Abstract
Despite the progress in immunotherapy and targeted therapy for patients with cutaneous malignant melanoma not all patients with loco-regional recurrences will respond to treatment. Electrochemotherapy is a relatively new treatment modality where the efficacy of a chemotherapeutic drug is enhanced by an electrical field. Here we report a case of a 68-year-old woman with a large therapy resistant inguinal lymph node melanoma metastasis complicated by bleeding that was successfully treated with electrochemotherapy.
Not all melanoma patients respond to targeted therapy/immunotherapy.
Electrochemotherapy (ECT) is a local chemotherapy treatment.
In ECT the effect of the chemotherapeutic drug is enhanced by an electrical field.
One of the effects of ECT is an antivascular effect.
A patient with a bleeding melanoma metastasis was successfully treated with ECT.
Previous treatment with BRAF inhibitor had been unsuccessful.
The effect was probably due to combination of ECT and BRAF inhibitor.
The last decade has seen a lot of progress in the treatment and prognosis of cutaneous malignant melanoma as a consequence of a deeper understanding of both the underlying tumor biology and the immune system response. The emergence of immunotherapy with checkpoint inhibitors, and also targeted therapy have reduced the risk of recurrence and increased life expectancy for patients with metastatic malignant melanoma [Citation1–5]. However, not all patients are cured and the success of the new therapies can paradoxically lead to an increased need for local palliative treatments. There are many such options including surgery, radiotherapy, talimogene laherparepvec (T-VEC) and isolated limb perfusion [Citation6,Citation7]. In some cases, these modalities cannot be used or have already been used without a successful outcome.
Electrochemotherapy (ECT) is a local chemotherapy treatment where the effects of the chemotherapeutic agents used; cisplatin or bleomycin, are greatly increased by an applied electrical field causing an increased cell membrane permeability by a mechanism called electroporation. There are three known clinical effects of ECT: a direct cytotoxic effect, an antivascular effect and, in some patients, an immunological effect [Citation8–10]. In the last decade ECT has been established mainly as a palliative treatment of cutaneous and subcutaneous metastases from a wide variety of primary tumors: head and neck cancer, breast cancer, gynecological cancer, gastrointestinal cancer and malignant melanoma [Citation11]. In a systematic review by Mali et al. a cut-off for treatment efficacy at tumor sizes of 3 cm was reported [Citation12]. The European Standard Operation Procedures of Electrochemotherapy (ESOPE) is a standardized protocol for ECT treatment [Citation13,Citation14]. In the Swedish national guidelines, ECT is recommended for palliative treatment of cutaneous and subcutaneous metastases [Citation15]. Recent clinical studies have implied a role for ECT as an adjuvant treatment for malignant melanoma [Citation16,Citation17]. Here we report a case of a therapy-resistant ulcerating melanoma lymph node metastasis where ECT treatment was successful, achieving a nearly complete remission and a total control of the patient’s main complaint, bleedings.
The case
A 68-year-old woman with stage IV cutaneous malignant melanoma but otherwise healthy was referred from the oncology department at another university hospital in November 2015. The patient had been treated surgically for a 3.3 mm thick stage T3b malignant melanoma of the left hallux. The surgery included amputation with adequate negative margins and a sentinel lymph node biopsy with a metastasis in one lymph node in the left inguinal region. A lymph node dissection was scheduled but had to be cancelled when the patient developed a postoperative infection. The infection was treated with flucloxacillin that caused a hepatotoxic reaction leading to reversible liver failure. In the meantime, the patient developed several metastases in the inguinal and pelvic regions. The lymph node manifestation was at this point considered unresectable and radiotherapy of the inguinal and pelvic regions was performed However, with further progression and ulceration of the inguinal metastases (). The patient’s quality of life was poor because of constant bleeding from the ulceration which severely limited her daily activities and she rarely left her home. Treatment with the antifibrinolytic drug cyklokapron had had limited effects on the bleeding. Since the tumor had a BRAF-V600E gene mutation dabrafenib was administered, with some effect causing tumor shrinkage but none on her main complaint: bleeding (). She was then referred to our institution for assessment for ECT treatment. At presentation, she had a 5.5 x 3.5 cm ulcerating tumor in the left inguinal region (). During examination, there were several oozing bleedings present. Her liver function was normalized and the dabrafenib treatment had been discontinued for 5 days after taking it for 8 weeks. She had no other medication besides cyklokapron.
