Abstract
Objective: This research aimed to assess the efficacy and safety of pembrolizumab (PBL) combined with albumin-bound paclitaxel (ab-Pac) and nedaplatin (NDP) for advanced esophageal squamous cell carcinoma (ESCC). Methods: A total of 47 ESCC patients were administered PBL or NDP on day 1 and ab-Pac on days 1 and 8, every 21 days for one cycle. Tumor and toxicities were evaluated every two cycles and every cycle, respectively. Results: The objective response rate was 68.1% and the disease control rate was 100%. The median follow-up was 16.7 months; median progression-free and overall survival were 12.6 and 19.9 months, respectively. Conclusion: The combination of PBL with ab-Pac and NDP proved to be an effective and safe treatment regimen for advanced ESCC.
Esophageal cancer (EC) is a prevalent malignancy originating in the upper gastrointestinal tract. In Europe and North America, over 50% of EC cases are of the adenocarcinoma histological type, while in China, more than 95% of cases are squamous cell carcinoma [Citation1,Citation2]. The early diagnostic rate of EC is low, and most EC patients are diagnosed at an advanced stage. EC remains an exceedingly aggressive and deadly malignant disease with a poor prognosis and low survival rate [Citation3].
At present, the most commonly used global treatment regimen for EC recommended by the National Comprehensive Cancer Network is chemotherapy using fluoropyrimidine + oxaliplatin/cisplatin [Citation4]. In China, chemotherapy using paclitaxel (Pac) + platinum (the TP regimen) is widely employed [Citation5]. Pembrolizumab (PBL), a PD-1 inhibitor, was approved for marketing in China and has been widely used for treating advanced non-small-cell lung cancer and metastatic melanoma. In 2020, PBL was also approved for the treatment of advanced esophageal squamous cell carcinoma (ESCC) and included in the Drug Assistance Program [Citation6].
The KEYNOTE-590 trial stands as the pioneering phase III trial aimed at assessing the effectiveness and safety of combining PBL with chemotherapy (comprising 200 mg of PBL on day 1, 800 mg/m2 of 5-fluorouracil [5-FU] on days 1–5 and 80 mg/m2 of cisplatin on day 1 in a 21-day cycle) as a first-line treatment for unresectable locally advanced or metastatic EC [Citation7]. The results of the study revealed that the chemoimmunotherapy group achieved a significantly higher objective response rate (ORR) at 45.0% compared with 29.3% in the chemotherapy group. Moreover, the progression-free survival (PFS) and overall survival (OS) of the experimental group were notably superior to those of the control group, with a 2-year PFS rate of 12 versus 3% and a 2-year OS rate of 26 versus 16%. These improvements were observed irrespective of PD-L1 expression. It is worth noting that the incidences of grade 3 or higher adverse events (AEs) in the experimental and control groups were 71.9 and 67.6%, respectively [Citation8]. Subsequent analysis focused on the Chinese population, revealing that up to 82.4% of participants in the chemoimmunotherapy group had a performance status (PS) score of 1, with 98% of them having ESCC [Citation9]. However, PFS, ORR and OS in the Chinese cohort were lower than those in the global cohort, suggesting that the standard 5-FU plus cisplatin regimen was not effective for the Chinese population.
In the ESCORT-1st study, the efficacy and safety of camrelizumab + TP as a first-line treatment for advanced EC were evaluated [Citation10]. The camrelizumab + TP group exhibited a higher ORR (72.1 vs 62.1%) and significantly longer median PFS (6.9 vs 5.6 months) and median OS (15.3 vs 12.0 months) compared with the placebo + TP group. The incidence of grade 3 or higher AEs in the two groups was consistent at 63.4 versus 67.7%, with a decreased neutrophil count being the predominant grade 3 treatment-associated AE. The ORIENT-15 study investigated the efficacy and safety of sintilimab + chemotherapy (cisplatin + Pac/5-FU) as a first-line treatment for unresectable locally advanced or metastatic EC. The results of the study indicated that the sintilimab + chemotherapy group had a longer median PFS (7.2 vs 5.7 months) and median OS (16.7 vs 12.5 months) compared with the placebo + chemotherapy group, while the incidence rates of grade 3 or higher AEs (59.9 vs 54.5%) in the two groups were similar [Citation11]. The JUPITER-06 study assessed the efficacy and safety of toripalimab + TP as a first-line treatment for advanced or metastatic ESCC compared with a placebo + TP regimen. Interim results presented at the European Society for Medical Oncology Congress 2021 showed that the toripalimab group had longer median PFS (5.7 vs 5.5 months) and median OS (17.0 vs 11.0 months) compared with the placebo group [Citation12]. In the RATIONALE-306 study, the efficacy and safety of tislelizumab + chemotherapy as a first-line treatment for advanced or metastatic ESCC were investigated against a placebo + chemotherapy regimen. The results demonstrated that tislelizumab + chemotherapy achieved longer median OS (17.2 vs 10.6 months) and median PFS (7.3 vs 5.6 months), as well as a higher ORR (63 vs 42%). The incidence of AEs in the tislelizumab group and the placebo group was consistent at 97 versus 96% [Citation13]. It is noteworthy that the JUPITER-06 study and the ESCORT-1st study exhibited similar experimental designs and targeted populations. Both studies involved the use of PD-1 inhibitor + TP in Chinese ESCC patients and reported reasonable PFS and OS. These clinical trials collectively suggested that immunotherapy in combination with TP is more effective, safer and better tolerated compared with 5-FU plus cisplatin. The maximum median OS reported in phase III trials for EC patients was 17.2 and 11.0 months in the chemoimmunotherapy group and the TP group, respectively, aligning with prior studies [Citation10,Citation12]. However, these outcomes were considered suboptimal. It is imperative to further refine the treatment regimen to enhance tolerance and improve the quality of life for EC patients.
