Abstract
Aim: Personalized medicine (PM) is revolutionizing biomedical and clinical research while improving the ways healthcare is delivered. The EU is at the forefront of science and innovation in this field, increasing collaborations worldwide. This paper aims to assess the status of recent collaborations between Europe and China in PM-related science, technology and funded research. Methods: We analyze scientific literature, patents and funding programs, respectively. Results: PM is a scientific and industrial priority in both geographical areas, but current levels of collaboration are suboptimal. To increase these levels, policy makers should promote cooperation between researchers, innovators, industries, regulators, funding agencies and healthcare systems, while providing a forum to exchange best practices, define common guidelines for PM implementation and promote public–private partnerships.
The term personalized medicine (PM) first appeared in published works in 1999 [Citation1], highlighting how the new technical advances in predicting health risks, tracking disease development and predicting response to therapy could enable tailored approaches to care. Over time, PM has served as a catch-all term that is often used synonymously with genomic medicine or precision medicine [Citation2]. In 2014, the Horizon 2020 Advisory Group defined PM as a medical model using characterization of individuals’ phenotypes and genotypes (e.g., molecular profiling, medical imaging and lifestyle data) to: tailor the right therapeutic strategy to the right person at the right time, determine their predisposition to disease and deliver timely and targeted prevention. This definition was adopted by the European Council Conclusion on Personalized Medicine for patients, which specified that ‘Personalized Medicine relates to the broader concept of patient-centered care, which takes into account that, in general, healthcare systems need to better respond to patient needs’ (2015/C 421/03).
The concept of PM has recently morphed into personalized healthcare, intended as, but not limited to, personalized, predictive, preventative, participatory and person-centered approaches that enable system transformation through the direct involvement of citizens, patients, policy makers, academia and industries for an effective and sustainable planning, implementation and delivery of PM services [Citation3].
Since the human genome was sequenced, the potential role of PM on patients and healthcare systems was clear. Despite the results that have been obtained through genome-based technologies, only a few of these have been adopted in clinical practice [Citation4].
With time, the reduction in cost and the increased throughput of DNA sequencing [Citation5] coupled with the growth of bioinformatics tools as artificial intelligence and machine learning [Citation6] greatly influenced the introduction of PM in clinical practice. These approaches enable the possibility to cross-link a wide amount of medical data to extrapolate hidden information.
The analysis of genetics, genomics and epigenetics, as well as deep clinical phenotyping and digital biomarkers, represent innovative tools to classify disease states (diagnostic tests) and to predict future clinical outcomes (prognostic tests). In future, periodic patients profiling based on innovative technologies will be routinely used in healthcare to shift toward a preventive and proactive approach [Citation4].
Although the main focus in PM is related to the role of omics, a significant role of social and environmental determinants of health has also been shown [Citation6]. On the other hand, there are currently more than 70,000 unique genetic testing products on the market with an average of ten new products added each day [Citation7].
Over the last two decades, Europe and China ranked among the global leaders in PM [Citation8].
The European Union coordinates research in all the Member States (MS) by developing and implementing Europe-wide programs to support research and innovation [Citation9], as illustrated by the 7th EU Research Framework Program (FP7, 2007–2013), Horizon 2020 (2014–2020) and the recently launched Horizon Europe (2021–2027).
In 1998, China started collaborating with the EU on science and technology by signing the EU–China Science and Technology Agreement [Citation10]. Since then, multiple science and technology agreements have been established between China and EU at the community, individual MS and private enterprise level. These agreements have been fundamental to promote research collaboration. Chinese researchers have been granted the possibility to be active participants in the EU Framework Programmes for Research and Innovation [Citation8].
In Horizon 2020 (https://ec.europa.eu/programmes/horizon2020/en/horizon-2020-whats-it-china) call for proposals, several topics have been included for targeted cooperation with China in the fields of food, agriculture and biotechnology, water, energy, IT technologies, nanotechnology, space and polar research. China is now one of the EU’s key international partners in science.
As of 2005, China is the second largest spender on research and development (R & D) globally (Organisation for Economic Co-operation and Development data in millions of USD$ – accessed in April 2021). The business sector funds 74% of all domestic R & D and performs 76% of all R & D. The Chinese government funds 22% of R & D, half of which is provided by the central government. Public research institutes perform 15% of all R & D, followed by universities (8%) [Citation11]. The 2016 announcement of the China Precision Medicine Initiative – a 15-year program worth $9.2 billion – has radically changed the healthcare regime in the country and aims to ensure China remains a driver in PM [Citation12].
China’s PM revolution is primarily focused on to genome sequencing approaches and cloud-based genomics. Other key areas are big data collection and analytics, IT infrastructures and mobile health. With big data in health and medicine being treated as a national priority, China is building national and regional health and big data centers in cities such as Fuzhou, Xiamen, Nanjiang and Changzhou. The growing attention of PM in China is confirmed by the $312 million investments in molecular diagnostics in the US between 2000 and 2017, making this segment the fourth largest between all Chinese biotech investments, most of which are related to diagnostic-testing services [Citation13].
As of 2016, the main policy forum on PM in Europe is the International Consortium on Personalized Medicine (ICPerMed), which provides guidelines and supports the communitarian policy frameworks [Citation14], as well as national and regional strategies in PM [Citation15]. In addition, a European action plan was developed toward the integration of big data into policy development, biomedical research and clinical practice for health and wellness management [Citation16]. It is clear that access to well-curated and high-quality health-related data [Citation17] will lead to improved medical diagnosis and treatments. On this aspect, the Health Research and Innovation Cloud was launched [Citation18] with the purpose of contributing to the development of a secure, flexible and decentralized digital health infrastructure.
Other PM-related initiatives of fundamental importance for the EU is the European Health Data Space [Citation19], which will start at the end of 2021 with the aim to harness data for better healthcare, better research and better policy making for the benefit of patients.
There are many challenges to overcome in the acquisition and analysis of large amounts of omics data. These challenges include the development of infrastructures capable to store and share biological samples and the related data (e.g., biobanks), as well as ethical and legal requirements (e.g., informed consent from patients) necessary for PM to become a reality in our healthcare systems. To overcome these challenges, it is crucial that countries collaborate on all aspects of PM [Citation20].
