Keywords::
Changing established clinical practices in healthcare systems, such as the UK National Health Service (NHS), can be a challenge. Introducing innovation into practice or disrupting pathways has the potential to deliver a more efficient and higher quality health service. However, with research findings typically taking around 17 years to translate into clinical practice, the NHS has recognized a need to speed up innovation, as highlighted in the long-term NHS plan [Citation1]. The transformation of service delivery through innovation requires clear pathways for effective implementation, but there are known to be many challenges. It is, therefore, important to learn from examples that can be used as a template to facilitate adoption of innovation in the future.
Warfarin is the most commonly used oral anticoagulant worldwide with annual prescriptions in the western world typically equaling 0.5–1.5% of the population [Citation2]. Achieving target anticoagulation and avoiding adverse drug reactions [Citation3,Citation4] depends on the correct drug dose for each person. Several factors contribute to the variability in therapeutic warfarin dose requirement including clinical (10–20% variability) and genetic factors (40% variability) [Citation5]. Two main genes have been strongly associated with warfarin response: CYP2C9 and VKORC1 [Citation6].
The clinical utility of point-of-care genotype guided dosing (POCT-GGD) of warfarin was shown in our EU-PACT randomized controlled trial (RCT) which was carried out in the UK and Sweden with a 7% improvement in time in therapeutic range in genotyped patients [Citation7], with the greatest effect seen in those carrying CYP2C9 and VKORC1 variants [Citation8]. At the same time, another RCT (COAG), performed in the US, showed negative results [Citation9], for which there were many different reasons [Citation8]. Since then, however, other trials have shown positive results [Citation10,Citation11]. The EU-PACT RCT [Citation7] also demonstrated that POCT-GGD was cost-effective when compared with usual clinical dosing [Citation12].
However, demonstration in a RCT does not necessarily mean that it will work in real-world clinical practice. For this reason, we undertook a project to implement POCT-GGD in routine clinical practice. This article describes the operational processes and highlights the challenges encountered in implementing a personalized medicine pathway in outpatient anticoagulation clinics in the NHS. The results of the implementation study have been published elsewhere [Citation13].
Project overview
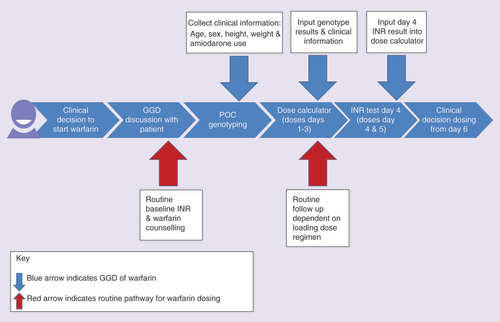
The aim of the project was to conduct a matched cohort study in order to evaluate whether POCT-GGD of warfarin was superior to standard dosing in ‘real world’ clinical practice. Six NHS anticoagulation clinics in the North West Coast of England agreed to participate. Three clinics (St Helens and Knowsley Teaching Hospitals NHS Trusts, Aintree University Hospital NHS Foundation Trust and Lancashire Teaching Hospitals NHS Trust, Preston, UK) acted as comparator sites and undertook routine practice and additional data collection for the evaluation. Three other clinics were implementation sites (The Royal Liverpool and Broadgreen University Hospitals NHS Trust, [lead site]; Countess of Chester Hospital NHS Foundation Trust; and Warrington and Halton Hospitals NHS Foundation Trust, UK) that undertook genetic testing on patients prior to starting warfarin treatment for atrial fibrillation or venous thromboembolism (the process is described in ).
When a decision is made to anticoagulate, patients are given a loading dose of warfarin, with subsequent INR testing utilized to modify doses according to the different types of dosing software available in different anticoagulant clinics. Genotype guided dosing involves the use of two algorithms, one at initiation (which takes into account clinical and genetic factors) and another on day 4 (which takes into account not only the clinical and genetic factors but also the INR). After day 5, the dosing follows the standard procedures utilized by the clinics.
GGD: Genotype-guided dosing; INR: International Normalized Ratio; POC: Point-of-care.
