Abstract
Aim: To evaluate methylnaltrexone for opioid-induced constipation in patients with and without cancer. Methods: This post hoc analysis comprises two Phase III, multicenter, double-blind, randomized studies of advanced-illness patients who received methylnaltrexone subcutaneous injection or placebo. Results: Significantly more patients treated with methylnaltrexone than placebo experienced laxation within 4 (cancer = 55.5 vs 15.5%; noncancer = 55.6 vs 12.8%) and 24 (cancer = 64.7 vs 29.8%; noncancer = 64.4 vs 30.8%) h after the first dose (p < 0.01 vs placebo). Regardless of cancer status, methylnaltrexone reduced median time to laxation and improved constipation relief without impacting opioid analgesia or withdrawal symptoms. Conclusion: Methylnaltrexone provided significant improvements in opioid-induced constipation over placebo in advanced-illness patients with and without cancer. Clinical trial registration numbers: study 301: NCT00401362; study 302: NCT00402038.
Approximately, 39–66% of patients with cancer experience pain, and in more than a third of these patients the pain is moderate to severe [Citation1]. Opioid medications have long been a cornerstone of cancer pain treatment [Citation2], despite the risks of adverse effects, addiction and development of tolerance associated with chronic use [Citation3]. Constipation is one of the most common side effects of opioid treatment, with an incidence up to 60% in patients treated for cancer-related pain [Citation4]. Surveys have shown that approximately one quarter to one half of patients have skipped, decreased or discontinued opioid use because of constipation, in essence accepting one discomfort in hopes of relieving another [Citation5–10].
The motivation of patients to sacrifice analgesia in pursuit of relief from opioid-induced constipation (OIC) can stem from limited success with over-the-counter constipation remedies [Citation8]. Prescription-strength laxatives also reduce OIC only slightly better than placebo [Citation11]. The modest efficacy of these treatments in OIC likely stems from their failure to address the activation of intestinal μ-opioid receptors that play a primary role in OIC [Citation12,Citation13]. Blocking activation of these receptors peripherally without reversing the action of opioids on CNS μ-receptors that produce analgesia is an essential strategy of OIC treatment [Citation12,Citation13].
Although OIC is common in patients receiving opioids for cancer-related pain, there are several other factors that may contribute to constipation in this population. Chemotherapy (vinca alkaloids, alkylating agents, thalidomide, taxanes), gastrointestinal (GI) obstructions including postoperative adhesions related to abdominal surgeries, autonomic dysfunction, nutritional/dietary deficiencies, fluid restriction, concomitant noncancer treatments (diuretics, antacids, calcium channel blockers, antihypertensive agents, general anesthesia), and immobility are all factors that are often more prevalent in cancer patients and may contribute to constipation [Citation14–16].
Methylnaltrexone (Relistor®, Salix Pharmaceuticals, a division of Bausch Health US, LLC, NJ, USA) is a selective, peripherally acting μ-opioid receptor antagonist that improves GI motility and transit time without affecting μ-opioid receptor-mediated analgesia [Citation17–20]. The oral and subcutaneous (SC) injectable formulations are indicated for patients with chronic pain related to prior cancer or its treatment who do not require frequent opioid dosage escalation. The injectable formulation is also indicated for the treatment of OIC in adults with advanced illness or pain caused by active cancer who require opioid dosage escalation for palliative care [Citation17].
Methylnaltrexone is the only peripherally acting μ-opioid receptor antagonist indicated for patients with cancer; however, published studies to date have only included patients with noncancer pain [Citation21–23] or advanced illnesses including but not limited to cancer [Citation24–26]. In previous studies evaluating SC methylnaltrexone in patients with advanced illnesses, methylnaltrexone demonstrated robust efficacy and was well tolerated in treating OIC without affecting central analgesia or precipitating opioid withdrawal [Citation24–26]. However, constipation experienced by cancer patients taking opioids may be attributable to several factors other than opioid use; therefore, evaluation of the efficacy and safety of methylnaltrexone in this population is warranted. This post hoc analysis compared the effects of methylnaltrexone among advanced illness patients with and without active cancer to determine if a diagnosis of cancer has an impact on the efficacy and safety of SC methylnaltrexone.