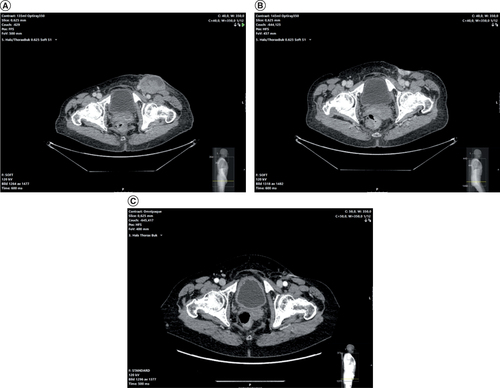
(A) Large ulcerating lymph node in the left inguinal region after radiation therapy. (B) Persisting ulceration after dabrafenib treatment. (C) Complete regress of metastasis 7 months after electrochemotherapy.
(A) Ulcerating melanoma metastasis before electrochemotherapy treatment. (B) Electrochemotherapy treatment with intralesional cisplatin. (C) The patient had significantly less bleeding 2 weeks after treatment. (D) The ulceration was now almost healed and the bleeding had stopped 6 weeks after treatment.
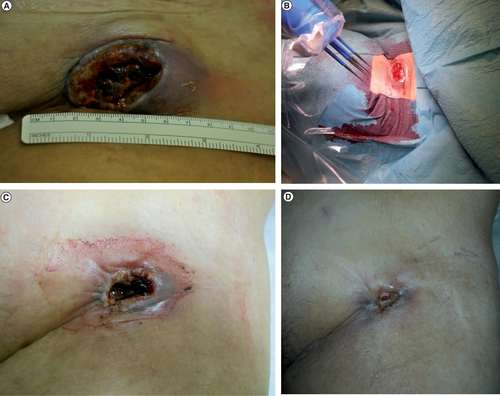
Since bleeding was her main complaint and this had a negative effect on her quality of life it was decided to treat with ECT primarily because of its antivascular effect [Citation9]. Since the tumor was quite large a two-session treatment with a 3-week interval was planned. The treatment was performed under general anesthesia with muscle relaxation. Local injection of 18 ml cisplatin (Sandoz, Copenhagen, Denmark) with a concentration of 1 mg/ml in accordance with the ESOPE protocol was administered. Cisplatin was used since in our clinical experience bleomycin causes more necrosis increasing the risk of a femoral artery blow-out bleeding. Ultrasound with the BK Medical 8870 system (Peabody, MA, USA) was used during injection to ensure an even distribution of cisplatin including a 0.5 cm margin. Electroporation was then performed using the Sennex electroporation system (Bionmed, Saarsbrucken, Germany) with 16 complete applications using the six-needle applicator (). The Sennex system delivers eight 0.1 ms square-wave pulses per application with electric field strength 1100 V/cm. A 0.5 mm treatment margin was achieved again using ultrasound. Great care was taken not to penetrate the femoral vessels. Two weeks after treatment, the patient came back for a follow-up visit. She had significantly less bleeding and the tumor six was now 4.5 × 2.5 cm (). She had however had some side effects with pain of the adductor muscle group. She wanted to postpone the treatment and a new follow-up visit was scheduled. About 4 weeks later the tumor size was 0.7 × 0.5 cm and the bleeding had stopped completely (). Dabrafenib treatment had been restarted two weeks earlier because of newly diagnosed lung metastases. No additional ECT treatment was performed. Her quality of life was greatly improved and she could even travel abroad. She died 11 months after treatment from cerebral metastases. A CT scan performed 7 months after ECT treatment showed a lasting response ().
Discussion
Undoubtedly, the progress of immunotherapy and targeted therapy have greatly improved the prognosis and quality of life in patients with cutaneous malignant melanoma. However, some patients are not responding to treatment leaving the patient with sometimes severe local symptoms. Here, we have reported a case of a patient with an ulcerating unresectable lymph node metastasis that had not responded to previous treatment with radiotherapy and only partially to targeted therapy with dabrafenib. In fact, the tumor and the patient’s symptoms with bleeding that severely impaired her quality of life had increased during both treatments. ECT was successful in achieving a near total local remission with a total control of the bleedings after one treatment. Since dabrafenib was unsuccessful in decreasing the bleedings when first administered it can be assumed that the clinical effect was caused by a synergistic effect of ECT and dabrafenib. This assumed synergistic effect has previously been reported in case reports [Citation18]. In vitro studies have reported that ECT is effective in cell lines with the BRAF mutation and show a synergistic effect with BRAF inhibitors (vemurafenib) [Citation19]. ECT as a successful mono-modality treatment have also been reported in a case report of two cases [Citation20]. Clinically, synergistic effects between ECT and immunotherapy resulting in increased survival have been reported [Citation16,Citation17]. Although, there is a role for ECT in the treatment of malignant melanoma recognized in the Swedish guidelines it is the authors opinion that its future role should not be limited to treatment of in-transit metastases but instead should be subjected to clinical studies as both a mono-modality treatment and as an adjuvant treatment with targeted therapy and/or immunotherapy in the metastatic setting.