To date, there are multiple initiatives that aim to promote the collaboration between China and Europe. Recently, bioXclusters [Citation21] plus, an initiative dedicated to improve the internationalization of European personalized healthcare, signed a gateway agreement with Fenglin Group. This established that European companies receive a free consultancy package from Fenglin BioMedical Center to ease the European life-sciences small and medium enterprises (SMEs) entering into the Chinese market.
Moreover, government-promoted networks and initiatives have been launched throughout the years. These include:
In 2011, Germany’s Federal Ministry of Education and Research (BMBF) and the Chinese Ministry of Science and Technology (MoST) agreed to create a Sino-German Life Science Platform aiming at intensifying the cooperation between the two countries, as a tool for consolidating and developing existing collaborative activities in the life sciences sector [Citation22,Citation23];
UK–China Research and Innovation Partnership Fund that supports research and cooperation in areas including stem cells, health, food security and other topics [Citation24].
In this context, our work aims to outline the status of EU–China collaborations in the field of PM through the mapping of patents and publications and providing an overview of the main funding programs in PM. The results of our analysis will help identify strategic areas of interest for collaboration to further strengthen Europe–China cooperation and meet the global health challenges of the future.
Patent mapping: methodology
For the mapping, we first identified the definitions of PM commonly accepted by the scientific community, from which we extrapolated a set of keywords, functions, expressions and concepts of the overall scopes and characteristics of PM.
In particular, to identify PM-related patent documents, we adopted the functional approach developed by Bonaccorsi et al. [Citation25], which integrates schemes already in place, with a full scale functional classification. This is based on the main functions performed by a technology, and not on inventive solutions or potential applications. Such approaches reduce many limitations of traditional patent classification, considering the representation of the function’s generality and abstraction. In particular, it has been observed that sometimes relevant patents are published under Cooperative Patent Classification (CPC) or International Patent Classification (IPC) that are different from those of the starting patent application, and traditional queries are not usually able to detect them, either because they rely too much on the CPC/IPC patent classification, or because the keywords used are too domain dependent. Furthermore, the functional approach allowed us to classify patents in categories that are not defined in the IPC or CPC patent classification system. Moreover, for several reasons, a concept could be expressed in patents using periphrastic constructions instead of PM-related terms. Traditional semantic approaches could represent a hurdle in a very complex and multidisciplinary sector like PM. To overcome this problem, we applied the above-mentioned functional approach devised to also enrich and refine the definition iteratively while improving the recall of the patent set. The analysis has been carried out using ErreQuadro S.r.l.’s proprietary tools on documents from their proprietary database, based on the data provided by the Worldwide Patent Statistical Database (Patstat) service by the European Patent Office.
Definition of precision medicine as a comprehensive concept
PM often serves as a catch-all term [Citation26] and therefore, in order to develop a scientifically sound approach for our mapping, we adopted the following definitions:
Personalized medicine
A medical model using characterization of individuals’ phenotypes and genotypes (e.g., molecular profiling, medical imaging and lifestyle data) for tailoring the right therapeutic strategy for the right person at the right time, and/or to determine the predisposition to disease and/or to deliver timely and targeted prevention, which makes up the 4P medicine concept (European Council Conclusion 2015/C 421/03) [Citation27].
Precision medicine
Treatments targeted to the needs of individual patients on the basis of genetic, biomarker, phenotypic or psychosocial characteristics that distinguish a given patient from other patients with similar clinical presentations [Citation28,Citation29].
Focus on process and used data: precision medicine as ‘a model that integrates clinical and other data to stratify patients into novel subgroups’.
Personalized healthcare & precision public health
The application of clinical know-how, concepts of systems medicine and PM technologies to improve health and minimize disease [Citation30,Citation31]. Within the manuscript, we used PM to refer to all the above definition interchangeably. We then extrapolated the following keywords to be used within the searches: ‘personalized medicine, precision medicine, preventive medicine, predictive medicine, systems biology, systems medicine, stratified medicine, targeted therapy, tailored treatment/therapy, deep phenotyping, omics sciences, big data, machine learning techniques’.
Once the query was developed, all the patent documents coherent with the definitions and keywords deployed were retrieved and a first PM patent dataset was built. This dataset was further refined by eliminating the patent documents that, despite falling into the dataset, were clearly not coherent with the definitions and approaches of PM. Specifically, considering the wide range of results from the first non-refined dataset we applied filters, starting with the below assumptions to determine what type of research was sufficiently PM-related.
PM-related is:
Drugs, devices, methods for patient-tailored treatment/therapy;
Procedures using an individualized approach based on genetic evaluation and treatment;
Treatment strategies based on individual data of patient genotype, phenotype, lifestyle;
Methods for synthetizing or designing customized and patient-specific drugs;
Methods for predictive medicine (e.g., statistical method for predicting life expectation, therapy response based on patient genotype, phenotype, lifestyle).
Not PM-related is:
Customized prosthesis/implants;
Medical instrument designed for specific group of patients (e.g., obese patients);
Diagnostic, therapeutic and prophylactic approaches for a specific disease (and not for an individual patient);
Genetic analysis for general purposes.
Other fields excluded:
Veterinary;
Personalized nutrition plans.
Through the indications related to the geolocalization of the patent assignee, it was possible to divide the dataset into two: Europe (intended as patents of assignees located in EU) and China (intended as patents of assignees located in China).
Patent mapping: findings
Global patent set: statistical outcomes
The results from the first round of querying showed the following statistics for PM-related patents worldwide:
Approximately 73,000 patents;
12,000 patent families (this set was used for classification). Each family may include more than one patent in relation to the territories in which the title is extended;
94.5% of precision (or positive predictive value): intended as the fraction of relevant patents among the global patent set. This value should be high enough to grant the proper significance to statistical data retrieved from the set. Precision is evaluated by manually analyzing a sample subset of patents;
High level of recall: the fraction of relevant patents that were included in the set (total number of documents retrieved that are relevant/total number of relevant documents in the database).