Nanoparticle albumin-bound Pac (nab-Pac), a solvent-free Pac formulation employing human serum albumin rather than specialized solvents to encapsulate hydrophobic Pac molecules within nanoparticles of approximately 130 nm. The use of albumin has been observed to facilitate Pac uptake by binding to the albumin-binding membrane receptor protein gp60 in preclinical models, thereby enhancing transendothelial drug transport, enhancing delivery efficiency and elevating Pac concentrations within tumor tissues [Citation14]. While nab-Pac has earned approval for the treatment of breast, pancreatic and non-small-cell lung cancers, its application in the context of EC has yet to be fully investigated. Specifically, the clinical outcomes associated with nab-Pac following progression on immunotherapy, as well as its potential in combination with immunotherapy for advanced ESCC patients, remain uncharted territory, with no prior reports available [Citation14].
Notably, albumin-bound paclitaxel (ab-Pac) and nedaplatin (NDP) represent newer generations of taxane and platinum agents with demonstrated superior treatment efficacy against squamous cell carcinoma [Citation15,Citation16]. In a prior study of first-line ab-Pac and NDP (ab-TN regimen) treatment involving 31 patients with advanced ESCC, the results showed that one (3.2%) patient achieved complete response (CR), and only one patient exhibited progressive disease (PD). The partial response (PR) and stable disease (SD) rates were 64.5 (20/31) and 29.0% (9/31), respectively. Furthermore, the study reported an impressive ORR of 67.7%, a disease control rate (DCR) of 96.8% and a median PFS of 9.4 months. The incidence of grade 3 or higher treatment-associated AEs was less than 10% [Citation17]. It was noted that the ab-TN regimen outperformed previous TP regimens in terms of median PFS [Citation18]. Last year, we reported the result of 35 ESCC patients receiving PBL combined with NDP and nab-Pac in a single center [Citation19]. Even with a very small population, this real-world study demonstrated an outstanding result with ORR of 71.4% and median PFS of 13.4 months.
In the present study we included more patients based on the previous database and updated the OS data to provide more valuable real-world evidence for ESCC treated with immunotherapy combined with chemotherapy. The results indicated that this combination therapy is both effective and safe, suggesting promising prospects for the treatment of advanced ESCC.
Patients & methods
Clinical data
Clinical data from 47 chemotherapy-naive advanced ESCC patients who underwent PBL + ab-TN at Changhai Hospital (Shanghai, China) between June 2020 and November 2022 were retrospectively collected and analyzed. Inclusion criteria were as follows: locally advanced unresectable or metastatic ESCC with at least one measurable lesion by imaging assessment; age ≤80 years old; no prior systemic therapy, or more than 6 months since postoperative adjuvant therapy; Eastern Cooperative Oncology Group (ECOG) PS score of 0–1; normal liver and hematological function with no contraindications for chemotherapy; completion of at least two treatment cycles and one treatment evaluation. The exclusion criteria were as follows: patients who currently or previously participated in any other antitumor clinical study; any prior treatment for EC with systemic anticancer therapy as the primary treatment, including cytotoxic therapy, targeted therapy (including tyrosine kinase inhibitors or monoclonal antibodies) and immunotherapy, except for neoadjuvant use; patients who had received adjuvant therapy within 6 months prior to enrollment; patients who were assessed as inappropriate for enrollment by the investigator (e.g., patients with neurological disorders or metabolic disorders, suspected possible diseases by physical examination or laboratory tests, or contraindications to the use of the study drug, or high risk of treatment-related complications).
Treatment regimen
A treatment cycle lasting 21 days involved the administration of 200 mg of PBL (intravenous drip, on day 1), 130 mg/m2 of ab-Pac (intravenous drip, on days 1 and 8) and 70 mg/m2 of NDP (intravenous drip, on day 1). Patients could receive a maximum of eight cycles of treatment, depending on the occurrence of intolerable toxicity or PD. Maintenance therapy with PBL was administered in patients with stable response or better and without intolerable toxicity after combination therapy cycles, complemented by routine supportive treatments such as hepatoprotective agents and antiemetics. Prior to and after each chemotherapy cycle, participants underwent assessments of liver and hematological functions, while myocardial enzyme profiles and thyroid function were monitored throughout the treatment. Moreover, patients received G-CSF supportive therapy when myelosuppression occurred. In cases of grade 4 toxicity, the subsequent cycle’s dosage was reduced by 20–25%.
Efficacy & AEs
Radiological assessments of treatment efficacy occurred after every two treatment cycles, following the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). The tumor responses according to RECIST 1.1 were defined as follows [Citation20,Citation21]. CR was defined as the disappearance of all target lesions, and any pathological lymph nodes (whether target or nontarget) must have reduction in short axis to <10 mm; PR was defined as a decrease of at least 30% in the sum of diameters of target lesions, taking as reference the baseline sum diameters; PD was defined as an increase of at least 20% in the sum of diameters of the target lesions, and in addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm (note that the appearance of one or more new lesions is also considered progression); and SD was defined as neither sufficient shrinkage to qualify as PR nor sufficient increase to qualify as PD. A clinical complete response (cCR) is defined as tumor residue not visible on esophagogram, computed tomography, endoscopy, positron emission tomography–computed tomography, or by other nonsurgical methods after treatment. The ORR denoted the ratio of CR and PR cases. The DCR represented the ratio of CR, PR and SD cases. PFS referred to the duration from the treatment’s initiation to disease progression, but for patients with locally advanced disease who achieved CR after immunochemotherapy and received surgery subsequently, PFS was defined as the duration from surgery to the first observed recurrence or metastasis, while OS indicated the time from the conclusion of treatment to either demise or the last follow-up. Additionally, AEs were assessed based on the National Cancer Institute Common Toxicity Criteria (version 5.0) [Citation20,Citation22].