Two senior research nurses oversaw the implementation of POCT-GGD technology in the intervention sites. The project manager from the Academic Health Sciences Network coordinated the comparator sites. A number of obstacles and delays were encountered because structural support and defined process for innovation in clinical settings are lacking, despite innovation guidance [Citation14–19].
The Research Ethics Committee determined that the work was an implementation program of a proven technology and therefore an NHS service evaluation, which did not need ethical approval but needed approval by the sites in accordance with their local governance.
Technology development & testing
The project began in March 2016. The point-of-care genotyping technology (ParaDNA) used in the EU-PACT trial [Citation7], provided by Laboratory of the Government Chemist Limited (LGC Ltd, Teddington, UK), had been updated to provide genotype results for CYP2C9*2 (rs1799853), CYP2C9*3 (rs1057910) and VKORC1 -1639G→A (rs9923231) in approximately 45 min, and rather than a blood sample, a noninvasive buccal sample was taken from the patient. Further refinement of the technology included the software, the assay and the introduction of a platform that could run four concurrent samples. This meant that the technology (including the software and the assay) required field testing before use in a clinical setting. However, initial internal quality assurance tests returned a high proportion of failed calls. A multiplex permutation assay plate was then developed that enabled duplicate analyses of each sample. A rigorous testing schedule was undertaken until the reliability of the results reached acceptable clinical levels (>95% reliability).
This process took approximately 1 year, which was longer than anticipated. The dose calculator used in the EU-PACT trial was adapted for easy use by clinic staff and to facilitate data collection. We used the same algorithms demonstrated to be effective in the EU-PACT trial [Citation7], but the interface of the program was made more user-friendly for the clinical teams and the instructions were simplified. It was password protected and only members of the clinical team who required access were provided with login details.
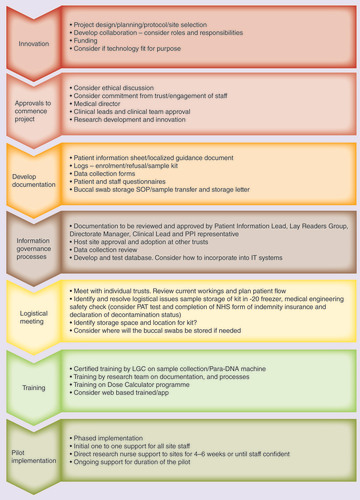
During this testing period and to sustain the momentum of the project, documentation was developed, and information governance processes identified and approvals sought at individual sites.
Governance to undertake the implementation project
The approval processes for an implementation project that changes practice with new technology were unclear and differed in each site. For the purpose of this pilot, approval from the trust medical director, clinical lead, clinical team and the research development and innovation department was sought. All three implementation NHS Trust sites informed the project team that this was their first implementation project in the personalized medicine area.
Several documents were specifically developed to support the innovation operational processes pathway (). The research nurse’s expertise played a pivotal role in the documentation design with support from the wider team. Questionnaires to capture patient and staff experience were designed with public advisor input and underwent review from the lead trust’s lay readers group.
These are used as an example of the processes we used in our implementation project in the UK NHS anticoagulation clinics. These processes would need to be modified for other healthcare systems.
NHS: National Health Service; PAT: Portable appliance testing; PPI: Patient and public involvement; SOP: Standard operating procedure.
The patient/staff facing documents (patient information form and patient and staff questionnaires) were reviewed by the information governance team in the hospitals.
There were no guidelines or template for content on type of patient information required for the project. Therefore, we undertook a consultation with patient advisors within National Institute for Health Research, Collaboration for Leadership in Applied Health Research and Care (CLAHRC), North West Coast; the lay readers group and the patient information forum at the lead site which ensured patient and public involvement. The directorate manager and lead nurse practitioner of the anticoagulation service were also consulted. The information governance team advised on the correct format and layout for document approval required by hospitals. Approval for both patient information documents was gained at the lead hospital site and were adopted and localized by the five other NHS hospital sites involved. LGC Ltd provided the standard operating procedure for the cleaning and decontamination of equipment.
In order to collect data on health-related quality of life, all patients completed the EQ-5D-5L at the enrollment date and at week 12. The EQ-5D-5L, which is a standard validated tool for measuring generic health status, was registered with EuroQol prior to use.