Conclusion
The combination of electrochemotherapy and vemurafenib was successful in stopping and preventing further bleeding from a large melanoma metastases where vemurafenib alone had not been successful.
Financial & competing interests disclosure
SJ Kristiansson and FJ Landström have been paid by OnMed Oncological Medical Devices AB for educating physicians about the Sennex System and product development. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Weber J , Mandala M , Del Vecchio M et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377, 1824–1835 (2017).
- Long GV , Hauschild A , Santinami M , Atkinson V et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 377, 1813–1823 (2017).
- Weber JS , D’Angelo SP , Minor D et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16, 375–384 (2015).
- Larkin J , Minor D , D’Angelo S et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label Phase III trial. J. Clin. Oncol. 36, 383–390 (2018).
- Robert C , Schachter J , Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372(26), 2521–2532 (2015).
- Andtbacka RH , Kaufman HL , Collichio F et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33(25), 2780–2788 (2015).
- Moreno-Ramirez D , de la Cruz-Merino L , Ferrandiz L , Villegas-Portero R , Nieto-Garcia A . Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety. Oncologist 15(4), 416–427 (2010).
- Sersa G , Miklavcic D , Cemazar M , Rudolf Z , Pucihar G , Snoj M . Electrochemotherapy in treatment of tumours. Eur. J. Surg. Oncol. 34(2), 232–240 (2008).
- Jarm T , Cemazar M , Miklavcic D , Sersa G . Antivascular of electrochemotherapy: implications in treatment of bleeding metastases. Expert Rev. Anticancer Ther. 10(5), 729–746 (2010).
- Falk H , Lambaa S , Hjort Johannesen H , Wooler G , Venzo A , Gehl J . Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma - a case report. Acta Oncol. 56(8), 1126–1131 (2017)
- Morley J , Grocott P , Pursell E , Murrells T . Electrochemotherapy for the palliative management of cutaneous metastases: a systematic review and meta-analysis. Eur. J. Surg. Oncol. 45(12), 2257–2267 (2019)
- Mali B , Jarm T , Snoj M , Sersa G , Miklavcic D . Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur. J. Surg. Oncol. 39(1), 4–16 (2013)
- Mir LM , Gehl J , Sersa G et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator (TM) by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 4, 14–25 (2006)
- Gehl J , Sersa G , Matthiessen et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 57, 874–882 (2018)
- Regionala Cancercenter i samverkan . Nationellt vårdprogram Malignt melanom 2019. https://kunskapsbanken.cancercentrum.se/diagnoser/melanom/vardprogram/systemisk-behandling/#chapter-11-3-1-6-Elektrokemoterapi
- Theurich S , Rothschild SI , Hoffmann M et al. Local tumor treatment in combination with systemic ipilimumab immunotherapy prolongs overall survival in patients with advanced malignant melanoma. Cancer Immunol. Res. 4, 744–754 (2016).
- Campana LG , Peric B , Mascherini M et al. Combination of pembrolizumab with electrochemotherapy in cutaneous metastases from melanoma: a comparative retrospective study from the InspECT and Slovenian Cancer Registry. Cancer 13(17), 4289 (2021).
- Valpione S , Campana LG , Pigozzo J , Chiarion-Sileni V . Consolidation electrochemotherapy with bleomycin in metastatic melanoma during treatment with dabrafenib. Radiol. Oncol. 49, 71–74 (2015).
- Dolinsek T , Prosen L , Cemazar M , Potocnik T , Sersa G . Electrochemotherapy with bleomycin is effective in BRAF mutated melanoma cells and interacts with BRAF inhibitors. Radiol. Oncol. 50, 274–279 (2016).
- Gallagher Chin KY , MacKenzie-Ross A . Bleomycin electrochemotherapy for the management of locally advanced metastatic melanoma: two notable clinical cases potentially indicating a greater therapeutic role in the era of targeted and immuno-therapy. JPRAS Open 26, 43–48 (2020).
