Abstract
Osteoarthritis (OA) is a progressive disease and OA pain intensity is related to ongoing pathophysiological changes. However, OA pain is complex and multimodal; its characteristics, including severity, localization and the stimuli that elicit it, can change as the disease progresses and differ greatly among patients. Understanding mechanisms underlying specific pain characteristics may help guide clinicians in choosing appropriate treatments, targeting treatments to those patients most likely to benefit. Associations have been demonstrated between biomarkers and some characteristics of OA pain, and to processes linked to the shift in pain characteristics over the course of OA. This article examines how understanding OA pain characteristics and their relation to the disease process could inform treatment choice when applying well-established treatment guidelines.
Lay abstract
Patients with osteoarthritis (OA) suffer from pain that is likely to increase in severity over the years. But how a patient experiences that pain – what causes it, where it hurts and whether they would describe it, for example, as aching or stabbing, throbbing or burning – will differ for each patient and can change as the disease progresses. We know that OA pain is difficult to treat, and many patients are unable to find medication that provides enough relief. Could the specific characteristics of a patient’s pain provide clues as to how to treat it? In this article, we describe connections that have been found between pain characteristics and various proteins and signaling molecules in patients with OA. Importantly, some of those same pain-related molecules are reduced in patients with OA treated with pain medications. More research will be needed to connect specific pain characteristics with potential treatments, but studies like these suggest that OA pain descriptors could one day provide useful information for doctors who are trying to choose from among various recommended medications for their patients with OA.
Recommended osteoarthritis (OA) pain medications may provide meaningful improvement in pain for some patients with OA but little to no effect for others, or response to a treatment may vary over time for a given patient.
Matching OA drugs to subsets of patients most likely to benefit could improve patient outcomes.
Characteristics of OA pain, including severity, localization and the stimuli that cause pain, differ among patients and can change as the disease progresses from early to late stages.
Several biomarkers have been associated with joint degradation or overall pain severity in patients with OA as well as with specific pain characteristics.
Nonsteroidal anti-inflammatory drugs used in the treatment of OA can alter levels of some of those same biomarkers in serum or synovial fluid from patients with OA.
Understanding mechanisms underlying specific pain characteristics in patients with OA may help guide clinicians in choosing appropriate treatments; biomarkers provide a potentially useful tool for exploring those connections.
Osteoarthritis (OA) is a progressive disease characterized by degeneration of synovial joints [Citation1], most commonly affecting the knees, hands, feet and spine and, relatively commonly, the shoulder and hip joints [Citation2]. It is among the leading causes of disability worldwide, particularly in older individuals [Citation3,Citation4]. Pain is a key symptom of OA, along with stiffness, swelling and impaired joint function [Citation5]. Patients can suffer pain for years or decades, with impacts across physical, emotional and quality-of-life domains [Citation5–7]. As OA cannot yet be cured, the treatment goals are to manage pain, improve quality of life, slow disease progression and delay total joint replacement [Citation8–10]. Critical outcomes for the treatment of OA are pain relief and improved function, whereas function is known to often decline with increasing pain severity [Citation10].
Characteristics of pain symptoms, including severity, localization and the stimuli that cause pain, differ among patients and can change as OA progresses from early to late stages (). The factors that underlie differences in pain symptoms may impact the effectiveness of a chosen treatment. Pain management might therefore be improved by matching treatment to pain symptoms, based on an understanding of the underlying pathophysiology [Citation11–13]. The aims of this article are to explore how OA pain can vary between patients and shift over the course of the disease, to examine relationships between biomarkers and OA pain characteristics and the effects of OA treatments on biomarker levels and to consider an approach to treatment based in part on the characteristics of the patient’s pain symptoms.
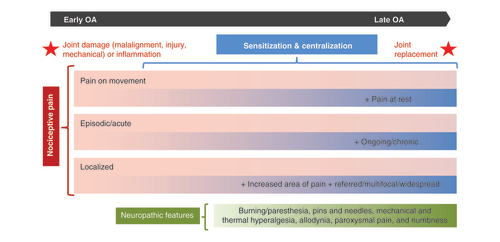
This schematic summarizes various pain characteristics that may or may not be present in an individual patient with osteoarthritis pain over the course of the disease. Pink/blue bars represent possible shifts in nociceptive pain that a patient could experience. Characteristics observed in early osteoarthritis (pain on movement, episodic pain, localized pain) continue in later stages as new characteristics arise. Sensitization and centralization processes underlie possible shifts in nociceptive pain characteristics. Pain with neuropathic features (green bar) may also be present.