Statistical analysis
Statistical analysis was conducted using SPSS v. 24.0 software (IBM Corp., NY, USA). Categorical variables such as gender, primary tumor site, site of metastasis, ECOG score, surgery and radiotherapy were characterized by the frequency. Meanwhile, continuous variables (e.g., age) were described using the median value. Additionally, the median rates of PFS and OS were estimated through the Kaplan–Meier method and were compared by the log-rank test. A value of p < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 47 patients diagnosed with advanced ESCC were included in the study, with a median age of 64 years (range: 45–79). Among them, 37 (78.7%) patients were male and 10 (21.3%) patients were female. Regarding their ECOG PS scores, 26 (55.3%) patients had a score of 0, while 21 (44.7%) patients had a score of 1. Of the total, 24 (51.1%) patients had experienced postoperative recurrence and metastasis, while 23 (48.9%) patients presented with distant metastasis at their initial diagnosis. Furthermore, 21 (44.7%) patients had a history of prior radiotherapy. Only lymph node metastases were found in 21 (44.7%) patients, and organ metastases were identified in 26 (55.3%) patients. Notably, the top three sites of organ metastases were the lung (n = 17; 36.2%), liver (n = 7; 14.9%) and bone (n = 4; 8.5%), as detailed in .
Table 1. Baseline characteristics of the 47 participants in this study.
Efficacy
The number of immunochemotherapy treatment cycles of the 47 patients ranged from two to eight, with a median treatment duration of six cycles. Among these participants, seven (14.9%) achieved CR (four cCR + three pathological complete response [pCR]) (Supplementary File), 25 (53.2%) showed PR and 15 (31.9%) had SD, with none exhibiting PD. The ORR was 68.1% and the DCR reached 100% (). Among the seven patients who achieved CR (Supplementary Figures 1–7), five had locally advanced unresectable disease and two had multiple metastases (one patient had postoperative lymph node metastasis, with the supraclavicular node as the target lesion; the other patient had postoperative anastomotic recurrence with multiple lung metastases) after surgery. Among the five patients with locally advanced unresectable disease, two underwent surgery after receiving three cycles of treatment and one underwent surgery after receiving six cycles of treatment, all of whom were confirmed to achieve pCR after surgery; the other two patients achieved cCR without surgery. All locally advanced patients have not yet experienced recurrence or metastasis. The patient with lung metastases achieved cCR and presented with retroperitoneal lymph node metastases with a PFS of 28 months; the other patient with supraclavicular lymph node metastases achieved cCR after treatment and has been without progression until now.
Table 2. Efficacy of pembrolizumab + albumin-bound paclitaxel and nedaplatin in the treatment of advanced esophageal squamous cell carcinoma.
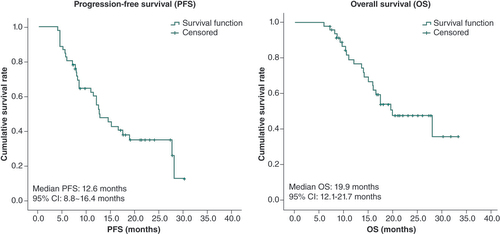
As of 15 May 2023, the median follow-up time for the study participants was 16.7 months (range: 6.0–33.3). Among the 47 participants, 30 experienced PD and 22 participants had passed away. In addition, four participants completed a 2-year PBL maintenance treatment, while eight participants withdrew from the study for various reasons, including surgery and achieving a state of SD or better, COVID-19, personal reasons, radiation pneumonitis after radiotherapy or diarrhea. Furthermore, seven participants are currently receiving PBL maintenance therapy, and two participants passed away due to non-cancer-related causes (cerebral infarction and obstructive jaundice, respectively). The median PFS was 12.6 months (95% CI: 8.8–16.4) and the median OS was 19.9 months (95% CI: 12.1–27.7), as illustrated in .
ab-Pac: Albumin-bound paclitaxel; ESCC: Esophageal squamous cell carcinoma; NDP: Nedaplatin; OS: Overall survival; PBL: Pembrolizumab; PFS: Progression-free survival.
Adverse events
In this study, major AEs were closely monitored, and the following AEs were observed: myelosuppression, abnormal thyroid function (including hypothyroidism and hyperthyroidism), skin rash, pyrexia, joint/muscle soreness, pneumonia, abnormal liver function, vitiligo and alopecia. It is noteworthy that most of these AEs were of grade 1 or grade 2 severity. However, a subset of participants (8.5%) experienced grade 3 AEs, which included myelosuppression in two cases and single cases of diarrhea and skin rash. Importantly, there were no AE-related fatalities. The most common hematological AEs were decreased neutrophil count (48.9%), reduced white blood cell count (46.8%) and anemia (31.9%) (). Among the nonhematological AEs, skin rash (25.5%), joint/muscle soreness (25.5%), hypothyroidism (19.1%) and fever (19.1%) were the most prevalent. These findings contribute to our understanding of the safety profile associated with the treatment.
Table 3. Adverse events of patients who received pembrolizumab + albumin-bound paclitaxel and nedaplatin.