It was necessary to provide information governance teams with comprehensive information about the project, especially in relation to collection, transfer and storage of data from the sites, a process which was slowed by a range of factors including staff changes and a lack of clarity in how to deal with governance around this type of project, which led to conflicting advice being given. For example, we needed to emphasize that this was a pilot implementation project and not a research study; therefore, a nondisclosure agreement was not required as all data collected were unidentifiable. However, to ensure safe transfer, storage of the data and consistency of data, it was necessary for a bespoke template and database to be developed for data collection. We used the OpenClinica database that housed the data for the project, which allowed all sites to upload data remotely.
Setting up the implementation sites
Logistics meeting
Discussions were held with all clinical teams at each implementation sites prior to going live. This enabled the project team to identify the clinic’s current routine processes, patient pathway and the changes that would be required to accommodate POCT-GGD, which were different in each site. It was important to establish if the clinics could accommodate the new technology and the additional patient appointment that was needed for dosing on day 4 (). There were differences in the operational processes of the individual clinical teams which had to be accommodated for the project.
Training
Our aim in conjunction with LGC Ltd was to provide training for all the staff in relation to the new technology and the processes used for sample collection. Further training by the Liverpool research team included documentation, utilizing the dose calculator program, data entry in the OpenClinica database and changes to the patient pathway. The number and roles of staff involved in the clinics varied between the sites, and included administrative assistants, healthcare assistants, anticoagulation nurses and clinical leads. Therefore, training had to be personalized based on local requirements, depending on the site and staff involved.
The implementation sites were trained individually with at least two training sessions to accommodate all staff. The innovation was rolled out one site at a time in order to ensure that research nurses were available to fully support the clinical teams. The research nurses were present at each site as the project went live and worked with members of staff on a one-to-one basis to ensure competency and confidence. This ensured continuous support for the first 4–6 weeks, after which time, the nurses withdrew but maintained regular contact.
The dose calculator program had to be redesigned to be fit-for-purpose for the implementation. It was then retested and revalidated and guidance developed for the clinical staff to use. The data manager and research nurses trained the sites on how to log in and input the genotype information from the ParaDNA machine into the dose calculator to generate a genotype guided dose of warfarin for the first 5 days.
A site file was prepared for all sites which contained all the necessary documentation localized to their own NHS Hospital Trust. In addition, the research nurses developed a localized guidance document for each implementation site to incorporate local processes. LGC Ltd developed a laminated guidance flow chart on how to obtain sample collection, which was displayed in the clinical rooms to guide staff.
Medical engineering
Each implementation site also needed to liaise with the medical engineering department. The ParaDNA Machine required a portable appliance test (PAT; an examination of electrical appliances and equipment to ensure they are safe to use), completion of NHS form of indemnity insurance document and a declaration of decontamination status, which needed to be signed by LGC Ltd and the medical engineering department. The research nurses were able to share the knowledge gained from the lead site with the other implementation sites and guide them through the processes needed.
Reaction kit storage/buccal sample storage
The project required buccal samples to be taken from patients, analyzed and subsequently stored. Reaction kits developed by LGC Ltd needed to be stored at -20°C in a fridge freezer. However, none of the anticoagulation clinics had existing freezer storage available to store the reaction trays. The storage of the kit at the lead site took from May 2015 until February 2016 to organize. Numerous solutions were discussed, and freezer space was secured in a research laboratory. An agreement letter was drawn up to confirm this and clarify the processes for freezer failure. The research nurses advised the clinical teams at the other sites to investigate available storage space, which was also secured in laboratories.
All buccal samples obtained were stored by the clinical teams and transferred to University of Liverpool for quality assurance measures and validation. Initially, it was unclear if a material transfer agreement was required because there were no processes to follow for an innovation. After discussions, a letter was devised and signed between both parties (the lead site and University of Liverpool) to confirm that a material transfer agreement was not needed. The other two implementation sites adopted this letter. The standard operating procedure for the storage and transfer of samples from the implementation sites to University of Liverpool was developed by one of the laboratory scientists.
Discussion
Balancing the need for effective anticoagulation with a reduction in the time needed to reach a therapeutic INR and without concomitant increases in adverse events is important, not only for patients but also for the healthcare systems in terms of economic costs [Citation20–22]. There are now other anticoagulants available (dabigatran, rivaroxaban, apixaban and edoxaban), which provides clinicians and patients with a choice, but there is still a place for warfarin in clinical practice [Citation23].