Innovation Index (I.I.) of PM (defined as the ratio between the global number of patents and the number of bibliographic data [DOCDB {document database}] families of a patent set) = 6.1. I.I. provides qualitative information about patenting strategies. The higher the level of patents extended, the higher the value generated by those patents. To compare, the I.I. of other technological fields are as follows: the two-wheel vehicles I.I. = 2.3; machine learning I.I. = 2.7; wind turbines I.I. = 3.2. This indicates that, unlike other technology areas, in PM field there is a high tendency to extend the patent beyond the region where the invention was generated.
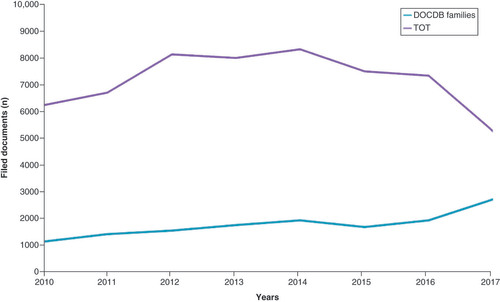
The mapping refers to the period of 2010–2019. It is important to note that the analysis was performed in the first months of 2020 and, due to the blind period, the patents filed in the previous 18 months (till 2017) are not fully available, therefore we have cut them out from the figure. We observed that the number of patent families (DOCDB families) showed a slow growth from 2010 (about 1000 documents) to 2016 (about 2000 documents) and then reached a peak in the year 2017 (about 3000 documents). As expected, the total number of patents is higher than the number of DOCDB families. clearly shows the high index of innovation; in fact, the number of documents (purple line) is significantly higher than the number of patent families (blue line) throughout the decade (2010–2019), net of the blind period (that was cut out of the figure). This confirms there is a high tendency to extend the patent even outside the state in which the research was produced. At single country level, there is a higher concentration of patent applications in the US, followed by Europe, Japan and China (data not shown).
DOCDB simple patent family: A simple patent family is a collection of patent documents that are considered to cover a single invention. The technical content covered by the applications is considered to be identical. Members of a simple patent family will all have the same priorities. The TOT line indicates the total number of filed documents.
DOCDB: Document database; TOT: Total.
The assignees of a patent are either private companies or public research institutes. The results also show that the patenting activity (which can be understood as a primary indicator of climate for innovation in PM) is mainly pursued by companies (∼60% of total patents applications), followed by universities (∼25%) and finally by other categories (i.e., not for profit, hospitals, government – ∼15%). The main assignees are Harvard University (US), Roche (CH), University of California (US), Inserm (FR), Genentech (US) and Novartis (CH) among others (data not shown).
EU–China comparison
After gathering an overview of the patenting activity at a global level, we filtered the results to focus on a detailed comparison between EU and China. As explained above, the EU/China attribution is based on the geolocation of the patent family assignee.
For this purpose, as a first step we defined EU countries as follows:
European Union MS;
European Free Trade Association;
European Economic Area;
Canada;
Israel.
We decided to include third countries such as Canada and Israel in consideration of the important ties and the already established collaboration within the European PM-related initiatives.
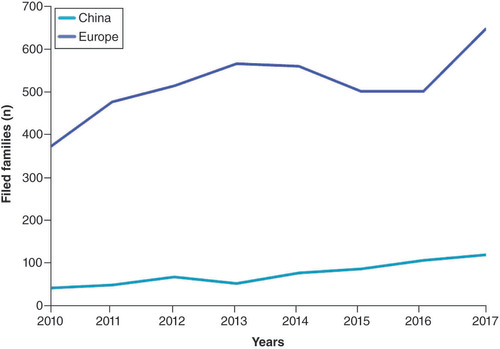
First, we compared the patent activity for the time periods selected for the mapping. Again, the trend for 2018 and 2019 is not reliable due to the blind period, and therefore, these data were cut out from the figure and analysis. clearly demonstrates that PM patenting activity is more active in Europe (purple line) than in China (blue line), with peaks of number of filed families of approximately 650 and 100, respectively, in 2017.
Both in EU and China patent filing on PM is widely done by private companies although, universities play a greater role in China than in Europe. In this regard, it should be noted, unlike Europe, in China many companies are state-owned enterprises, such as the Beijing Genomic Institute Group (BGI Group), formerly known as the Beijing Genomics Institute, a public Chinese genome sequencing company formed in 1999 to participate in the Human Genome Project.
The key players in EU are Roche (Switzerland), Inserm (France), Novartis (Switzerland), Philips (The Netherlands), CNRS (France), Immatics Biotech (Germany) (Supplementary Table 1), while in China we have University of Hong Kong (Hong Kong), Fudan University (Shanghai), BGI Shenzhen Company (Beijing), Chinese Academy of Sciences (Beijing) and Sun Yat Sen University (Guangzhou) (Supplementary Table 2).
In an attempt for a more detailed overview on patenting activity in the EU, we highlighted the countries where the headquarters of the assignees are based. We found that the headquarters are mostly located in Germany (19.5%), followed by Switzerland (16.4%), France (13.1%), UK (12.0%), Canada (8.4%) and Israel (6.2%).
In regard to the patent activity of the five main assignees in China, the data showed interruptions through the last 10 years in inventive activity, whereas in Europe all owners have been active since 2010 and there are no interruptions. Moreover, there was almost no activity in 2009 and 2010 except for Sun Yat Sen University. In most cases, the greatest inventive activity is between 2013 and 2015. An analysis was performed to determine how many EU-owned patents have been extended in China and vice versa, as an indication of a market interest for PM solutions. We found that around 3.7% of EU-owned patents are filed in China and around 9.8% of Chinese-owned patents are filed in an EU country. This confirms the significant interest that Chinese patent owners have in the Western market. On the other hand, European countries have only recently started to enter the Asian market; this delay might be due to cultural barriers, to the challenges that foreign companies face when entering the Chinese market for the first time, and/or to the rapid growth and expansion of the Chinese economy.
Finally, in regard to the IPC classes, which provide hierarchical system of language-independent symbols for the classification of patents and utility models according to the different technical fields to which they belong, we observed that the main class related to patent families of European assignees is G01N, which covers process, product or methods developed to investigate or analyze materials by determining their chemical or physical properties, whereas for the Chinese assignees it is C12Q, which is related to measuring or testing processes involving enzymes, nucleic acids or microorganisms.