In this study, there were several notable instances of immunotherapy-related AEs. One participant developed grade 3 skin rash with pruritus after completing four treatment cycles. Although this participant experienced relief from the symptoms following intravenous administration of dexamethasone and oral prednisone and ebastine, immunotherapy was discontinued. Another participant encountered grade 3 diarrhea after six treatment cycles, which was considered to be related to immunotherapy, and therefore immunotherapy was permanently discontinued. Furthermore, two participants faced grade 3 myelosuppression, which led to a 20% reduction in chemotherapy doses during the subsequent cycle. Among the noteworthy AEs, six participants developed pneumonia. Notably, two of these participants had received radiotherapy within the preceding 6 months. While the exact cause of pneumonia remained unclear, immunotherapy was suspended but resumed after improvement with steroid hormone and antibiotic treatment. Three participants who developed pneumonia were also diagnosed with COVID-19 during the study. Immunotherapy was temporarily halted, and it was subsequently resumed for two of these participants upon recovery from COVID-19; however, the third participant opted to terminate immunotherapy for delay caused by COVID-19 and for personal reasons. In addition, nine participants experienced fever, with temperatures reaching up to 39.5°C on the day of administration. However, no significant chills were observed, and these participants recovered after physical cooling and rehydration. Importantly, no evidence of infection was detected in their blood tests, suggesting that the fever might be related to an infusion reaction. Additionally, five patients encountered grade 2 hypothyroidism, necessitating thyroid hormone replacement therapy. Furthermore, three patients experienced hyperthyroidism, and three more patients developed vitiligo approximately 10 months after commencing treatment. Notably, specific treatment for vitiligo was not administered. These findings shed light on the spectrum of immunotherapy-related AEs encountered in the study, providing valuable insights into their management and implications for treatment.
Discussion
Chemotherapy remains the primary treatment for advanced EC in China, with 5-FU or Pac + platinum (TP) being the dominant regimen. From a pragmatic standpoint, the administration of a taxane can be accomplished within a single day, while 5-FU necessitates continuous infusion over a 48-h period. Consequently, for the sake of patient convenience and the conservation of medical resources, many Chinese physicians tend to favor taxane-based regimens over fluoropyrimidine regimens. Furthermore, variations in the reimbursement status of chemotherapeutic agents among Asian countries may also influence the choice of first-line regimens. For instance, 5-FU, Pac and cisplatin have been reimbursed in China for an extended period, whereas Pac may not receive reimbursement in several Asian countries for ESCC treatment due to a lack of approval for this specific indication. However, such a regimen shows low efficacy and severe AEs, resulting in limited application over the past decades [Citation23,Citation24]. With a high mutation burden, EC is supposed to benefit from immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors), and a series of randomized studies of immune checkpoint inhibitor treatment of EC marked a new era of EC treatment [Citation7,Citation14,Citation25,Citation26]. However, the choice of chemotherapy backbone for trials involving immunotherapy plus chemotherapy is likely to reflect local preferences. Given the absence of a consensus on the optimal first-line chemotherapy for East Asian patients with advanced ESCC, the selection of first-line treatment might impact the choices available for subsequent treatment lines. For example, if platinum-based fluoropyrimidine chemotherapy is used as a first-line treatment, both taxanes and irinotecan can be considered as second-line chemotherapy options. Conversely, opting for platinum-based chemotherapy combined with taxanes in the first-line setting limits the choice of second-line chemotherapy to irinotecan [Citation23]. Based on all the consideration above, phase III studies like ESCORT-1st, ORIENT-15, JUPITER-06 and RATIONALE-306 chose the TP regimen as the backbone [Citation10–13].
But how we can refine the treatment pattern in clinical practice is a question that needs to be answered. As we reported before, first-line ab-TN treatment in advanced ESCC was commonly used in our center with promising results [Citation17]. Based on this, the update results of this study also revealed a high ORR (68.1%) and DCR (100%), with the numbers of CR/PR/SD cases being seven (14.9%), 25 (53.2%) and 15 (31.9%), respectively. The median follow-up was 16.7 months, while median PFS and median OS were 12.6 and 19.9 months, respectively. The incidence of grade 3 or higher treatment-associated AEs was 8.5%, and the most common AEs were reduced neutrophil count (48.9%), decreased white blood cell count (46.8%), anemia (31.9%), abnormal thyroid function (25.5%), joint/muscle soreness (25.5%), skin rash (25.5%) and fever (19.1%). Notably, immunotherapy had to be terminated for two patients due to grade 3 skin rash (one patient) and diarrhea (one patient).
Although the ORR (68.1%) demonstrated by the current study was comparable to that in the ESCORT-1st study (72.1%), the incidence of grade 3 or higher AEs in this study was only 8.5%, indicating that the AEs and PFS of the proposed regimen were significantly superior to those observed in previous phase III trials [Citation10]. This improvement can be attributed to the enhanced PS (PS score of 0 in 55.3% of participants), secondary prevention and supportive therapy for myelosuppression, a reduced percentage of participants with distant lymph node metastases only (44.7%) and dose reduction for NDP. Additionally, the administration of 130 mg/m2 of ab-Pac on days 1 and 8 in this study contributed to the reduced incidence of AEs, while the JUPITER-06 and ESCORT-1st studies involved the administration of 175 mg/m2 of Pac on day 1 [Citation10,Citation12]. Notably, the weekly administration of Pac was shown to be superior to the 3-weekly administration of Pac, with comparable efficacy and a reduced incidence of AEs [Citation27,Citation28]. Notably, the proposed treatment regimen demonstrated a significantly higher ORR, a lower incidence of AEs and enhanced patient tolerance compared with the KEYNOTE-590 trial [Citation7]. Moreover, the CR rate in the proposed regimen group was higher than that observed with ab-TN alone, and no cases of PD were identified in the proposed regimen group. Further subgroup analysis revealed that the primary tumor site, surgery and radiotherapy had minimal influences on PFS.
However, retrospective studies, by their nature, rely on the available medical records and may not capture AEs as comprehensively as prospective studies. In our study, we collected data on AEs based on medical records, and there could be cases where events were not documented or were under-reported. This limitation is duly noted and should be considered when interpreting the safety outcomes presented in this study.