Methods
Study design
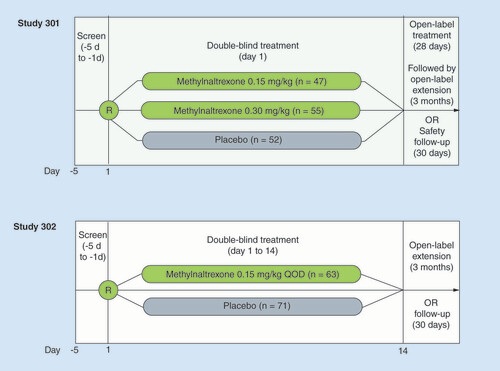
This post hoc analysis comprises two Phase III, multicenter, double-blind, randomized, placebo-controlled studies to assess the safety and efficacy of methylnaltrexone SC injection in patients with advanced medical illness and OIC (study 301: NCT00401362; study 302: NCT00402038). The trial designs have been described in detail elsewhere [Citation24,Citation26] and are summarized briefly here.
Participants
The patients were aged ≥18 years with advanced medical illness (e.g., terminal illness such as incurable cancer or end-stage acquired immunodeficiency syndrome) with a life expectancy of ≥1 month who were on a stable opioid regimen for at least 3 days before study drug administration. Eligible patients had a poor response to commonly administered laxatives. If a patient was currently taking a laxative, the regimen was to be stable for at least 3 days before study drug administration. In study 301, patients could not have had clinically significant laxation within 48 h prior to study drug administration. In study 302, patients had fewer than three bowel movements in the previous week and no clinically significant bowel movements in the previous 24 h before the first dose of study drug.
Patients were excluded if they had any disease process suggestive of GI obstruction; any potential nonopioid cause of bowel dysfunction based on the investigator’s opinion that may have been responsible for the constipation (e.g., medications that might interfere with GI motility, ischemic bowel, postsurgical adhesions, bowel loops protruding through hernias, rectocele, intussusception and tumor); or history of or current peritoneal catheter for intraperitoneal chemotherapy or dialysis, clinically significant active diverticular disease, evidence of fecal impaction by physical examination or x-ray, surgically acute abdomen or fecal ostomy.
Interventions
In study 301, following Institutional Review Board approval and after a 5-day screening period, patients were randomized 1:1:1 to receive a single SC injection of methylnaltrexone 0.15 mg/kg, methylnaltrexone 0.30 mg/kg or placebo (). In study 302, following Institutional Review Board approval and a 5-day screening period, patients were randomized to receive SC injections of methylnaltrexone 0.15 mg/kg or placebo every other day for 2 weeks (; dose escalation to 0.30 mg/kg was possible starting on day 9 at the discretion of the investigator). Patients in study 301 who completed the double-blind period were eligible for a 4-week, open-label, as-needed treatment period at an initial dosage of 0.15 mg/kg that could be subsequently decreased to 0.075 mg/kg or increased to 0.30 mg/kg at the discretion of the investigator. Patients who completed the open-label period in study 301 or the double-blind period in study 302 were eligible to enroll in 3-month, open-label extension studies.
Outcomes
Assessments in this post hoc analysis included laxation response within 4 and 24 h after the first dose of study drug, median time to rescue-free laxation assessed at 4 and 24 hours, and rating scales for constipation distress, pain and opioid withdrawal at 4 h after study drug administration. Constipation distress was assessed using a 5-point scale (1, none; 2, little bit; 3, somewhat; 4, quite a bit; 5, very much), and pain intensity was assessed using a scale from 0 (no pain) to 10 (worst possible pain). Opioid withdrawal was evaluated using a modified Himmelsbach Withdrawal Scale, in which patients rated opioid withdrawal symptoms (rhinorrhea, tremor, piloerection, yawning, perspiration, restlessness and lacrimation) on a 4-point scale (1, none; 2, mild; 3, moderate; 4, severe). Global Clinical Impression of Change (GCIC) ratings (patient and clinician) were assessed at 24 h (study 301) or 7 days (study 302), and the patient and investigator or staff member rated the overall change in bowel status since baseline using a 7-point scale (1, much worse; 2, somewhat worse; 3, slightly worse; 4, no change; 5, somewhat better; 6, slightly better; 7, much better).
Statistical analyses
Data from both studies were pooled, and patients were stratified by those with active cancer and those without active cancer. Efficacy analyses were performed on the intent-to-treat (ITT) analysis set, which was defined as all randomized patients who received at least one double-blind dose of study drug; the safety analysis set was the same as the ITT analysis set. Comparisons between the methylnaltrexone and placebo groups were analyzed using the χ2 test for the percentage of patients with laxation response and Fisher’s exact test for the percentage of patients with improvement from baseline in constipation distress scores. The mean changes from baseline in constipation distress and pain scores were summarized using descriptive statistics. Kaplan–Meier curves were generated to show the median time to first laxation within 4 and 24 h of study dose. The nominal level of significance was 0.05 with no adjustment for multiplicity.