The fact that patients can differ significantly in their capacity to metabolize drugs is now recognized, yet implementation of this knowledge is limited [Citation5,Citation24]. Finkelstein et al. [Citation25] suggest that although the availability of genomic testing has grown, its clinical application is still in the early stages. Momentum for the implementation of personalized medicine in clinical practice is growing [Citation26]. Pharmacogenomics is certainly one area of personalized medicine which is already delivering in other areas such as cancer and drug safety [Citation27]. More recently, in the USA, there have been several case studies of implementation of pharmacogenetic testing into clinical practice focusing on individual genes, for example, CYP2C19 for antiplatelet therapy [Citation28,Citation29] and CYP2D6 genotyping for various drugs [Citation30], or establishing a pharmacogenetic service within healthcare settings [Citation31–33]. Experience within a Spanish health system of implementing a 180 SNP panel has also been reported [Citation34]. All of these papers highlight the challenges faced, and careful processes that need to be adopted which differ with the type and setting of the healthcare organization, the type of test being implemented and the processes required for interpretation.
In the UK NHS, the most successful example of implementation of pharmacogenetic testing has been with HLA-B*57:01 in the prevention of abacavir hypersensitivity [Citation35]. TPMT testing prior to azathioprine use has also been implemented but the uptake of testing has varied with specialty [Citation36]. The recent Accelerated Access Review [Citation15] has highlighted the need for innovation in the NHS. Therefore, the opportunity to translate the work from the EU-PACT RCT [Citation7] into clinical practice was timely.
Even though the project focuses on a specific area, in other words, implementation of genotype-guided warfarin dosing, it provides a number of valuable and generic insights into how pharmacogenetic innovation can be introduced into the UK NHS. There are similarities to the experience in the USA, most importantly, the need for careful planning, but of course, the processes for governance approvals and reimbursement vary according to the healthcare setting. It is also important to note that our study provides the first example, to the best of our knowledge, of the challenges in implementing a point-of-care genotyping pharmacogenetic test.
One of the significant barriers encountered in our project related to the NHS processes for innovation as opposed to research. It became evident that the processes required for implementation of new technology were unclear. Even though several policy reports [Citation14–19] have been published to encourage the adoption of innovation in the NHS, adoption can be challenging. The implementation of the project, which was driven by senior research nurses, thus had to be flexible and utilize their experiences (and transferable skills) within the NHS to identify the best pathways to ensure that all governance processes were satisfied. The adoption of innovation is not an ordered sequential process, but should be recognized as complex, iterative and organic [Citation37]. Technological implementation can involve the transformation of organizations and individuals and it always has unforeseen issues which may not be predicted in advance [Citation38]. Given that innovation and adoption processes are not yet fully developed, hospitals should use the expertise of research staff, including research nurses and other healthcare professional staff, in facilitating the adoption of innovation and negotiating some of the complexities we have highlighted.
Although the initial unexpected challenges with the technology resulted in a delay in starting the project, it enabled the project team to develop closer working relationships with LGC Ltd to ensure that the quality of the results obtained would be compatible with clinical practice. This is similar to the experience of O’Donnell et al. [Citation39] who also found that the biggest challenge was validating the performance of the pharmacogenetic testing platform to meet an agreed upon quality threshold for clinical return.
The reality of organizing and coordinating training sessions was difficult due to the pressures of the busy anticoagulation clinics, staffing issues and the workload of the clinical teams. These issues were resolved through collaborative working between the project team, industry partner LGC Ltd and the clinical teams. Training was tailored to fit each Trusts needs but standardized as much as possible for consistency across the Trusts. The clinical teams needed education not only about pharmacogenetics and the benefits of pharmacogenetic testing, but also on how to introduce and discuss genetic tests with patients and families. For innovation to be introduced in any healthcare system, including the NHS, it is important to ensure that staff are provided with the time and flexibility required for training in the adoption of a new technology. Indeed, it is accepted that the level of general knowledge of genomics among medical practitioners is one of the most pressing challenges preventing broad implementation of personalized medicine [Citation26]. If innovation of interventions such as personalized medicine is to become routine in the NHS, we need to assess the level of knowledge nurses and other professionals have and respond with appropriate support. Recognizing innovation as a professional development activity could support and facilitate the activity of frontline innovators [Citation15].