Following disease progression in advanced ESCC patients who received first-line immunotherapy in combination with chemotherapy, the routine approach involves transitioning to salvage chemotherapy featuring a different regimen (irinotecan, S-1, capecitabine, 5-FU or anlotinib), despite the absence of robust evidence from prospective clinical trials. However, the limited options for pretreated advanced ESCC patients might make the continuation of the initial PD-1 inhibitor treatment along with an alternative anticancer agent a viable option, particularly for those who initially responded positively to first-line therapy. In a retrospective study that encompassed 82 advanced ESCC patients treated with a PD-1 inhibitor as their first-line therapy, 61 patients pursued ongoing immunotherapy, combining it with antiangiogenic drugs in their second-line treatment. This approach resulted in an ORR of 22.9% and a DCR of 75.4% [Citation14]. In contrast, using antiangiogenic drugs as monotherapy exhibited limited efficacy in pretreated advanced ESCC patients, with ORRs hovering around 7%. Consequently, a portion of patients may derive benefits from continuing immunotherapy after initial progression [Citation14]. In a previous study, 25 patients received serplulimab in conjunction with chemotherapy. Among these patients, those who continued immunotherapy alongside chemotherapy showed a numerically lower ORR compared with patients who received chemotherapy alone, with respective rates of 28.6 (2/7) and 38.9% (7/18) [Citation14]. Numerous unresolved queries persist within this domain. Despite the common practice of employing various cytotoxic agents after the failure of the initial PD-1 blockade in combination with chemotherapy, the establishment of a standardized second-line treatment approach remains elusive. Furthermore, the outcomes related to response and survival prove suboptimal when utilizing a single-agent PD-1 inhibitor in second-line treatment for patients without prior immunotherapy exposure [Citation29]. The augmentation of chemotherapy alongside the conventional PD-1 blockade may offer enhanced efficacy, prompting further exploration into the optimal choice of companion drug to accompany PD-1 inhibitors in the second-line or subsequent treatment phases.
At present, the standard treatment for locally advanced EC is neoadjuvant concurrent chemoradiotherapy, which was confirmed by the CROSS [Citation30] and NEOCRTEC5010 [Citation31] studies, but the prevalence of this combination in China is less than 10% due to the fear of AEs caused by radiotherapy. Therefore, neoadjuvant chemotherapy is more commonly used in clinical practice. However, neoadjuvant chemotherapy only achieves modest results. In a study by Klevebro et al., neoadjuvant chemotherapy was employed for EC patients, and for those with ESCC, the pCR rate following neoadjuvant chemotherapy was found to be 9%, with a major pathological response rate of approximately 15% [Citation32]. Additionally, in Fan et al.’s single-arm phase II study, the neoadjuvant chemotherapy approach, involving nab-Pac combined with cisplatin, only resulted in a pCR rate of 13.3% for patients with locally advanced ESCC [Citation33].
With the success of immunotherapy in patients with advanced EC, more and more studies have investigated the efficacy of immunotherapy in patients with locally advanced disease. Now, both neoadjuvant chemoradiotherapy combined with immunotherapy and neoadjuvant chemotherapy combined with immunotherapy are being explored. Preliminary study results have shown different pCR rates (24–56%) [Citation34–44]. Furthermore, some phase III clinical studies such as Keystone-002 [Citation45] and phase II studies like NICE-2 [Citation46] are being conducted to compare different regimens head-to-head, to further investigate which regimen is superior.
The NIC-ESCC study, which involved 51 ESCC patients treated with a neoadjuvant regimen incorporating camrelizumab alongside nab-Pac and carboplatin, reported a remarkable pCR rate of 39.2% [Citation47]. Similarly, the SIN-ICE study, administering sintilimab along with nab-Pac and NDP, achieved a pCR rate of 35.5% [Citation48]. Recent developments in immunological neoadjuvant therapy for EC have been quite promising [Citation39,Citation49]. An open-label, single-arm, single-center phase II clinical trial introduced sintilimab in combination with chemotherapy, involving nab-Pac and cisplatin, as neoadjuvant therapy for ESCC. A total of 30 patients were enrolled in this study, and based on RECIST assessments, the ORR reached 67% (20/30), with a DCR of 97% (29/30). Ultimately, 23 of these patients underwent McKeown minimally invasive radical esophagectomy, and the pCR rate for primary tumors stood at 21.7%, with a significant major pathological response rate of 52.2% for primary tumors [Citation50]. In a previous investigation, it was determined that the rate of severe AEs (grade 3–4) resulting from the combination of neoadjuvant immunotherapy and chemotherapy for patients with locally advanced ESCC was 21.2% (14/66), and there were no treatment-related fatalities [Citation23]. The mentioned study indicated that neoadjuvant immunotherapy combined with chemotherapy, followed by surgical resection, might represent a safe, practical and effective treatment option for patients with locally advanced ESCC.
In another comprehensive study, a larger cohort of patients was included, and the follow-up period extended to nearly 2 years [Citation51]. The results showed a 1-year disease-free survival (DFS) rate of 91.1% for neoadjuvant sintilimab combined with chemotherapy, with a 2-year DFS rate of 78.3%. Moreover, the 1-year OS rate stood at an impressive 97.8%, and the 2-year OS rate registered at 88.0%. These follow-up outcomes collectively provide initial evidence of a survival benefit and reinforce the potential of combining neoadjuvant PD-1 inhibitors with chemotherapy. Notably, a multivariable Cox regression analysis highlighted the number of neoadjuvant treatment cycles as an independent predictor of DFS. Patients who completed three or four cycles of neoadjuvant treatment demonstrated a significant survival advantage compared with those who received only two cycles, as evidenced by 2-year DFS rates of 88.1 versus 68.0% [Citation51]. In contrast, a study by Sihag et al. reported a significantly higher postoperative complication rate (22/25; ~88%) within the neoadjuvant immunotherapy combined with radiotherapy and chemotherapy group [Citation52]. This variance in outcomes may be attributed to Sihag et al.’s utilization of immunotherapy in conjunction with radiotherapy and chemotherapy.