Results
Participants
The pooled study populations (n = 287) consisted of 123 patients who were randomized to placebo, of whom 84 had cancer, and 164 patients who were randomized to methylnaltrexone, 119 of whom had cancer. Among the 109 methylnaltrexone-treated patients who received 0.15 mg/kg, 74 had cancer. Baseline patient characteristics stratified by presence of cancer are presented in . Although baseline opioid use was higher in patients with cancer versus patients without cancer, constipation distress affected each group similarly. Despite taking an average of at least two types of laxatives at baseline, patients in all groups had a high level of constipation distress before taking study medication. Baseline current and worst pain scores were also similar among all study groups.
Table 1. Baseline characteristics stratified by the presence of cancer in patients (pooled intent-to-treat population).
Outcomes
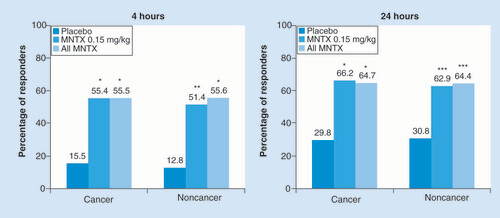
The presence or absence of cancer did not appear to have any effect on whether patients experienced laxation within 4 or 24 h after the first dose of study medication (). At 4 h the proportions of patients treated with methylnaltrexone experiencing a laxation response were three to four times greater compared with placebo, and at 24 h the response remained more than twice as high; all differences were statistically significant (p < 0.01).
Among patients with cancer, n = 84 for placebo, n = 74 for MNTX 0.15 mg/kg and n = 119 for all MNTX. Among patients without cancer, n = 39 for placebo, n = 35 for MNTX 0.15 mg/kg and n = 45 for all MNTX.
*p < 0.0001; **p < 0.001; ***p < 0.01 vs placebo.
MNTX: Methylnaltrexone.
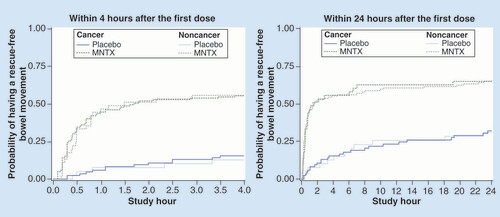
Improved and rapid laxation with methylnaltrexone after initial dosing was reflected in median times to first rescue-free laxation at 4 and 24 h that were shorter compared with placebo (). The median times to first rescue-free laxation ranged from 1.5 to 2.2 h in methylnaltrexone-treated patients with or without cancer, whereas in the placebo groups the median times were greater than 4 h at the 4-h assessment and greater than 24 h at the 24-h assessment.
Among patients with cancer, n = 84 for placebo, n = 74 for MNTX 0.15 mg/kg, and n = 119 for all MNTX. Among patients without cancer, n = 39 for placebo, n = 35 for MNTX 0.15 mg/kg and n = 45 for all MNTX.
MNTX: Methylnaltrexone.
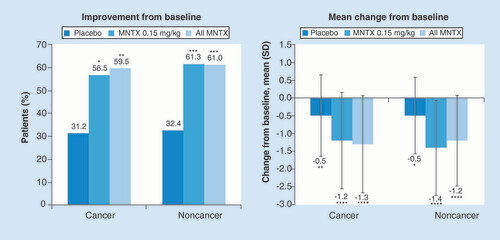
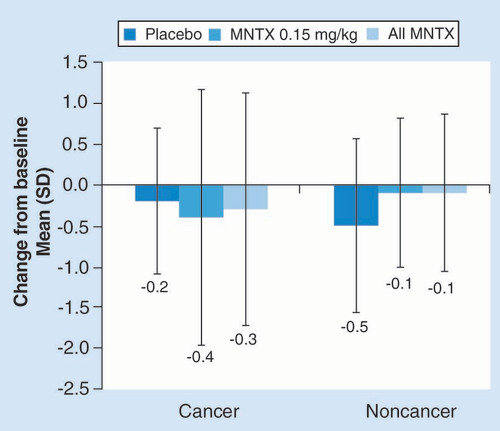
The proportions of patients experiencing improvements from baseline and the degrees of constipation distress reduction were also similar among patients who had cancer and those who did not have cancer (). Significantly more patients in the methylnaltrexone group than the placebo group reported improvements in constipation distress (p < 0.05), and the mean changes in constipation distress scores from baseline also were statistically significant in the methylnaltrexone groups (p < 0.0001) as well as the placebo groups (p < 0.01).