EU–China collaboration
After identifying the main players in PM in both EU and China, we proceeded with a deeper analysis to find patents derived from a cooperation between EU and China and to understand the level of collaboration on PM technologies between the two.
For this purpose, we assumed that cooperation on patents happen in cases of:
Coassignation
A patent family (or a patent) having at the same time at least one Chinese assignee and one EU assignee.
Collaboration
A patent (or set of patents) linked to a patent family having at least one Chinese assignee and one EU assignee.
From the application of these criteria, 26 EU–China coassigned patents and 140 EU–China collaboration patents were found. In both sets, the main EU country players were France, the UK and Switzerland. In Supplementary Tables 3 & 4, there is an overview on the main country and principal private/public assignees, respectively, for coassigned patents and collaboration patents.
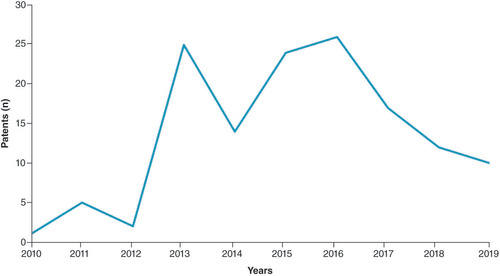
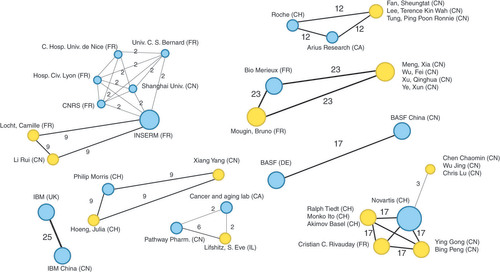
We were also able to track the level of collaboration through the years () and the cross-collaboration (), which uncovers the transversal collaboration between two entities cooperating on the same patents. As we can see, EU–China collaboration in PM has increased in the last 10 years with peaks in 2013, 2015–2016. However, the matrix shows that the highest level of collaboration is between subsidiaries of the same company, for example, IBM China and IBM UK (25 patents) and BASF China and BASF DE (17 patents).
It depicts the collaboration network established by European and Chinese assignees (nationality specified on every single label). Each assignee is represented by a circle – blue circle if the assignee is a company, yellow circle if the assignee is the inventor itself. The lines show interactions between assignees. The thicker the line is, the stronger such interactions are (collaborations/coassignations – number of patents shown above the line).
Traditional Chinese medicine
Traditional Chinese medicine (TCM) is an ancient system of health and wellness that has been used in China for thousands of years [Citation32]. Since TCM is very well established, not only in China but also across Europe, we decided to add TCM in our mapping to assess whether PM collaboration was also happening within this concept. Therefore, we included ‘procedures of TCM’ (defined as a branch of ‘alternative medicine’ in the Western world) as:
Acupuncture, cupping therapy, moxibustion, reflexology;
Traditional Chinese herbal medicine;
Traditional Chinese massage methods and instruments;
Procedures or instruments declared as TCM in the relevant patents.
A set of approximately 197,000 patents (∼175,000 families) were retrieved, with a level of precision of about 97%. The innovation index is approximately 1 as most patent families have only one application filed in the Chinese national patent office. Moreover, such patent applications are rarely ever granted. A peak of patents related to TCM were registered in the mid-2010s and were filed by Chinese companies/institutions in 97% of the cases (data not shown).
The top five assignees for TCM patents are universities or public research institutions and among them Guangxi University holds the highest number of patent families (n = 591). Moreover, we found 206 patents that met PM and TCM criteria. These data suggest that TCM, such as herbal medicine and acupuncture, presents some common ground with PM approaches to treat medical disorders.
Scientific literature mapping: methodology
The approach and process undertaken for scientific literature mapping followed the same logic of patent searches with some adaptations to the PubMed database. PubMed comprises more than 26 million citations for biomedical literature from MEDLINE, life science journals and online books. We started with the Medical Subject Headings (MeSH) definition of PM to build the query for retrieving relevant articles in the database. The database that has been chosen for the search is PubMed, since it is free and public.
The query strategy, in this case, has been based on a hybrid approach, merging two different sets:
Set (1) ‘precision medicine’ and ‘personalized medicine’ as MeSH terms (untagged terms that are entered in the PubMed search box are automatically mapped to the MeSH vocabulary when a match is found);
Set (2) a customized query (similar to the one elaborated for the patent database), containing the keywords highlighted above.
Such approach provides a higher level of recall to the set. This set also included non-English written articles if, among title, abstract or keywords, at least one is available in English. Regarding the time frame, we have included all the publications between 2010 and 2020. Nevertheless, as the analysis of the database has been conducted in the first quarter of 2020, the set of papers for 2019 and 2020 may not be complete. In order to have a more precise and reliable dataset, we applied ‘human’ as a searching filter. As a result, we had a total of 41,535 scientific papers (and 200,835 affiliations) that fall under our umbrella of PM. The results that emerged from the query have been downloaded in an .xls format. More precisely, we downloaded the global paper set in a MEDLINE format and through EndNote – the commercial reference management software used to manage bibliographies and references when writing essays and articles we have been able to download it in an .xls format including information about authors, affiliation, year of publication, journal, volume and other relevant information.
The mapping was then divided into three main steps: global paper set analysis, EU–China compared statistics and EU–China collaboration statistics.
Global paper set analysis
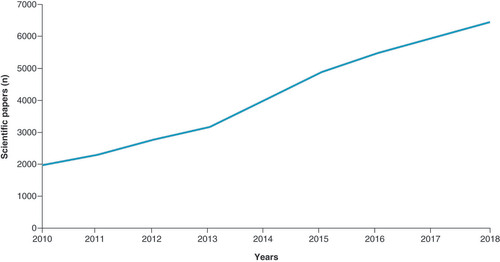
The quantitative analysis shows that from 2010 there has been a constant increase of publications related to PM, and in 2018 it reached the peak of about 7000 papers. The PubMed database is only periodically updated and therefore might be partially complete with 2019 and 2020. For this reason, we have excluded 2019 and 2020 from & .