In patients with locally advanced ESCC, neoadjuvant triplet chemotherapy significantly improved OS and PFS compared with neoadjuvant double chemotherapy. Zhang et al. assessed the efficacy and safety of a triple-drug neoadjuvant chemotherapy regimen, which included nab-Pac, cisplatin and capecitabine, for locally advanced ESCC [Citation53]. Among 21 patients who received this regimen and later underwent surgery, eight (38.1%) achieved pCR. However, a noteworthy 35.5% of patients experienced grade 3/4 chemotherapy-related AEs. Several factors impact the efficacy of triplet chemotherapy, including disease stage, tumor burden and the patient’s physical condition and tolerance of treatment [Citation24]. A possible explanation for the apparent absence of OS improvement with triplet versus doublet chemotherapy in advanced ESCC patients could be the relatively higher incidence of AEs compared with earlier disease stages. Patients experiencing AEs are more likely to discontinue treatment, thus negatively affecting survival outcomes. Generally, in EC, triplet chemotherapy regimens are reserved for select patients with an excellent PS [Citation24]. The JCOG-1109 trial, which assessed the superiority of neoadjuvant docetaxel, cisplatin and 5-FU (DCF) therapy over cisplatin + 5-FU, demonstrated the significant OS advantage of neoadjuvant DCF over cisplatin + 5-FU for ESCC (hazard ratio: 0.868; 95% CI: 0.770–0.978; p = 0.02) [Citation54]. However, more questions – such as whether a three-drug regimen is enough and whether immunotherapy is necessary – will be raised and need to be answered in the future.
In our study, 17 out of 47 patients had locally advanced unresectable EC. Of these 17 patients, five eventually achieved cCR (29.4%), while three had surgery and were confirmed to have achieved pCR. Another patient was converted successfully after chemotherapy and confirmed to be tumor regression grade 2 (TRG2), which defined as more than single cells or rare small groups of cancer cells with evident tumor regression) patient has achieved favorable results until now. Therefore, the combination of PBL with ab-Pac and NDP is also a potential treatment option for neoadjuvant treatment and worth further exploration.
As far as we know, this is the first study to provide real-world evidence of a PD-1 inhibitor combined with ab-Pac and NDP in first-line treatment of metastatic ESCC with a superior median PFS and median OS than phase III studies that have been reported [Citation7,Citation10–13], even with a relatively small population. Nevertheless, it is important to acknowledge several limitations of this study. Firstly, this study was retrospective, and it had a short follow-up time and a small sample size. Secondly, the OS data may be compromised by delayed treatment due to COVID-19, which has the potential to impact the power of the findings. Thirdly, as a single-arm study without comparing the chemotherapy group alone, the conclusion of the article needs to be confirmed in a controlled trial in the future. Finally, it is essential to recognize that the under-reporting or incomplete documentation of AEs is a common challenge in retrospective studies. These limitations highlight the necessity for additional research through prospective, controlled trials to confirm efficacy and safety of the PBL + ab-Pac and NDP regimen.
Conclusion
In conclusion, this study suggests that the combination of PBL with ab-Pac and NDP represents a promising strategy for the first-line treatment of ESCC, accompanied with satisfactory efficacy and more favorable safety and tolerability profile. However, to solidify this conclusion, additional randomized controlled prospective studies are warranted.
Chemoimmunotherapy with pembrolizumab (a PD-1 inhibitor) and albumin-bound paclitaxel demonstrates significant efficacy and tolerable safety in the treatment of advanced esophageal squamous cell carcinoma (ESCC).
In this retrospective study, a total of 47 ESCC patients were included, and the median age of the participants was 64 years.
High response rates were observed, with 68.1% of patients achieving objective responses and a disease control rate of 100%.
The treatment regimen had a range of two to eight cycles, with a median treatment duration of six cycles.
Seven patients (14.9%) achieved complete responses, and the majority exhibited partial responses (53.2%).
The most common adverse events were myelosuppression, abnormal thyroid function, skin rash and fever, with most being grade 1 or 2.
No treatment-related deaths were reported, and only 8.5% of patients experienced grade 3 adverse events.
Median progression-free survival was 12.6 months, and median overall survival was 19.9 months, with a median follow-up of 16.7 months.
This study suggests that the proposed chemoimmunotherapy regimen may provide a promising strategy for first-line ESCC treatment.
Randomized controlled prospective studies are needed to confirm these findings and establish the regimen’s place in ESCC therapy.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by F Yan, L Chen, M Ying and J Li. The first draft of the manuscript was written by F Yan and L Chen. All the authors read and approved the final manuscript.
Ethical conduct of research
This study does not have direct contact with the subjects, does not involve the personal privacy of the subjects and does not provide test reports to the subjects; the test results are only used for this study and are not used as an auxiliary diagnostic basis; this study itself does not cause any possible damage to the health of the subjects; nor does it intervene in the diagnosis, examination and treatment of the subjects in the real world. Therefore, this study has no additional risk that compromises the health and other interests of the subjects. This clinical study meets the following conditions for applying for exemption from informed consent: the risk of the study to the subjects is no greater than the minimum risk; the exemption from informed consent will not adversely affect the health and rights of the subjects; the privacy and personal identity information of the subjects are protected; if informed consent must be obtained, the study will not be conducted. Based on the above, an exemption from signing the subject’s informed consent has been requested. This study is a single-center study and has been approved by Changhai Hospital (Shanghai, China) Ethics Committee.
Supplemental Figure 1
Download JPEG Image (9.9 MB)Supplemental Figure 2
Download JPEG Image (1.5 MB)Supplemental Figure 3
Download JPEG Image (5.5 MB)Supplemental Figure 4
Download JPEG Image (5.4 MB)Supplemental Figure 5
Download JPEG Image (1,008.4 KB)Supplemental Figure 6
Download JPEG Image (2.7 MB)Supplemental Figure 7
Download JPEG Image (1.1 MB)Acknowledgments
The authors sincerely thank all investigators and patients who contributed to this study. The authors also thank Q Hao from the Department of Radiology (Changhai Hospital, Shanghai, China) and W Zhang and J Li from MSD GMA China for their scientific support.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/imt-2023-0188
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Sung H , Ferlay J , Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
- Zheng RS , Zhang SW , Zeng HM et al. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Center 2(1), 1–9 (2022).