For the percentage of patients with improvement in constipation distress: among patients with cancer, n = 84 for placebo, n = 74 for MNTX 0.15 mg/kg and n = 119 for all MNTX; among patients without cancer, n = 39 for placebo, n = 35 for MNTX 0.15 mg/kg and n = 45 for all MNTX. For the mean change from baseline in constipation distress: among patients with cancer, n = 77 for placebo, n = 69 for MNTX 0.15 mg/kg and n = 111 for all MNTX; among patients without cancer, n = 37 for placebo, n = 31 for MNTX 0.15 mg/kg and n = 41 for all MNTX.
*p < 0.01; **p < 0.001; ***p < 0.05; ****p < 0.0001 vs placebo for percentage of patients with improvement and versus baseline for mean change from baseline.
MNTX: Methylnaltrexone.
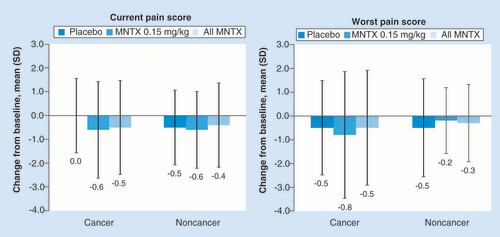
Treatment with methylnaltrexone had no apparent effect on opioid pain intensity in either patients with cancer or those without cancer, as there were no significant differences in current or worst pain scores with active treatment compared with placebo (). Opioid withdrawal symptoms were no more evident in patients receiving methylnaltrexone regardless of cancer status, than in those receiving placebo ().
For the analysis of current pain: among patients with cancer, n = 76 for placebo; n = 71 for MNTX 0.15 mg/kg; and n = 114 for all MNTX; among patients without cancer, n = 38 for placebo; n = 31 for MNTX 0.15 mg/kg; and n = 41 for all MNTX. For the analysis of worst pain: among patients with cancer, n = 76 for placebo, n = 69 for MNTX 0.15 mg/kg and n = 112 for all MNTX; among patients without cancer, n = 38 for placebo, n = 30 for MNTX 0.15 mg/kg and n = 40 for all MNTX.
MNTX: Methylnaltrexone.
Among patients with cancer, n = 75 for placebo, n = 71 for MNTX 0.15 mg/kg and n = 114 for all MNTX. Among patients without cancer, n = 38 for placebo, n = 32 for MNTX 0.15 mg/kg and n = 42 for all MNTX.
MNTX: Methylnaltrexone.
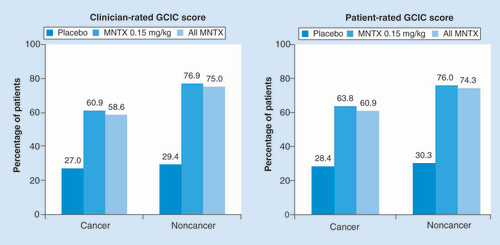
A larger proportion of methylnaltrexone-treated patients without cancer experienced clinician- and patient-rated global improvement in OIC, and patients receiving active treatment were more likely than those receiving placebo to have experienced global improvement (). There was consistent agreement between clinician and patient GCIC scores for each group.
Percentages based on number of patients with nonmissing measures: on the clinician GCIC among patients with cancer, n = 74 for placebo, n = 69 for MNTX 0.15 mg/kg and n = 111 for all MNTX; among patients without cancer, n = 34 for placebo, n = 26 for MNTX 0.15 mg/kg and n = 36 for all MNTX. On the patient GCIC among patients with cancer, n = 74 for placebo, n = 69 for MNTX 0.15 mg/kg and n = 110 for all MNTX; among patients without cancer, n = 33 for placebo, n = 25 for MNTX 0.15 mg/kg and n = 35 for all MNTX. Patient and Clinician GCIC ratings were recorded at 24 h and 7 days after administration of study drug in the 301 and 302 studies, respectively.
GCIC: Global Clinical Impression of Change; MNTX: Methylnaltrexone.
Cancer patients were more likely than noncancer patients to experience treatment-emergent adverse events (TEAE; ), and TEAEs were more common in methylnaltrexone-treated patients than in those receiving placebo. One of these events, malignant neoplasm progression, was virtually exclusive to cancer patients, who also were more likely to experience vomiting and increased sweating. Abdominal pain was the most frequent TEAE, with rates that were similar among all patients receiving placebo but higher for the cancer versus noncancer patients receiving methylnaltrexone. Flatulence and nausea were the next most common TEAEs, affecting methylnaltrexone-treated patients more frequently than placebo-treated patients and occurring at comparable rates in cancer patients and noncancer patients regardless of treatment.