Implementation of technology requires a multidisciplinary team approach and the involvement of people with a range of skills potentially including clinical and nonclinical staff, service users, laboratory/pathology, industry, regulatory and research. An important question that arises from this innovation journey is whose role is it to introduce innovation into the clinical area? Over the last decade, many new organizations charged with improving innovation in the NHS have emerged. The landscape has been fragmented, cluttered and confusing, but the NHS has started developing processes to simplify this [Citation14], further emphasized in the NHS long-term plan [Citation1]. Despite the need to work collaboratively, more clearly defined roles at the outset of the project would have been beneficial. The Accelerated Access Review [Citation15] clearly sets out the role of Academic Health Science Networks in supporting the testing and diffusion of technologies in the NHS, which clearly articulates what is expected. However, it is also important to include research teams who developed the innovation as part of multidisciplinary teams in order to facilitate the process of adoption, not only for academic innovations but also for industry.
It is also important to remember that implementation can be a social as well as a technical process [Citation40] in that the research nurses were able to share operational experiences of the technology with the clinical teams which complimented the formal training delivered by LGC Ltd. This helped to build the confidence of the clinical teams in their techniques to obtain a genotype result and respond to their needs.
In this project, research nurses were diverted from their role of patient recruitment into research studies. Although there may be a possibility of diversifying the role of the research nurse and using their transferable skills [Citation41], it should not be at the expense of undertaking research. Given that the clinical staff involved in implementation may not have the time, resources or skills necessary to undertake implementation work the NHS should be considering developing implementation teams, with specific skills to facilitate widespread implementation and adoption of innovation and technology into clinical practice. This should also include developing the relevant expertise in implementation among research development & innovation departments, academic health science networks together with guidance relating to the necessary documentation and processes needed to facilitate projects.
The processes required to initiate, coordinate, problem solve and complete this pilot implementation project has provided a powerful learning opportunity. It has enabled the development of an operational pathway to show systematically the specific steps required for a pilot innovation (). Importantly, the process pathway is grounded in the real-world processes of the NHS, one of the biggest healthcare systems in the world.
Conclusion
It is accepted that patient care can be improved through the implementation of evidence-based innovations and the translation of research findings into clinical practice. Implementation requires processes which are different (), but complementary, to those of research [Citation42,Citation43]. In relation to improving the use of warfarin, which was the focus of this project, incorporation of genetic information has the potential to shorten the time to stable INR, increase the time within therapeutic INR range and reduce under-dosing and over-dosing during the initial treatment period [Citation2,Citation20]. The results of this implementation project were similar [Citation13] to our EU-PACT RCT [Citation7], highlighting the success of our approach. A limitation of our approach is that the results only apply to northern European populations, as the algorithm has not been validated in other ethnic groups. It is important that similar work is performed in other ethnic groups to ensure that we do not exacerbate health inequalities. Our experience of implementing this project has highlighted the challenges that abound in setting up innovation and the lack of processes to facilitate innovation. This is not to criticize the NHS Trusts involved in this project; indeed, they should be congratulated for their perseverance and patience in working with us to successfully deliver this project. It is clear that operational processes and guidance for implementing innovation into the NHS, and indeed in any healthcare system are complicated. Tackling the issues outlined in this report so that streamlined and transparent processes can be developed to enhance implementation will be crucial in facilitating the aims, objectives and ambition of the UK Accelerated Access Review [Citation15], the Industrial Life Science Strategy [Citation44] and the NHS long-term plan [Citation1]. This also applies to healthcare systems in other countries. New partnerships will be crucial in driving forward personalized medicine into healthcare [Citation18], and a participatory approach which puts patients at the center of the whole pathway is most likely to succeed.
Adapted from [Citation42].