- Yang H , Li X , Yang W . Advances in targeted therapy and immunotherapy for esophageal cancer. Chin. Med. J. (Engl.) 136(16), 1910–1922 (2023).
- National Comprehensive Cancer Network . NCCN Guidelines in esophageal and esophagogastric junction cancers, version 3 (2023). www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Zhao J , Zhang S , Guo X et al. PD-1 inhibitors combined with paclitaxel and cisplatin in first-line treatment of esophageal squamous cell carcinoma (ESCC): a network meta-analysis. BMC Cancer 23(1), 1221 (2023).
- Pembrolizumab Injection (Keytruda) instruction . China, Approval: 2018-07-20, Update: 2023-09-05. www.msdchina.com.cn/Product
- Sun JM , Shen L , Shah MA et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced esophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398(10302), 759–771 (2021).
- Metges JP , Kato K , Sun JM et al. First-line pembrolizumab plus chemotherapy versus chemotherapy in advanced esophageal cancer: Longer-term efficacy, safety, and quality-of-life results from the phase 3 KEYNOTE-590 study. American Society of Clinical Oncology-Gastrointestinal Cancers Symposium, CA, USA, Abstract #241 (2022).
- Li Z , Sun Y , Ye F et al. First-line pembrolizumab plus chemotherapy versus chemotherapy in patients with advanced esophageal cancer: Chinese subgroup analysis of KEYNOTE-590. American Society of Clinical Oncology, IL, USA, Abstract #4049 (2021).
- Luo H , Lu J , Bai Y et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA 326(10), 916–925 (2021).
- Lu ZH , Wang JY , Shu YQ et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic esophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ 377, e068714 (2022).
- Wang ZX , Cui CX , Yao J et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell 40(3), 277–288 (2022).
- Xu J , Kato K , Raymond E et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 24(5), 483–495 (2023).
- Xin D , Song Y , Mu L et al. The efficacy and safety of nanoparticle albumin bound-paclitaxel-based regimen as second- or third-line treatment in patients with advanced esophageal squamous cell carcinoma. Thorac. Cancer 14(15), 1392–1397 (2023).
- Wang HY , Yao ZH , Tang H et al. Weekly nanoparticle albumin-bound paclitaxel in combination with cisplatin versus weekly solvent-based paclitaxel plus cisplatin as first-line therapy in Chinese patients with advanced esophageal squamous cell carcinoma. Onco. Targets Ther. 30(9), 5663–5669 (2016).
- Zhang F , Wang Y , Wang ZQ et al. Efficacy and safety of cisplatin-based versus nedaplatin-based regimens for the treatment of metastatic/recurrent and advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis. Dis. Esophagus 30(2), 1–8 (2017).
- Fang Y , Mingzhen Y , Longpei C et al. Clinical observation of albumin-bound paclitaxel plus nedaplatin as first-line treatment in advanced esophageal squamous cell carcinoma patients. Chin. Oncol. 30(8), 632–635 (2020).
- Chen Y , Ye J , Zhu Z et al. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: a randomized, multicenter, phase III clinical trial. J. Clin. Oncol. 37(20), 1695–1703 (2019).
- Yan F , Ying M , Chen L , Fu Q et al. Efficacy and safety of pembrolizumab plus albumin-bound paclitaxel and nedaplatin as a first-line therapy for advanced esophageal squamous cell carcinoma. Chin. J. Cancer Biother. 29(9), 801–806 (2022).
- Eisenhauer EA , Therasse P , Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45(2), 228–247 (2009).
- Schwartz LH , Litière S , de Vries E et al. RECIST 1.1 – update and clarification: from the RECIST Committee. Eur. J. Cancer 62, 132–137 (2016).
- National Cancer Institute Common Toxicity Criteria (version 5.0). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Xia P , Li P , Wu S et al. Evaluation of the safety and effectiveness of neoadjuvant combined chemoimmunotherapy in the treatment of locally advanced esophageal squamous cell carcinoma: a retrospective single-arm cohort study. Ann. Transl. Med. 10(18), 991 (2022).
- Xu J , Bai Y , Li E , Xu N , Shi D , Qian J . Efficacy and safety of chemotherapy regimens for first-line treatment of advanced esophageal squamous cell carcinoma in Asia: a systematic review. Expert Rev. Anticancer Ther. 22(9), 981–998 (2022).
- Li Y , Zhou A , Liu S , He M et al. Comparing a PD-L1 inhibitor plus chemotherapy to chemotherapy alone in neoadjuvant therapy for locally advanced ESCC: a randomized phase II clinical trial: a randomized clinical trial of neoadjuvant therapy for ESCC. BMC Med. 21(1), 86 (2023).
- Hirano H , Kato K . Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J. Clin. Oncol. 49(5), 412–420 (2019).
- Osman MA , Elkady MS , Nasr KE . Weekly paclitaxel versus three-weekly paclitaxel in recurrent platinum-resistant epithelial ovarian and peritoneal cancers: a phase III study. Clin. Med. Insights Oncol. 10, 35–41 (2016).
- Dalton HJ , Yu X , Hu L et al. An economic analysis of dose dense weekly paclitaxel plus carboplatin versus every-3-week paclitaxel plus carboplatin in the treatment of advanced ovarian cancer. Gynecol. Oncol. 124(2), 199–204 (2012).
- Kojima T , Shah MA , Muro K et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. 38(35), 4138–4148 (2020).