Methods
For this narrative review, we conducted Medline and Cochrane database searches on pain symptoms in OA, biomarkers associated with OA symptoms and typical drugs used to treat OA pain. Terms for pain characteristics included inflammatory, nociceptive, neuropathic, nociplastic, acute, chronic, ‘pain at rest,’ ‘pain on activity’ and ‘pain on movement,’ hyperalgesia, peripheral, central, sensitization, progression and stage. Search terms for biomarkers included cytokine, chemokine, growth factor, inflammatory, immune, and biomarker, as well as specific biomarkers known to be related to pain (e.g., TNF-α, C-reactive protein [CRP], and interleukin [IL]). Drug search terms included nonsteroidal anti-inflammatory (NSAID), cyclooxygenase 2 (COX-2) inhibitor, specific NSAIDs and COX-2 inhibitors, acetaminophen (or paracetamol) and aspirin (or salicylate or rubefacient).
Articles that reported statistically significant differences in biomarker levels with radiographic progression or with pain severity (based on pain scale scores), or a significant association with specific pain characteristic were included; markers that differed in individuals with versus without OA or were related to OA symptoms other than pain were not included. Articles on analgesic effects were included if they reported a significant difference in biomarker level with OA treatment versus baseline or versus control. The pathophysiology of pain over the course of OA progression is outlined based on comprehensive reviews of the literature on peripheral and central pain mechanisms in OA [Citation11–22].
Hypothetical patients with OA used to illustrate the consideration of pain characteristics in treatment selection (Boxes 1 & 2) were developed based on articles characterizing pain experienced by patients with OA, and demographic and clinical characteristics were selected to reflect known risk factors for OA. Treatment options mentioned in Boxes 1 & 2 are based on published OA treatment guidelines [Citation1,Citation9,Citation10,Citation23].
History
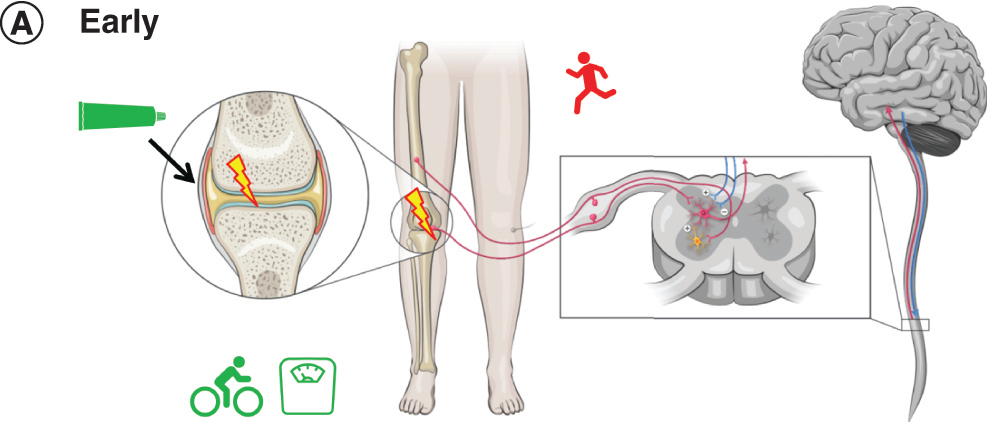
Patient A, a slightly overweight woman in her 40s with no concomitant diseases, sustained a traumatic injury to her knee in a fall from her bike in her 30s [Citation24]. Pain and bruising resolved after the injury but she continued to have stiffness in her knee after long periods of sitting or driving [Citation25,Citation26]. Several years ago, Patient A started experiencing recurrent episodic pain on exercising. As the pain would go away with some days of rest, she did not seek treatment at that time.
Figure 2A. Early OA: pain
Patient A now experiences moderate pain localized to the joint when standing and walking [Citation27]. She no longer runs, as that makes the pain flare up, and has gained additional weight. Radiographic examination indicates minimal OA (KL stage 2).
Treatment
Patient A uses a topical NSAID gel on an as-needed basis to alleviate nociceptive pain in the knee with little systemic exposure to the drug. The topical NSAID also reduces inflammation at the joint. Her clinician advises her to manage her weight and to consider a nonweight-bearing exercise like cycling.