![Figure 3. Some of the barriers which can exist in implementing an innovation into any healthcare system.Adapted from [Citation42].](/cms/asset/b8387046-8c26-41f0-9c7f-e4f07fb5dd87/ipgs_a_12348852_f0003.jpg)
Disclaimer
The views expressed are those of the author(s) and not necessarily those of the NHS, the individual NHS Trusts or the NIHR or the Department of Health and Social Care. Funding for the project was provided by the NIHR Collaboration for Leadership in Applied Health Research and Care, North West Coast, UK and the Innovation Agency (Academic Health Science Network for the North West Coast). Infrastructure support, inlcuding research nurse support, was provided by the Wolfson Centre for Personalised Medicine and the MRC Centre for Drug Safety Science.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- NHS England . The long term plan. (2019). https://www.longtermplan.nhs.uk/
- Johnson JA , CaudleKE , GongLet al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin. Pharmacol. Ther.102(3), 397–404 (2017).
- Budnitz DS , LovegroveMC , ShehabN , RichardsCL. Emergency hospitalisations for adverse drug events in older Americans. N. Engl. J. Med.365(21), 2002–2012 (2011).
- Pirmohamed M , JamesS , MeakinSet al. Adverse drug reactions as cause of admission to hospital, prospective analysis of 18 820 patients. BMJ329, 15–19 (2004).
- Shahabi P , DubeMP. Cardiovascular pharmacogenomics; state of current knowledge and implementation in practice. Int. J. Cardiol.184, 772–795 (2015).
- Bourgeois S , JorgensenA , ZhangEJet al. A multi-factorial analysis of response to warfarin in a UK prospective cohort. Genome Med.8(1), 2 (2016).
- Pirmohamed M , BurnsideG , ErikssonNet al. A randomized trial of genotype-guided dosing of warfarin. N. Engl. J. Med.369(24), 2294–2303 (2013).
- Pirmohamed M , KamaliF , DalyAK , WadeliusM. Oral anticoagulation: a critique of recent advances and controversies. Trends Pharmacol. Sci.36(3), 153–163 (2015).
- Kimmel SE , FrenchB , KasnerSEet al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N. Engl. J. Med.369(24), 2283–2293 (2013).
- Gage BF , BassAR , LinHet al. Effect of genotype-guided warfarin dosing on clinical events and anticoagulation control among patients undergoing hip or knee arthroplasty: the GIFT randomized clinical trial. JAMA318(12), 1115–1124 (2017).
- Syn NL , WongAL , LeeSCet al. Genotype-guided versus traditional clinical dosing of warfarin in patients of Asian ancestry: a randomized controlled trial. BMC Med.16(1), 104 (2018).
- Verhoef TI , RedekopWK , LangenskioldS et al. Cost–effectiveness of pharmacogenetic-guided dosing of warfarin in the United Kingdom and Sweden. Pharmacogenomics J.16(5), 478–484 (2016).
- Jorgensen AL , PrinceC , FitzgeraldGet al. Implementation of genotype-guided dosing of warfarin with point-of-care genetic testing in three UK clinics: a matched-cohort study. BMC Med.17, 76 (2019).
- Department of Health . Innovation, health and wealth: accelerating adoption and diffusion in the NHS. (2011). https://webarchive.nationalarchives.gov.uk/20130107070708/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_134597.pdf
- UK Department of Health . Accelerated access review: final report (2016). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/565072/AAR_final.pdf
- NHS England . Five year forward view (2014). www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf
- NHS England . NHS innovation into action: supporting delivery of the NHS five year forward view (2015). www.england.nhs.uk/wp-content/uploads/2015/10/nhs-inovation-into-action.pdf
- NHS England . Improving outcomes through personalised medicine (2016). www.england.nhs.uk/wp-content/uploads/2016/09/improving-outcomes-personalised-medicine.pdf
- Office for Life Sciences . A guide to navigating the innovation pathway in England (2016). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/525787/AAR_how_to_guides.pdf
- Burn J , PirmohamedM. Direct oral anticoagulants versus warfarin: is new always better than the old?Open Heart5(1), e000712 (2018).
- Forde D , O’ConnorMB , GilliganO. Potentially avoidable inpatient nights among warfarin receiving patients; an audit of a single university teaching hospital. BMC Res. Notes2, 41 (2009).
- Shah A , ShewaleA , HayesCJ , MartinBC. Cost–effectiveness of oral anticoagulants for ischemic stroke prophylaxis among nonvalvular atrial fibrillation patients. Stroke47(6), 1555–1561 (2016).