- Shapiro J , van Lanschot JJB , Hulshof MCCM et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 16(9), 1090–1098 (2015).
- Yang H , Liu H , Chen Y et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. 156(8), 721–729 (2021).
- Klevebro F , Alexandersson von Döbeln G , Wang N et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann. Oncol. 27, 660–667 (2016).
- Fan Y , Jiang Y , Zhou X et al. Phase II study of neoadjuvant therapy with nab-paclitaxel and cisplatin followed by surgery in patients with locally advanced esophageal squamous cell carcinoma. Oncotarget 7(31), 50624–50634 (2016).
- Li C , Zhao S , Zheng Y et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur. J. Cancer 144, 232–241 (2021).
- Shang X , Zhao G , Liang F et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage III) esophageal squamous cell carcinoma: a study protocol for a prospective, single-arm, single-center, open-label, phase-II trial (Keystone-001). Ann. Transl. Med. 10(4), 229 (2022).
- Li Z , Liu J , Zhang M et al. A phase II study of neoadjuvant immunotherapy combined with chemotherapy (camrelizumab plus albumin paclitaxel and carboplatin) in resectable thoracic esophageal squamous cell cancer (NICE study): interim results. J. Clin. Oncol. 39(Suppl. 15), Abstract 4060 (2021).
- Gu Y , Chen X , Wang D et al. A study of neoadjuvant sintilimab combined with triplet chemotherapy of lipo-paclitaxel, cisplatin, and S-1 for resectable esophageal squamous cell carcinoma (ESCC). Ann. Oncol. 31(Suppl. 6), S1287–S1318 (2020).
- Zhao L , Xing W , Yang Y et al. The sequence of chemotherapy and anti-PD-1 antibody influence the efficacy of neoadjuvant immunochemotherapy in locally advanced esophageal squamous cell cancer: a phase II study. J. Clin. Oncol. 39(Suppl. 15), Abstract 4051 (2021).
- Yan X , Duan H , Ni Y et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD-NICE). Int. J. Surg. 103, 106680 (2022).
- van den Ende T , de Clercq NC , van Berge Henegouwen MI et al. A phase II feasibility trial of neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: the PERFECT trial. J. Clin. Oncol. 37(Suppl.), Abstract 4045 (2019).
- Kelly RJ , Smith KN , Anagnostou V et al. Neoadjuvant nivolumab plus concurrent chemoradiation in stage II/III esophageal/gastroesophageal junction cancer. J. Clin. Oncol. 37(Suppl. 4), Abstract 142 (2019).
- Jiang N , Jiang M , Zhu X et al. SCALE-1: safety and efficacy of short course neoadjuvant chemo-radiotherapy plus toripalimab for locally advanced resectable squamous cell carcinoma of esophagus. J. Clin. Oncol. 40(Suppl. 16), Abstract 4063 (2022).
- Cowzer D , Wu AJC , Sihag S et al. Durvalumab (D) and PET-directed chemoradiation (CRT) after induction FOLFOX for esophageal adenocarcinoma: final results. J. Clin. Oncol. 40(Suppl. 16), Abstract 4029 (2022).
- Uboha NV , Eickhoff JC , Maloney JD et al. Phase I/II trial of perioperative avelumab in combination with chemoradiation (CRT) in the treatment of stage II/III resectable esophageal and gastroesophageal junction (E/GEJ) cancer. J. Clin. Oncol. 40(Suppl. 16), Abstract 4034 (2022).
- Shang X , Zhang W , Zhao G et al. Pembrolizumab combined with neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy followed by surgery for locally advanced oesophageal squamous cell carcinoma: protocol for a multicentre, prospective, randomized-controlled, phase III clinical study (Keystone-002). Front. Oncol. 12, 831345 (2022).
- Yang Y , Zhu L , Cheng Y et al. Three-arm phase II trial comparing camrelizumab plus chemotherapy versus camrelizumab plus chemoradiation versus chemoradiation as preoperative treatment for locally advanced esophageal squamous cell carcinoma (NICE-2 Study). BMC Cancer 22(1), 506 (2022).
- Liu J , Li J , Lin W et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, phase 2 study. Int. J. Cancer 151, 128 (2022).
- Duan H , Wang T , Luo Z et al. A multicenter single-arm trial of sintilimab in combination with chemotherapy for neoadjuvant treatment of resectable esophageal cancer (SIN-ICE study). Ann. Transl. Med. 9(22), 1700 (2021).
- Hong ZN , Gao L , Weng K et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemotherapy for locally advanced esophageal squamous cell carcinoma: a propensity score matching analysis. Front. Immunol. 13, 836338 (2022).
- Zhang Z , Hong ZN , Xie S et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, single-center, phase 2 trial (ESONICT-1). Ann. Transl. Med. 9, 16–23 (2021).
- Lv H , Huang C , Li J et al. The survival outcomes of neoadjuvant sintilimab combined with chemotherapy in patients with locally advanced esophageal squamous cell carcinoma. Front. Immunol. 13, 1100750 (2023).
- Sihag S , Ku GY , Tan KS et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J. Thorac. Cardiovasc. Surg. 161, 836–843; e1 (2021).
- Zhang W , Li Y , Xue L et al. Encouraging pathological complete response rate from neoadjuvant chemotherapy with albumin-bound paclitaxel plus cisplatin and capecitabine for locally advanced esophageal squamous carcinoma: preliminary outcome of a retrospective study. Cancer Manag. Res. 13, 2163–2170 (2021).
- Matsuda S , Kitagawa Y , Takemura R et al. Real-world evaluation of the efficacy of neoadjuvant DCF over CF in esophageal squa-mous cell carcinoma: propensity score matched analysis from 85 authorized Institutes for Esophageal Cancer in Japan. Ann. Surg. 278, e35–e42 (2022).