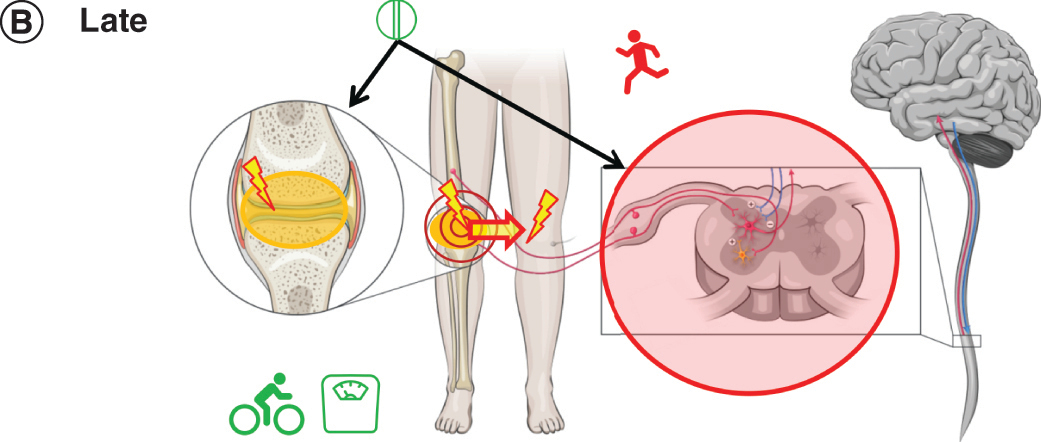
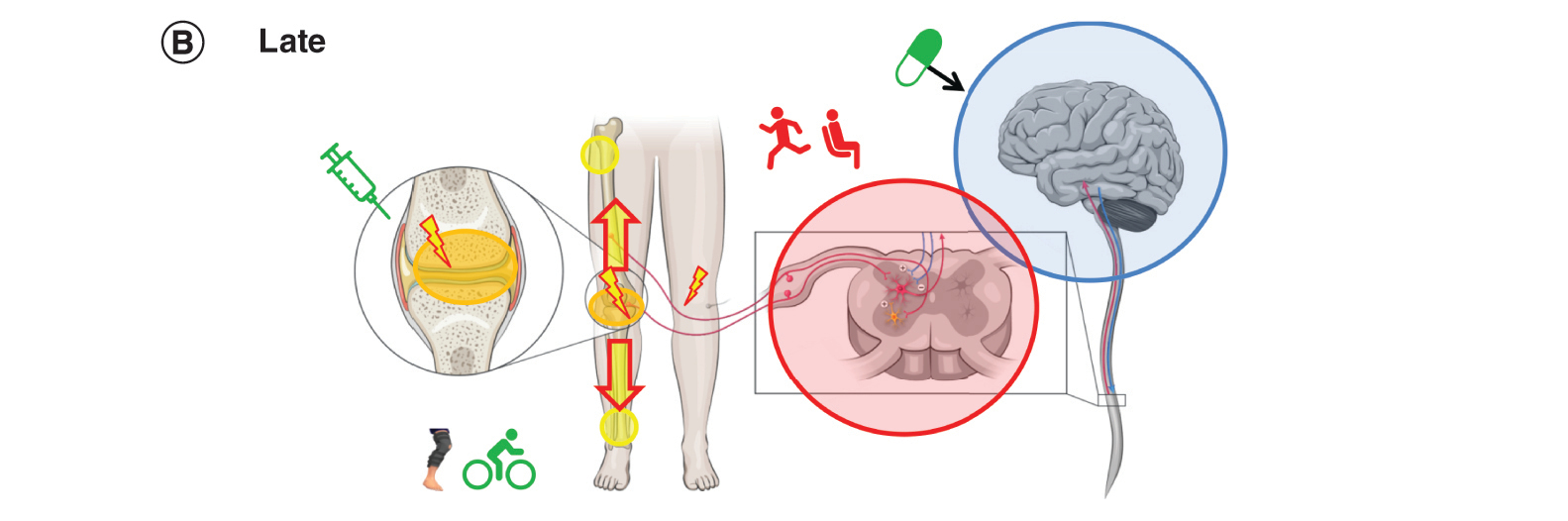
Figure 2B. Later OA: pain
More than a year after the onset of localized, intermittent pain, Patient A has ongoing chronic pain. Radiographic examination indicates moderate OA (KL stage 3). Her pain severity with movement has increased. Mild pressure outside of the joint (e.g., bumping knees together when sitting) is now painful, and she reports pain in her noninjured knee, all signs of peripheral and central sensitization.
