEZSPIRE® (tezepelumab, AstraZeneca), highlights of prescribing information, revised February 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf (Accessed: March10, 2023).
- TEZSPIRE® (tezepelumab) subcutaneous injection 210 mg. Interview form, 2nd edition, revised November 2022. www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/670227_2290403G1025_1_01 (Accessed: March10, 2023, In Japanese).
- Menzies-Gow A , BafadhelM , BusseWWet al. An expert consensus framework for asthma remission as a treatment goal. J. Allergy Clin. Immunol.145(3), 757–765 (2020).