Table 2. Treatment-emergent adverse events in >10% of cancer or noncancer patients in either treatment groupTable Footnote†.
The percentages of patients experiencing at least one serious TEAE also were higher among cancer versus non-cancer patients: 50.0 and 25.5%, respectively, in the methylnaltrexone group and 19.0 and 12.8%, respectively, in the placebo group. In cancer patients, the only serious TEAE occurring in more than 5% of patients was malignant neoplasm progression, which was reported in 39.5% of methylnaltrexone-treated patients and 14.3% of those receiving placebo. All serious TEAEs were relatively uncommon in noncancer patients, with only congestive cardiac failure aggravated and concomitant disease progression occurring in at least 5% of patients: both occurred in 5.5% of patients receiving methylnaltrexone versus 2.6% of placebo patients.
Discussion
In this post hoc analysis of two Phase III, double-blind, randomized, placebo-controlled trials of methylnaltrexone SC injections for the treatment of OIC in advanced illness patients stratified by those with and without cancer, methylnaltrexone effectively promoted laxation within 4 h of dosing and reduced time to rescue-free laxation in both cohorts relative to placebo. Although participants with cancer were receiving higher opioid doses at baseline than those without a cancer diagnosis, the improvements in symptoms of OIC they experienced after methylnaltrexone treatment were similar to those in patients without cancer. Furthermore, methylnaltrexone use enabled patients to continue their opioid treatment while experiencing a reduction in constipation distress with no signs or symptoms of opioid withdrawal and no increases in pain scores. These results show that methylnaltrexone effectively reduces OIC in cancer patients, despite several nonopioid-related factors that may contribute to constipation in this population, and support the use of SC methylnaltrexone in patients with active cancer taking opioids for cancer-related pain.
As demonstrated in this pooled analysis and other controlled trials, methylnaltrexone effectively blocks peripheral GI μ-opioid receptors yet does not cross the blood–brain barrier, preserving CNS μ-opioid receptor analgesia [Citation27,Citation28]. Higher opioid doses in patients with cancer in these studies did not appear to present greater competition for peripheral receptors, as evidenced by similar relief of OIC and its discomfort in methylnaltrexone-treated patients both with and without cancer. This may help reduce the inclination to modify opioid intake toward the goal of OIC mitigation, which has been associated with poorer health-related quality of life, greater pain-related resource use, and, ironically, worse constipation [Citation6].
The moderately higher current pain scores reported by patients without cancer may be explained by the higher median oral opioid equivalent doses reported in patients with cancer, particularly in view of similar worst pain scores in the two groups. Comparable relief of OIC following methylnaltrexone SC injection in both patient groups suggests that constipation risk can be reduced and may allow more aggressive opioid treatment of pain in appropriate patients having advanced noncancer illnesses.
The results of this analysis are in concert with findings observed in a randomized controlled trial evaluating the efficacy and safety of methylnaltrexone in patients with advanced illnesses experiencing OIC (of whom >66% of patients had a primary diagnosis of cancer) [Citation25]. Improvements in rescue-free bowel movements (RFBM; methylnaltrexone, 62.9 vs placebo, 9.6%; p < 0.0001) were observed relative to placebo, as well as significant improvements in secondary outcomes including first RFBM ≤4 h after initial dose, mean number of bowel movements ≤24 h after dosing, and the percentage of patients using rescue laxatives [Citation25]. Consistent with these results, a recent Cochrane Database systematic review of the effectiveness and safety of μ-opioid antagonists found moderate evidence of improved laxation and rescue-free laxation response following methylnaltrexone treatment, without increases in opioid withdrawal symptoms or serious adverse events [Citation29].
Interestingly, each cohort (cancer and noncancer patients) in the current study who received methylnaltrexone had a higher proportion of patients with laxation within 4 h relative to those receiving placebo. Likewise, the time to first rescue-free laxation was rapidly improved in both cancer and noncancer patients. Taken together, the rapid effects may potentially improve patients’ health-related quality of life and chronic pain management, a finding reflected in improved clinician and patient-related global improvements in GCIC scores. These findings support previous analyses of patient-reported outcomes that assessed methylnaltrexone for OIC. In a systematic review of randomized clinical trials of methylnaltrexone, patient-reported outcomes such as satisfaction with treatment after 4 h and after 7 days, Patient Assessment of Constipation – Quality-of-Life Questionnaire, and patient GCIC scales all improved relative to placebo [Citation30].