Treatment
Patient A replaces the topical NSAID with an oral NSAID as needed, which can suppress inflammation in the knee and spinal cord [Citation28]. Because oral NSAIDs should be used for the shortest duration possible at the lowest effective dose [Citation10,Citation29], nonpharmacologic management strategies are particularly important. She continues to manage her weight but has replaced cycling with tai chi, an activity combining slow movement and relaxation [Citation1].
History
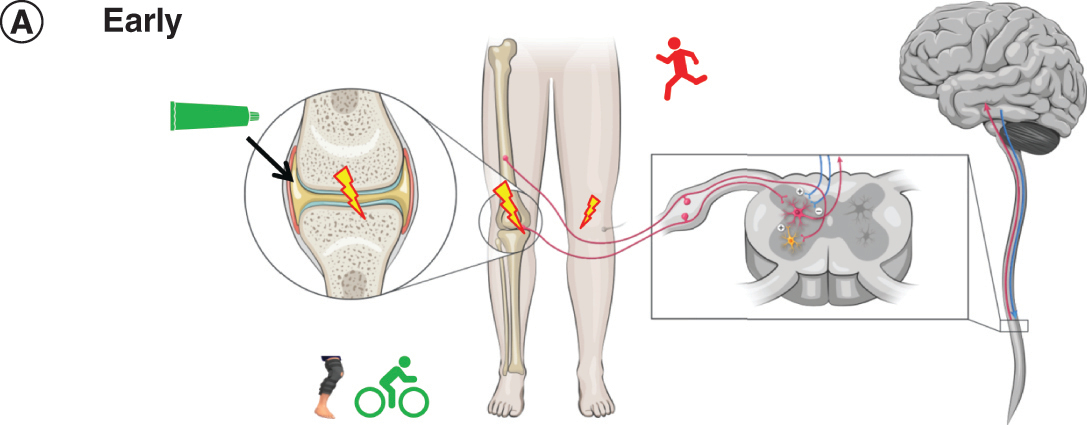
Patient B is a man in his mid 50s with hypertension who has worked in a warehouse for 20 years, primarily moving packing cartons, bending his knees repetitively. He does not recall a specific injury, but over time discomfort in both knees has increased during work [Citation30].
Figure 3A. Early OA: pain
Patient B now has aching pain in both knees, one more severe than the other. The pain occurs during movement and worsens with his level of activity [Citation27]. He also finds household activities like yardwork uncomfortable. Using stairs causes sharp pain and his more severely affected knee has buckled while climbing stairs [Citation27]. Radiographic examination indicates minimal OA (KL stage 2).
Treatment
Patient B has been able to obtain a desk-based job which no longer requires bending and lifting heavy loads. He uses a topical NSAID on his more painful knee to alleviate pain and reduce inflammation at the joint. He also uses braces on both knees to manage malalignment that is contributing to joint damage [Citation12,Citation31].
Figure 3B. Later OA: pain
At 66 years of age, Patient B’s radiographic findings now indicate moderate OA (K-L stage 3). He now experiences severe stabbing pain in his more affected knee, seemingly randomly on motion and at rest and his sleep is disrupted [Citation27]. His pain radiates out from the joint and extends as a burning pain up his leg, and he sometimes experiences pain in his entire lower limb. He has chronic fatigue and low mood. Patient B’s increasingly severe, multifocal pain and somatic symptoms are indicative of sensitization and centralization; the burning pain he describes suggests pain with neuropathic features as well [Citation32].

Treatment
Patient B has been able to obtain a desk-based job which no longer requires bending and lifting heavy loads. Due to his age and cardiovascular comorbidity, oral NSAIDs are contraindicated for Patient B; intra-articular glucocorticoid injection might be an option to treat his ongoing pain [Citation9,Citation10]. Based on his centralized pain and pain with neuropathic features, his physician also prescribes centrally acting duloxetine, which has efficacy for treating both components as well as mood [Citation1,Citation33]. If sleep issues do not resolve with improved pain and mood, the sleep disorder may need to be managed additionally. Referral to a multidisciplinary pain management program would also be appropriate for this patient [Citation10]. If these treatment steps fail to adequately control his pain, with a significant negative impact on his quality of life, Patient B might become a candidate for knee replacement surgery [Citation9].

Pain characteristics over the course of OA
The progression of OA is classified by radiographic evidence of joint damage, which is commonly described using the Kellgren-Lawrence (K-L) grading system [Citation34,Citation35]. In early OA (grade 2), bony projections and narrowing of the joint space may be observed. In later stages, damage to multiple tissues in the joint is apparent, with severe joint space narrowing, sclerosis of the bone beneath the cartilage and bone deterioration in severe OA (grade 4) [Citation35].