We established that a scientific article is assigned to a country if at least an author is affiliated to an institution of said country. Following this approach, the global patent set has shown that the most active countries in PM are the US (16,108 papers), the UK (4576 papers), China (3307 papers), Italy (3019 papers), Germany (3001 papers), Canada (2274 papers), France (2268 papers) and The Netherlands (2216 papers) among others. Analyzing the same dataset by the number of entities (affiliations) in each country (in a paper, more entities from the same country can be present) active in publishing papers on PM, the US confirmed to be on top with 58,639 affiliations and a 29.2% of total papers published, followed by the UK (15,131 affiliations and 7.5% of total papers) and China (with 10,178 affiliations and 5.0% of total papers).
Regarding the journals publishing articles on PM, theJournal of Pharmacogenomics ranks at the top with 2179 published papers, followed byPLoS ONE with 825 papers, the Journal of Clinical Pharmacology & Therapeutics with 395 papers, the Scientific Report with 380 papers,Oncotarget with 314 and Nature with 283 papers between the others.
EU–China compared statistics
To perform a comparison on scientific publication between EU and China, we used the same group of EU countries of the patent search (Austria, Belgium, Canada, Cyprus, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Latvia, Lithuania, Liechtenstein, Luxembourg, Malta, The Netherlands, Norway, Poland, Portugal, Slovakia, Spain, Romania, Slovenia, Sweden, Switzerland, Turkey and the UK).
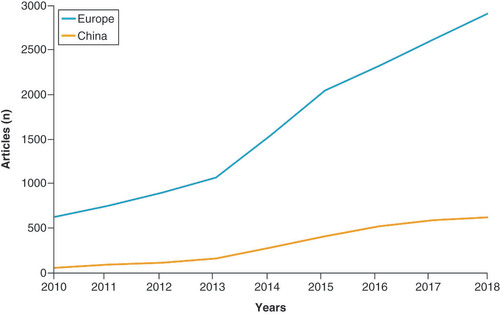
It emerged that the global set of articles contains around 41,535 articles, 16,823 of which have at least one European affiliation and 3307 at least one Chinese affiliation.
shows the trend of scientific publication related to PM in EU and China. The trend is constantly increasing, with an inflection point in 2013, 2015 and 2018, both for EU and China.
Looking deeper into the data of countries of affiliation, it emerged that the most active country in Europe is the UK (18.2% of total EU papers published), followed by Germany (12.18%), Italy (10.99%), France (10.31%), The Netherlands (7.58%) and Canada (7.62%) between the others. Most of the articles are published by Universities or Research Centers, both in EU and China.
By assessing the number of manuscripts published in the last 10 years by the top five European and Chinese players, we noted that, unlike patents, research activity has not been constant and has shown interruptions and resumes.
EU–China collaboration
To set the terms of collaboration for paper mapping, we established that a collaboration between EU and China happens when a scientific publication has at least one coauthor with a Chinese affiliation and at least one coauthor with a European affiliation. By applying this assumption to the global paper set, we found 434 total collaboration papers, representing 1.1% of the global papers set, the 13.5% of total Chinese papers and 2.6% of total EU papers. The EU countries publishing the most articles in collaboration with China (Supplementary Table 5) are the UK (220 papers), Germany (160), Canada (154), France (136), Italy (127) and The Netherlands (127). Moreover, as shown in Supplementary Figure 1, the collaboration started in 2013 and increased in a constant manner.
The main EU affiliation is the Department of Clinical Physiology and Nuclear Medicine of the Turku University Hospital (Finland) and for China is the Department of Clinical Pharmacology, Xiangya Hospital, Central south university (Changsha) that published, respectively, 14 and 115 coauthored papers.
Funding programs
At EU level, through the framework programs for research and innovation, several billion Euros have been invested in PM research [Citation33].
The comparison between EU and China has been finalized with the analysis of funding programs on PM in the two regions. For Europe, we liaised with the ICPerMed secretariat [Citation34], who granted us access to their database of European funding agencies financing PM. This provided us an extensive overview of the funding activities in the EU, with information on programs, funding organizations, countries and regions, as well as the number of funded projects and duration of the programs.
It emerged that Europe has invested on PM in a constant manner over the last years (2011–2018). In the past decade, it has, in fact, allocated more than 3 billion Euros to funding programs, for over 3000 projects and an average funding of 940,000 EUR per project.
The amount of funding has also been constant over the years, with no particular peaks or decreases over the analyzed decade. It also emerged that the most active organizations in funding in Europe are located in Germany (19 programs), Italy (19 programs), Austria (14 programs) and France (10 programs).
The analysis also showed that funding mostly goes to Universities, University Hospitals and Research Institutions. Very few organizations fund SMEs. Joint funding frameworks are being active not only between European countries but they are solid with extra-EU countries as well, like Canada, Israel and Turkey (which have been part of our mapping since they are member of ICPerMed). This means that the EU welcomes and fosters international collaboration.
For the Chinese funding programs mapping, we went through the Chinese National Innovation Funding Programs website [Citation35]. The website contains information on funding programs in China, where five funding pillars for science, technology and innovation, coordinated at national level, have been identified:
National Natural Science Fund: basic and applied research in natural sciences;
National S & T major projects: major key products, technologies and engineering of strategic importance for China’s economy and industrial competitiveness;
National Key R & D Programmes (NKPs): actively supporting well-defined and well-targeted R & D in areas of social welfare and people’s livelihood;
The Technology Innovation Guiding Fund(s): stimulating the transfer and commercialization of key results by investing in innovative start-ups and SMEs through venture capital funds, private equity and risk compensations;
The Bases and Talents Programme: aiming to establish top-notch innovation bases and to foster talents and teams with global competitiveness.
These five pillars of the Chinese National Innovation Funding Programmes have a unified management structure: priorities, strategy, directions and budgeting are coordinated by an interministerial joint council formed by 31 ministries and government agencies and are led by the MoST.