Cancer patients in this analysis experienced TEAEs at higher rates than noncancer patients, and events were more common in methylnaltrexone patients versus those receiving placebo. These differences were most apparent for malignant neoplasm progression and abdominal pain, which were each reported in approximately 40% of cancer patients receiving methylnaltrexone. Although progression of malignant neoplasm may be expected in a cohort of patients with advanced cancer, several studies have demonstrated that opioid use itself is associated with cancer progression [Citation31–34]. Higher μ-opioid receptor expression and increased opioid consumption were associated with significantly reduced progression-free survival and overall survival in patients with metastatic prostate cancer [Citation31]. Similarly, in a cohort of patients with newly diagnosed stage IV malignancies stratified by amount of opioid use, patients receiving high doses of opioids experienced significantly reduced overall survival rates compared with patients receiving low doses of opioids [Citation34]. Although cancer progression may be exacerbated by opioid use, several in vitro studies have suggested that this progression can be mitigated by treatment with μ-opioid receptor antagonists such as methylnaltrexone. In human pulmonary and dermal cells, clinically relevant doses of methylnaltrexone blocked angiogenesis and tumor progression caused by morphine use [Citation32]. In addition, methylnaltrexone treatment was shown to synergize with other cancer treatments (bevacizumab and 5-fluorouracil) in inhibiting proliferation and migration of a human epithelial cell line [Citation35]. These findings led to a post hoc analysis examining the effects of methylnaltrexone on patient survival, which yielded similar results. In a pooled, post hoc analysis of two randomized, placebo-controlled trials evaluating the effects of methylnaltrexone in advanced disease patients with OIC, methylnaltrexone-treated cancer patients experienced a significantly longer median overall survival time than those receiving placebo [Citation33]. Moreover, among methylnaltrexone-treated cancer patients, those who responded to methylnaltrexone (laxation within 4 h after the first administration) experienced a significantly longer median overall survival time than those who did not respond [Citation33]. No differences in overall survival were observed in patients without cancer treated with methylnaltrexone or placebo. Based on these collective findings, use of methylnaltrexone in a cohort of patients with cancer is not expected to adversely affect overall survival; however, further investigations and/or analyses are needed to confirm this hypothesis.
Cases of abdominal pain also may be expected in a cohort of patients with advanced illness and constipation who may be receiving concomitant treatments. AEs related to abdominal pain were predominately mild to moderate in severity, and the incidence of abdominal pain decreased with subsequent methylnaltrexone dosing [Citation36]. Interestingly, it was also found that among patients who reported abdominal pain, more patients (80%) who received methylnaltrexone reported an RFBM within 4 h of the first dose compared with placebo (33%), indicating that abdominal pain may be due to laxation [Citation36].
Limitations of this post hoc analysis include the fact that patients with and without cancer were not prespecified populations in these studies, which may have introduced greater heterogeneity to the pooled population. There was some degree of variability in the schedule of assessments between studies 301 and 302 that may have affected analyses of the pooled data. Patients in study 302 had a life expectancy of 1 month or more whereas study 301 inclusion criteria specified 1–6 months, which may have produced a more severely ill sample that skewed the pooled results. Furthermore, study 302 provided a somewhat broader OIC definition in the inclusion criteria, and this may affect the generalizability of the conclusions. Due to the nature of this post hoc analysis, no adjustments for multiplicity were performed. The p-values were nominal and for informational purposes only. Appropriate adjustments for multiplicity were performed for the primary endpoints for each individual, pivotal, Phase III study (study 301 and 302) that were pooled for this analysis.
Conclusion
This post hoc analysis suggests that patients with advanced cases of cancer or nonmalignant illness treated with methylnaltrexone SC injection may achieve similar relief of OIC. The effects appeared to be rapid regardless of opioid dose, affording patients continued pain management with reduced constipation distress. No signs of opioid withdrawal were observed. These results show that methylnaltrexone effectively reduces OIC in cancer patients, despite the several nonopioid-related factors that may contribute to constipation in this population, and support the use of SC methylnaltrexone in patients with active cancer taking opioids for cancer-related pain.
Opioid-induced constipation (OIC) is a common side effect of opioid treatment that often causes patients to change or discontinue their opioid dose.
Methylnaltrexone is approved for both the treatment of OIC in patients with a diagnosis of cancer who do not need frequent opioid dose escalation and for patients with active cancer receiving palliative care who require opioid dose escalation.
This post hoc analysis of pooled data from two pivotal studies showed that laxation within 4 or 24 h after the first dose was achieved among those receiving methylnaltrexone regardless of whether patients had cancer.