The K-L system is widely used in OA research, and it is also used to determine the need for surgical intervention [Citation35]. However, the pain experience of a patient is not necessarily linked to observable joint damage [Citation36]. For some patients, pain severity increases in line with joint degeneration, but others report pain long before there is visible damage. Some, conversely, report no pain even though they present with advanced K-L stages [Citation36]. Still others may have mild pain and little joint damage persisting over many years, with a clinical picture that deteriorates very little [Citation12]. Furthermore, the nature of a patient’s pain over the course of OA is not characterized solely in terms of its severity. The experienced pain can vary according to stimuli that prompt it (eg, pain on movement vs pain at rest), temporal characteristics (episodic vs chronic), or site (localized to the joint vs widespread or multifocal pain), as well as descriptive quality (e.g., dull or achy vs sharp, burning or shooting) [Citation27].
Pain quality, or type of pain, has been assessed using pain assessment scales such as the McGill Pain Questionnaire [Citation37,Citation38], in which respondents select descriptors from a list [Citation39], or as open-ended responses in focus group or one-on-one interviews (e.g., the Osteoarthritis Research Society International [OARSI]/Outcome Measures in Rheumatology initiative) [Citation27,Citation40]. Patients with OA describe pain in terms such as sharp or stabbing, shooting or knife-like, sometimes triggered by an action or activity [Citation27,Citation39–42]. It can also be a dull, heavy, throbbing, or aching pain, described as nagging or gnawing [Citation27,Citation39–42]. Some patients describe burning pain or tingling [Citation27,Citation42,Citation43]. Patients may feel tenderness at the affected joint or even excruciating pain on contact [Citation40,Citation41]. OA pain may come and go unpredictably or be constant [Citation27,Citation40]. It has been described as violent, brutal, fierce and unbearable [Citation27,Citation40].
Characteristics of OA pain often shift over the course of the disease [Citation27], with no apparent preset progression in individual patients [Citation44,Citation45]. Analyses of studies that characterize OA progression found too much variability between studies, and between patients within the same study, to describe a specific pain trajectory [Citation44,Citation45]. However, some patterns can be observed: pain on movement may occur at any stage of OA, pain exclusively localized at the joint may be more typical in early stages, and pain at rest, widespread pain and referred pain appear to be more common at late stages [Citation13]. Evidence suggests that OA is a mixed pain state with peripheral and centralized components [Citation46], and patients may experience those components differentially over the course of OA [Citation12–14,Citation45].
Mechanisms underlying the various pain characteristics and their shift during OA progression have been explored in detail in comprehensive reviews [Citation11–22]. Initially, OA pain derives from a nociceptive response to joint damage from injury, malalignment of the joint, or mechanical factors such as repetitive strain [Citation12,Citation13]. In early stages of OA, acute pain occurs episodically, is related to movement or pressure to the joint, and is localized at the injured joint [Citation27]. In some patients, nociceptor sensitization triggered by ongoing nociceptive stimulation can cause a shift in pain severity, its localization and in the types of stimuli that cause the pain [Citation19,Citation47]. This peripheral sensitization occurs when pro-inflammatory cytokines at the joint increase the reactivity of nociceptors, reducing pain thresholds and increasing pain severity [Citation19,Citation20].
In contrast, a central sensitization is characterized by increased descending excitatory input and reduced anti-nociceptive input to nociceptive fibers at the level of the spinal cord. This causes hyperexcitability, enhances the pain response to stimulation, and expands the receptive fields of nociceptive fibers, all of which allow a pain response to stimuli that are innocuous and/or outside the joint [Citation13,Citation21,Citation47]. Centralization of OA pain at later stages results from complex changes in the CNS and plays a key role in chronic pain [Citation22], which is defined by the Task Force for the Classification of Chronic Pain as persistent or recurrent pain lasting >3 months [Citation48]. It is characterized by global hypersensitivity to painful and nonpainful sensory stimulation, decreased endogenous analgesia, widespread and multifocal pain, and co-occurring somatic symptoms such as fatigue, poor sleep, memory problems, or depression [Citation22,Citation49]. Pro-nociceptive neurotransmitter levels are elevated in central pain processing areas and descending analgesic activity is reduced [Citation11,Citation21]. Sensitization and centralization of OA pain both contribute to the observed discordance between OA pain severity and radiographic staging [Citation36].