The growing interest of the Chinese in PM is also confirmed by the observation that around US$312 million have been invested in molecular diagnostics and PM in the US in the past two decades (2000–2017) by Chinese companies, making this the fourth largest segment among all Chinese biotech investments.
The Chinese NKPs, incorporate numerous previously existing programs, such as MoST’s ‘863 Programme’ for R & D, ‘Programme 973’ for basic research, Key Technologies R & D Programme and the International S & T Cooperation Programme, as well as National Development and Reform Commission and Ministry of Industry and Information Technology Industrial Technology R & D Fund.
NKPs support R & D with a focus on social welfare and people’s livelihood, like agriculture, energy and use of resources, health and environment. The programs invest on key-enabling technologies, featuring several well-targeted and defined objectives and deliverables to be achieved short–medium term (3–5 years) and reflecting a top-down industry–university–research cooperation design that integrates basic research, technology application, demonstration and commercialization. NKPs are currently among the most active and standardized national funding programs in China, with a total of 65 NKPs established to date, each of them funding numerous projects in different areas every year. Since their official launch, a total of 6.57 billion EUR has been allocated by the central government for 42 NKPs in 2016 and 2017. Around 1.25 billion EUR have been specifically dedicated to PM in 2016 and 2017, specifically, 642 million EUR in 2016 and 585 million EUR in 2017 [Citation35].
The legal framework of the NKPs encourages participation of international actors in the preparation and implementation of the projects, but in fact this is not very common. A 2016–2017 analysis showed that:
Only (0.9%) NKPs assigned were led by international entities;
Only two out of 2288 NKP assigned were led by a foreign principal investigators;
Very few foreign experts (from outside mainland China) were active in evaluation committees;
No evidence of foreign experts among the expert committees drafting tender guidelines;
No figures on international participation in wider project consortiums are available but is expected that these should be higher.
Conclusion
PM is increasingly fueling cross-sectorial and trans-national collaboration worldwide both at a scientific and technological level increasingly since the past decades. China and Europe are at the forefront of the PM rush, with increasing level of papers and patents produced yearly.
The outcomes of the mapping highlight that the interest in PM is a growing trend both in Europe and in China; they are among the top regions worldwide in terms of patents and publications dealing with PM, which suggests that PM is seen as a scientific and industrial priority in both areas. In particular, we observed that entities headquartered in Europe and China hold 14 and 6%, respectively, of the global patent set. Moreover, patent owners both in EU and China are private companies, with public entities playing a more relevant role in China than in Europe. Unlike Europe, in China many companies are state-owned enterprises, such as the BGI Group. Despite the respective efforts in PM, current levels of collaboration are not yet optimal, also in consideration of ethical (i.e., privacy), legislative and policy (i.e., intellectual property rights) differences that may slow down joint activities. The level of funding in both regions is increasing, although joint research and innovation projects are still at the stake.
Scientific collaborations account 448 Chinese–European coauthored papers with 6543 European and 1141 Chinese research teams, which have already worked together. The technological collaboration, on the other side, still lags behind with only 26 coassigned patents and 140 collaboration patents, the trend is decreasing between 2016 and 2019. Furthermore, in most cases, these patent documents refer to European and Chinese subsidiaries of the same company. Only two patents result from actual collaborations.
These findings need to become the cornerstone of further analysis to explore more in detail the reason of suboptimal collaboration levels, as well as further activities within the SINO-EU PerMed project to pave the way to the development of strategic actions. A PM approach will benefit both the patients by facilitating diagnosis, therapy and care, and on the other hand, the healthcare system by reducing, in the long run, costs associated with, for example, inappropriate diagnosis or ineffective therapy. However, PM will depend on the formation of appropriate organizational and regulatory infrastructure, and first of all, the development of algorithms able to predict and treat diseases based on a specific set of clinical, biohumoral, genetics and lifestyle data. To date, the PM programs collect mainly biological material, and of those that collected at least two types of data, only 42% referred to both electronic health records and biomaterial [Citation36]. A challenge for the future is integrating genetic data with other information, including lifestyle, environment, biohumoral data in a holistic approach able to improve the traditional symptom-driven practice of medicine, allowing earlier interventions using advanced diagnostics and tailoring better and economically personalized treatments.
In this framework, initiatives devoted to share data among research centers are a priority. In order to achieve this goal, the main issues that should be addressed are related to patient informed consensus, data confidentiality and intellectual property.
Great efforts have been made in China to promote the use of big data in PM, in particular, a centralized and integrated data platform is being developed, which will store biological samples and related metadata collected from research studies and will include at least 700,000 participants, 400,000 million from the general population and 300,000 million from patients carrying major noncommunicable diseases [Citation37]. Similar transnational initiatives should be developed addressing first of all common model for data harmonization.
To promote initiative between EU and China, another important step is to resolve linguistic differences between Chinese and English beyond the existing translation of the terms in order to use common key terminology, classification and coding standard. Confidentiality is an extremely important topic for big data use and unlike Europe, to the best of our knowledge, no law or regulation are currently present. Nevertheless, China published a draft law for personal data protection in the mid of 2020 and it will become effective during 2021. However at the current point, it is not sure what kind of impact it will have on EU–China cooperation.
It is clear that to bring the future medicine toward a personalized approach, an increased cooperation between multiple stakeholder is necessary as suggested by Horgan et al. [Citation38], the main actors involved are:
Policy makers that should promote the collaboration between different parties also by providing a forum to exchange best practice and defining common guidelines for data handling and sharing; reorganize healthcare organization and facilitate public–private partnership to create more value;
Researchers that should be prone to favor and support infrastructure and research project that could help the bench to bedside translation of PM technologies;
Patients that must be made aware of the results of the research in a way that ensures their data protection of the data they provide.
Future perspective
International collaborations help researchers to exchange information, data, best practices and materials from one country to another, with a benefit for the scientific community, healthcare and country productivity [Citation39]. The global pandemic itself suggests the importance of international collaboration and cooperation and highlights the need of solution that could facilitate the cross-border team working in order to overcome language, cultural and political barriers.
Coordination and cooperation between countries are needed to foster the introduction of PM approaches in the clinical management, which is a cross-disciplinary and strategic topic.