- Pirmohamed M . Warfarin: the end or the end of one size fits all therapy?J. Pers. Med.8(3), 1–9 (2018).
- Van Schaik RHN , IFCC Task Force on Pharmacogenetics. Clinical application of pharmacogenetics: where are we now?EJIFCC24(3), 105–112 (2013).
- Finkelstein J , FriedmanC , HripcsakG , CabreraM. Potential utility of precision medicine for older adults with polypharmacy: a case series study. Pharmgenomics Pers. Med.9, 31–45 (2016).
- Raby BA . Personalized medicine. UpToDate (2016). www.uptodate.com/contents/personalized-medicine
- Pirmohamed M . Personalized pharmacogenomics: predicting efficacy and adverse drug reactions. Annu. Rev. Genomics Hum. Genet.15, 349–370 (2014).
- Empey PE , StevensonJM , TutejaSet al. Multisite investigation of strategies for the implementation of CYP2C19 genotype-guided antiplatelet therapy. Clin. Pharm. Ther.104(4), 664–674 (2018).
- Cavallari LH , LeeCR , BeitelsheesALet al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc. Interv.11(2), 181–191 (2018).
- Cavallari LH , Van DriestSL , ProwsCAet al. Multi-site investigation of strategies for the clinical implementation of CYP2D6 genotyping to guide drug prescribing. Genet. Med. doi:10.1038/s41436-019-0484-3 (2019) (Epub ahead of print).
- Dunnenberger HM , BiszewskiM , BellGCet al. Implementation of a multidisciplinary pharmacogenomics clinic in a community health system. Am. J. Health Syst. Pharm.73(23), 1956–1966 (2016).
- Harada S , ZhouY , DuncanSet al. Precision medicine at the University of Alabama at Birmingham: laying the foundational processes through implementation of genotype-guided antiplatelet therapy. Clin. Pharmacol. Ther102(3), 493–501 (2017).
- Hicks JK , StoweD , WillnerMAet al. Implementation of clinical pharmacogenomics within a large health system: from electronic health record decision support to consultation services. Pharmacotherapy36(8), 940–948 (2016).
- Borobia AM , DapiaI , TongHYet al. Clinical implementation of pharmacogenetic testing in a hospital of the Spanish National Health System: strategy and experience over 3 years. Clin. Transl. Sci.11(2), 189–199 (2018).
- Hughes DA , VilarFJ , WardCC , AlfirevicA , ParkBK , PirmohamedM. Cost–effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics14(6), 335–342 (2004).
- Fargher EA , TrickerK , NewmanWet al. Current use of pharmacogenetic testing: a national survey of thiopurine methyltransferase testing prior to azathioprine prescription. J. Clin. Pharm. Ther.32(2), 187–195 (2007).
- Greenhalgh T , RobertG , MacFarlaneF , BateP , KyriakidouO. Diffusion of innovations in service organisations: systematic review and recommendations. Milbank Q.82(4), 581–629 (2004).
- Berg M . Implementing information systems in healthcare organisations: myths and challenges. Int. J. Med. Inform.64, 143–156 (2001).
- O’Donnell PH , DanaheyK , JacobsMet al. Adoption of a clinical pharmacogenomics implementation program during outpatient care – initial results of the University of Chicago “1,200 Patients Project”. Am. J. Med. Genet C Semin. Med. Genet.166C(1), 68–75 (2014).
- de Lusignan S , SingletonA , WellsS. Lessons from the implementation of a near patient anticoagulant monitoring service in primary care. Inform. Prim. Care12, 27–33 (2004).
- Hardicre J . An exploration of the role of the research nurse and its impact. Bri. J. Nurs.22(3), 168–169 (2013).
- Grol R , GrimshawJ. From best evidence to best practice: effective implementation of change in patients’ care. Lancet362(9391), 1225–1230 (2003).
- Pirmohamed M . Acceptance of biomarker-based tests for application in clinical practice: criteria and obstacles. Clin. Pharmacol. Ther.88(6), 862–866 (2010).
- Bell J . Life sciences industrial strategy: a report to the government from the life sciences sector (2017). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/650447/LifeSciencesIndustrialStrategy_acc2.pdf