Patients who used methylnaltrexone, regardless of a cancer diagnosis, reported significant improvements in constipation distress without any impact on pain intensity, suggesting that the central analgesic effects of the opioid are not compromised.
Adverse events were often gastrointestinal in nature and may have been an artifact of laxation.
Use of subcutaneous methylnaltrexone was efficacious for the treatment of OIC in patients with cancer, despite the several nonopioid-related factors that may contribute to constipation in this population.
This study supports the use of subcutaneous methylnaltrexone in patients with active cancer taking opioids for cancer-related pain.
Data sharing statement
The authors certify that this manuscript reports the secondary analysis of clinical trial data that have been shared with them, and that the use of this shared data is in accordance with the terms (if any) agreed upon their receipt. The source of this data is: Study 301: NCT00401362; Study 302: NCT00402038.
The datasets generated and/or analyzed during the current study are not publicly available at this time due to the proprietary nature of this information. Requests for additional information should be made to the corresponding author.
Financial & competing interests disclosure
This study was funded by Salix Pharmaceuticals, a division of Bausch Health US, LLC, Bridgewater, NJ, USA, which has licensed the rights to develop and commercialize Relistor® from Progenics Pharmaceuticals, Inc., New York, NY, USA. BH Chamberlain received funding from Wyeth Pharmaceuticals for the methylnaltrexone study 302, and M Rhiner and NE Slatkin received funding from Wyeth Pharmaceuticals for the methylnaltrexone studies 301 and 302. NE Slatkin has been employed by Salix Pharmaceuticals since July 2016; prior to that time, he worked on behalf of Salix as an unpaid consultant; and through February 2016 he served on the Salix speakers panel. N Stambler is a full-time employee and shareholder of Progenics Pharmaceuticals. RJ Israel is an employee of Bausch Health US, LLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Technical editorial and medical writing assistance were provided under the direction of the authors by DA Franznick, Echelon Brand Communications, LLC, an OPEN Health company, Parsippany, NJ, USA. Funding for this support was provided by Salix Pharmaceuticals.
Additional information
Funding
References
- Van Den Beuken-Van Everdingen MH , HochstenbachLM , JoostenEA , Tjan-HeijnenVC , JanssenDJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J. Pain Symptom Manage.51(6), 1070–1090.9 (2016).
- WHO. Cancer Pain Relief: With a Guide to Opioid Availability (2nd Edition). 1–63, World Health Organization, Geneva, Switzerland (1996).
- Kumar L , BarkerC , EmmanuelA. Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol. Res. Pract.2014, 141737 (2014).
- Glare P , WalshD , SheehanD. The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care.23(3), 229–235 (2006).
- Bell TJ , PanchalSJ , MiaskowskiC , BolgeSC , MilanovaT , WilliamsonR. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European patient survey (PROBE 1). Pain Med.10(1), 35–42 (2009).
- Gupta S , PatelH , ScopelJ , ModyRR. Impact of constipation on opioid therapy management among long-term opioid users, based on a patient survey. J Opioid Manag11(4), 325–338 (2015).
- Noble M , TregearSJ , TreadwellJR , SchoellesK. Long-term opioid therapy for chronic noncancer pain: a systematic review and meta-analysis of efficacy and safety. J. Pain Symptom Manage.35(2), 214–228 (2008).
- Pappagallo M . Incidence, prevalence, and management of opioid bowel dysfunction. Am. J. Surg.182(Suppl. 5A), 11S–18S (2001).
- Cook SF , LanzaL , ZhouXet al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment. Pharmacol. Ther.27(12), 1224–1232 (2008).
- LoCasale RJ , DattoC , WilsonH , YeomansK , CoyneKS. The burden of opioid-induced constipation: discordance between patient and health care provider reports. J. Manag. Care Spec. Pharm.22(3), 236–245 (2016).
- Nee J , ZakariM , SugarmanMAet al. Efficacy of treatments for opioid-induced constipation: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol.16(10)1569–1584 (2018).
- Camilleri M , DrossmanDA , BeckerG , WebsterLR , DaviesAN , MaweGM. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol. Motil.26(10), 1386–1395 (2014).
- Wald A . Constipation: advances in diagnosis and treatment. JAMA315(2), 185–191 (2016).
- McQuade RM , StojanovskaV , AbaloR , BornsteinJC , NurgaliK. Chemotherapy-induced constipation and diarrhea: pathophysiology, current and emerging treatments. Front. Pharmacol.7, 414 (2016).