A rebalancing process between the research leadership in developed and developing countries has been observed and China is quickly emerging as a new global leader in pharmaceutical sciences [Citation40], partly due to the increase in R & D expenditure in China during the last two decades [Citation41] and the evaluation of research output in China is primarily based on quantitative measures, which has spurred Chinese scientists to publish more and more. Despite these quantitative results, the academic impact of Chinese research entities remains behind the Western institutions [Citation40]. In February 2020, the Chinese MoST and Ministry of Education announced the adoption of more scientific and influence-driven research evaluation approaches [Citation42]. This transformation facilitate international collaboration between China and EU and joint-funding schemes and academic staff exchanges could be used to foster collaboration [Citation40].
The Europe–China relationship in health research and innovation are of core importance to advance PM-related science, innovation and technology. Enhanced cooperation between the two regions will have a tremendous impact on the way we perform research, promote innovation and deliver healthcare services, and the gain for the citizen could be unprecedented. Nevertheless, cultural differences remain, reverberating on ethical, legislative and policy frameworks that often appear inhomogeneous or even conflictive, hampering higher levels of collaboration. In order to identify and tackle major bottlenecks of the current suboptimal level of cooperation, additional studies on ethical, legal and social implication differences should be promoted and supported.
Relevant stakeholders should be identified in Europe and China and their priorities should be mapped with the aim to develop a strategic plan, which will be stepwise implemented. The mapping we have performed, represents the groundwork for such actions. The Sino-EU PerMed project can position itself as the right network to promote a stakeholder engagement pathway between Europe and China, by facilitating relevant discussions about the main barriers that will be identified. The EU–China strategy should enable a process to formulate and submit recommendations to the European Commission, Chinese authorities, ICPerMed and the European MS specifying, for example, how PM including suitable TCM approaches can be a future area for a fruitful cooperation with China. On the basis of our mapping, crucial stakeholders will be identified and the cooperation with experts from Europe and China will be set up and intensified by the activities of the Sino-EU PerMed project and by supporting the communication of ICPerMed toward China.
Background
Personalized medicine (PM) is revolutionizing biomedical and clinical research while improving the ways healthcare is delivered. Science and technology are at the core of PM, driving the research forward and yielding new understandings and discoveries at an unprecedented pace.
One important indicator of this development is the number of scientific publications and patents related to PM, which, in the last decade, have flourished.
The EU is at the forefront of science and innovation in this field and is strengthening collaborations worldwide.
Scope
We aimed to assess the status of recent collaborations between EU and China in PM-related science, technology and funded research based on published papers, patents and funding programs.
We analyzed the trends of scientific publications and patent applications related to PM worldwide by systematically mapping relevant information accessible through the European Patent Office database and PubMed online database in order to identify the degree of mutual collaborations between the two regions.
We also mined an available database of funding programs dedicated to PM in Europe and surveyed selected Chinese stakeholders to assess the level of funding activities related to PM in China.
Methodology
We identified the definitions of PM commonly accepted by the scientific community, from which we extrapolated a set of keywords, functions, expressions and concepts of the overall scopes and characteristics of PM.
A query was designed based on this approach and was used for both patents and publication searches. In order to identify relevant documents within the databases, we used a functional approach that integrates existing schemes with a full-scale functional classification.
Since a traditional semantic approach could represent a hurdle in a very complex and multidisciplinary sector such as PM, we applied the above-mentioned functional approach to enrich and refine the definition iteratively.
The data sources that were used are worldwide patent databases (such as esp@cenet) and PubMed Center of the National Center for Biotechnology Information.
For the funding programs, for Europe, we accessed the ICPerMed database that collects information about funding agencies funding PM and is open to the public upon registration.
For China, we carried out a survey among identified stakeholders and coupled this with desk research activities as the response level was low.
Conclusion
PM is fueling cross-sectoral and transnational collaborations worldwide, at an increased rate over the last decade. China and Europe are at the forefront of this ‘PM rush,’ with increasing levels of publications and patents produced yearly in the area of PM.
China and Europe are among the top global regions in terms of patents and papers production on PM, which suggests that PM is considered a scientific and industrial priority in both regions.
Current levels of collaboration are not yet optimal, due in part to ethical (i.e., privacy), legislative and policy (i.e., intellectual property rights) frameworks. These differences may slow down joint activities. To shift the future of medicine toward a personalized approach, increased cooperation between relevant stakeholders is necessary.
Supplemental Figure 1
Download PNG Image (41.2 KB)Supplemental Table 1
Download MS Word (16.9 KB)Supplemental Table 2
Download MS Word (18.5 KB)Supplemental Table 3
Download MS Word (17.6 KB)Supplemental Table 4
Download MS Word (19.2 KB)Supplemental Table 5
Download MS Word (15.5 KB)Acknowledgments
The authors thank J Egar, Canadian Institute of Health Research – Institute of Genetics, for help editing the manuscript. The authors would also like to thank Y Xu (X Yong), Guangzhou Institutes of Biomedicine and Health (GIBH), for help editing the manuscript.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/pme-2021-0030
Financial & competing interests disclosure
The Coordination and Support Action Sino-EU PerMed has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 874556. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Jain KK . From molecular diagnostics to personalized medicine. Meet. Rep.2(4), 299–301 (2002).
- Simmons LA , DinanMA , RobinsonTJ , SnydermanR. Personalized medicine is more than genomic medicine: confusion over terminology impedes progress towards personalized healthcare. Per. Med.9(1), 85–91 (2012).
- Nardini C , OsmaniV , CormioPGet al. The evolution of personalised healthcare and the pivotal role of European Regions in its implementation. Futur. Med.18(3), 283–294 (2021).
- Ginsburg GS , PhillipsKA. Precision medicine: from science to value. Health Aff.37(5), 694–701 (2018).
- Sabatini LM , MatthewsC , PtakDet al. Genomic sequencing procedure microcosting analysis and health economic cost-impact analysis: a report of the Association for Molecular Pathology. J. Mol. Diagnostics18(3), 319–328 (2016).
- Seyhan AA , CariniC. Are innovation and new technologies in precision medicine paving a new era in patients centric care?J. Transl. Med.17(1), 1–28 (2019).
- BBC Research . DNA sequencing applications. In: DNA Sequencing: Emerging Technologies and Applications (2016). www.bccresearch.com/market-research/biotechnology/dna-sequencing-emerging-tech-applications-report-bio045f.html
- Cheng CM . On ‘EU-China – a strategic outlook’. Tamkang J. Int. Aff.22(4), 145–152 (2019).
- European Union . Consolidated version of the treaty on the functioning of the European Union. Off. J. Eur. Union326, 344 (2012).
- European Commission . Roadmap for EU-Brazil S & T cooperation. Eur. Comm.October, 1–23 (2018).
- Grimpe C . Erawatch country reports 2012: Denmark (2013). http://erawatch.jrc.ec.europa.eu/erawatch/export/sites/default/galleries/generic_files/file_0366.pdf
- Cyranoski D . China embraces precision therapy. Nature529(7584), 9–10 (2016).
- Kazmierczak M , RittersonR , GardnerD , RosenDH , HanemannT , CasagrandeR. China’s biotechnology development: the role of US and other foreign engagement (2019). www.uscc.gov/sites/default/files/Research/US-China%20Biotech%20Report.pdf
- Vicente AM , BallensiefenW , JönssonJI. How personalised medicine will transform healthcare by 2030: the ICPerMed vision. J. Transl. Med.18(1), 1–4 (2020).
- International Consortium for Personalised Medicine . The ICPerMed vision for 2030. 28 (2019). www.icpermed.eu/media/content/Vision_Paper_2019.pdf
- Auffray C , BallingR , BarrosoIet al. Making sense of big data in health research: towards an EU action plan. Genome Med.8(1), 1–13 (2016).
- D’Errico G , BelloP. KA1 report – Interregional coordination for a fast and deep uptake of personalised medicine – Regions4PerMed (2019). www.regions4permed.eu/wp-content/uploads/2020/07/KA1_Best-Practices.pdf
- Aarestrup FM , AlbeyattiA , ArmitageWJet al. Towards a European health research and innovation cloud (HRIC). Genome Med.12(1), 1–14 (2020).
- European Commission . DG health and food safety. Assessment of the EU Member States’ Rules on Health Data in the Light of GDPR (2019). https://ec.europa.eu/health/sites/default/files/ehealth/docs/ms_rules_health-data_en.pdf
- Germain DP , FouilhouxA , DecramerSet al. Consensus recommendations for diagnosis, management and treatment of Fabry disease in paediatric patients. Clin. Genet.96(2), 107–117 (2019).
- Danieles A . BioXclusters plus paves the way for precision medicine companies to China (2017). www.clustercollaboration.eu/news/bioxclusters-plus-paves-way-precision-medicine-companies-china-market
- Federal Ministry of Education and Research . China strategy 2015–2020 strategic framework for cooperation with China in research, science and education (2015). www.readkong.com/page/china-strategy-2015-2020-strategic-framework-for-8655422
- European Commission . Review of the science and technology cooperation between the European Community and the Government of the People’s Republic of China (2008). http://ec.europa.eu/research/iscp/pdf/china_eu_en.pdf#view=fit&pagemode=none
- Newton Fund . UK-China Research and Innovation Partnership Fund. www.newtonfund.ac.uk/about/about-partnering-Countries/China/
- Bonaccorsi A , FantoniG , ApredaR , GabelloniD. Functional patent classification. Springer Handb. Sci. Technol. Indic.983–1003 (2019).
- Burnette R , SimmonsLA , SnydermanR. Personalized health care as a pathway for the adoption of genomic medicine. J. Pers. Med.2(4), 232–240 (2012).
- Carney SL , HoodL. Leroy Hood expounds the principles, practice and future of systems biology. Drug Discov. Today8(10), 436–438 (2003).
- Jameson LJ , LongoDL. Precision medicine – personalized, problematic, and promising. N. Engl. J. Med.362(5), 567–571 (2011).
- Song CH , HanJW , JeongB , YoonJ. Mapping the patent landscape in the field of personalized medicine. J. Pharm. Innov.12(3), 238–248 (2017).
- Khoury MJ , IademarcoMF , RileyWT. Precision public health for the era of precision medicine. Am. J. Prev. Med.50(3), 398–401 (2016).
- Ricciardi W , StefaniaB. New challenges of public health: bringing the future of personalised healthcare into focus. Eur. J. Public Health27, 36–39 (2017).
- Lü S , WangQ , LiG , SunS , GuoY , KuangH. The treatment of rheumatoid arthritis using Chinese medicinal plants: from pharmacology to potential molecular mechanisms. J. Ethnopharmacol.176, 177–206 (2015).
- Nimmesgern E , NorstedtI , Draghia-AkliR. Enabling personalized medicine in Europe by the European Commission’s funding activities. Per. Med.14(4), 355–365 (2017).
- ICPerMed Database. www.icpermed.eu/app/login
- Chinese National Innovation Funding Programmes. http://chinainnovationfunding.eu/chinese-national-innovation-funding-programmes/
- Lee J , HamidehD , NebekerC. Qualifying and quantifying the precision medicine rhetoric. BMC Genomics20(1), 1–12 (2019).
- Zhang L , WangH , LiQ , ZhaoMH , ZhanQM. Big data and medical research in China. BMJ360, 1–3 (2018).
- Horgan D , BorischB , RicherEet al. Propelling health care into the twenties. Biomed. Hub.5(2), 1–53 (2020).
- Bikard M , MurrayF , GansJS. Exploring trade-offs in the organization of scientific work: collaboration and scientific reward. Manage. Sci.61(7), 1473–1495 (2015).
- He C , WuJ , ZhangQ. Characterizing research leadership on geographical weighted collaboration network. Scientometrics126, 4005–4037 (2021).
- Basu A , FolandP , HoldridgeG , SheltonRD. China’s rising leadership in science and technology: quantitative and qualitative indicators. Scientometrics117(1), 249–269 (2018).
- Babbar S , KoufterosX , BeharaRS , WongCWY. SCM research leadership: the ranked agents and their networks. Supply Chain Manag.24(6), 821–854 (2019).