- National Cancer Institute . Gastrointestinal complications (PDQ®) – health professional version: constipation (2020). www.cancer.gov/about-cancer/treatment/side-effects/constipation/gi-complications-hp-pdq#link/_9_toc
- Wickham RJ . Managing constipation in adults with cancer. J. Adv. Pract. Oncol.8(2), 149–161 (2017).
- Salix Pharmaceuticals . Relistor®, package insert, NJ, USA (2018).
- Webster LR , BrennerDM , BarrettAC , PatersonC , BorteyE , ForbesWP. Analysis of opioid-mediated analgesia in phase III studies of methylnaltrexone for opioid-induced constipation in patients with chronic noncancer pain. J. Pain Res.8, 771–780 (2015).
- Yuan CS , FossJF , O’ConnorMet al. Effects of enteric-coated methylnaltrexone in preventing opioid-induced delay in oral-cecal transit time. Clin. Pharmacol. Ther.67(4), 398–404 (2000).
- Yuan CS , FossJF , O’ConnorM , ToledanoA , RoizenMF , MossJ. Methylnaltrexone prevents morphine-induced delay in oral-cecal transit time without affecting analgesia: a double-blind randomized placebo-controlled trial. Clin. Pharmacol. Ther.59(4), 469–475 (1996).
- Webster LR , MichnaE , KhanA , IsraelRJ , HarperJR. Long-term safety and efficacy of subcutaneous methylnaltrexone in patients with opioid-induced constipation and chronic noncancer pain: a Phase 3, open-label trial. Pain Med.18(8), 1496–1504 (2017).
- Rauck R , SlatkinNE , StamblerN , HarperJR , IsraelRJ. Randomized, double-blind trial of oral methylnaltrexone for the treatment of opioid-induced constipation in patients with chronic noncancer pain. Pain Pract.17(6), 820–828 (2017).
- Michna E , BlonskyER , SchulmanSet al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J. Pain12(5), 554–562 (2011).
- Thomas J , KarverS , CooneyGAet al. Methylnaltrexone for opioid-induced constipation in advanced illness. N. Engl. J. Med.328(22), 2332–2343 (2008).
- Bull J , WellmanCV , IsraelRJ , BarrettAC , PatersonC , ForbesWP. Fixed-dose subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: results of a randomized, placebo-controlled study and open-label extension. J. Palliat. Med.18(7), 593–600 (2015).
- Slatkin N , ThomasJ , LipmanAGet al. Methylnaltrexone for treatment of opioid-induced constipation in advanced illness patients. J. Support. Oncol.7(1), 39–46 (2009).
- Russell J , BassP , GoldbergLI , SchusterCR , MerzH. Antagonism of gut, but not central effects of morphine with quaternary narcotic antagonists. Eur. J. Pharmacol.78(3), 255–261 (1982).
- Yuan CS , FossJF. Methylnaltrexone: investigation of clinical applications. Drug Dev. Res.50(2), 133–141 (2000).
- Candy B , JonesL , VickerstaffV , LarkinPJ , StoneP. Mu-opioid antagonists for opioid-induced bowel dysfunction in people with cancer and people receiving palliative care. Cochrane Database Syst. Rev.6, CD006332 (2018).
- Siemens W , BeckerG. Methylnaltrexone for opioid-induced constipation: review and meta-analyses for objective plus subjective efficacy and safety outcomes. Ther. Clin. Risk Manag.12, 401–412 (2016).
- Zylla D , GourleyBL , VangDet al. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer119(23), 4103–4110 (2013).
- Singleton PA , MossJ , KarpDD , AtkinsJT , JankuF. The mu opioid receptor: a new target for cancer therapy?Cancer121(16), 2681–2688 (2015).
- Janku F , JohnsonLK , KarpDD , AtkinsJT , SingletonPA , MossJ. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann. Oncol.27(11), 2032–2038 (2016).
- Zylla D , SteeleG , ShapiroA , RichterS , GuptaP. Impact of opioid use on health care utilization and survival in patients with newly diagnosed stage IV malignancies. Support. Care Cancer26(7), 2259–2266 (2018).
- Singleton PA , GarciaJG , MossJ. Synergistic effects of methylnaltrexone with 5-fluorouracil and bevacizumab on inhibition of vascular endothelial growth factor-induced angiogenesis. Mol. Cancer Ther.7(6), 1669–1679 (2008).
- Slatkin NE , LynnR , SuC , WangW , IsraelRJ. Characterization of abdominal pain during methylnaltrexone treatment of opioid-induced constipation in advanced illness: a post hoc analysis of two clinical trials. J. Pain Symptom Manage.42(5), 754–760 (2011).