Additionally, approximately 5–25% of patients with OA may have pain with neuropathic features, which overlap with indicators of centralization [Citation14,Citation32,Citation49,Citation50]. Pain of this type is characterized by burning sensations, shooting pain, feelings of tingling and numbness, mechanical and thermal hyperalgesia, and allodynia (in which pain is provoked by stimuli that are not normally painful) [Citation14]. The International Association for the Study of Pain (IASP) defines neuropathic pain as a pain which arises from a lesion or disease to the peripheral or central nervous system [Citation51]; however, definitive signs of neuropathic pain generally are not observed in patients with OA [Citation52]. In a 2017 systematic review and meta-analysis [Citation50], the authors estimated that 23% of patients with OA have neuropathic pain. However, the reviewed studies used patient self-report neuropathic pain screening questionnaires (e.g., PainDETECT) to define neuropathic pain, meaning that patients were identified based on pain characteristics, and not on diagnosed nerve damage [Citation50]. The IASP uses the new term nociplastic to describe pain that arises from altered nociception despite no clear evidence of tissue damage or somatosensory disease or lesion. However, specific signs of nociplastic pain have not yet been formally characterized by the IASP [Citation51,Citation52].
Inflammation has been implicated both in the onset of OA and in the progression of pain symptoms [Citation53]. Low-grade inflammation or inflammation resulting from joint injury can contribute to early joint pain: pro-inflammatory cytokines, including TNF-α, can induce production of pain mediators such as prostaglandins, as well as directly activate nociceptors [Citation15,Citation16,Citation18,Citation20]. Inflammation can exacerbate joint damage in an ongoing cycle, with an imbalance between joint tissue repair and degradation [Citation17,Citation18]. As detailed above, inflammation has been implicated in the shift in pain symptoms over the course of OA via peripheral sensitization, causing increased reactivity of nociceptors at the joint [Citation19,Citation47]. It also can mediate central sensitization at the level of the spinal cord as demonstrated in animal OA models [Citation53]. In those models, infiltration and activation of inflammatory cells were observed in the dorsal root ganglion, and spinal hyperexcitability was induced by TNF-α via effects on spinal neurons and microglia [Citation16,Citation54,Citation55].
Biomarkers in OA
Understanding how pain characteristics are related to underlying OA disease processes could aid clinicians in making patient-focused treatment choices, and biomarkers provide a potentially useful tool for exploring those connections. The pathophysiology of OA that results in varying pain characteristics may be reflected in serum or synovial fluid biomarkers such as cytokines and chemokines associated with peripheral or central inflammation, or molecules that either cause or result from bone and cartilage turnover at the affected joint [Citation56,Citation57]. Identifying biomarkers that vary according to specific pain characteristics (e.g., pain on movement vs pain at rest vs pain with neuropathic features) would support the concept of tailoring treatment to those characteristics differentially, and could potentially point to therapeutic targets for future drugs.
Biomarkers & pain severity or radiographic progression
Several biomarkers have been associated with overall pain severity in patients with OA () [Citation56–76]. These markers are not specific to OA pain – indeed many, such as adhesion molecules, growth factors and matrix proteins, have roles in tissue growth and turnover [Citation62] and others are involved in inflammation and pain more generally (TNF-α, IL-1β) [Citation15]. However, their levels have been shown to vary significantly with severity of OA pain: Increasing levels of inflammatory markers and other cytokines, markers for cartilage and bone turnover, growth factors and adhesion molecules were associated with more severe pain scale scores [Citation56–59,Citation61–68], as were decreasing levels of IL-1β and macrophage-derived chemokine [Citation59,Citation61]. Both positive [Citation56,Citation59] and negative [Citation61] associations with pain severity have been reported for TNF-α and IL-6. Levels of biomarkers also vary with OA progression as measured by K-L staging: in multiple studies [Citation58,Citation62,Citation67–73], inflammatory markers and other cytokines, growth factors, adhesion molecules and markers for cartilage and bone turnover increased with K-L stage (). Synovial fluid levels of IL-1β and cytotoxic CD8+ T-cells showed an inverse relationship with K-L stage, suggesting involvement in joint damage early in OA [Citation61,Citation70]. Positive [Citation58,Citation71] and negative [Citation61,Citation70] associations with K-L stage have been reported for IL-6 and adiponectin. Collagen catabolic markers as a class were observed to increase with OA progression, whereas levels of a collagen anabolic marker (a collagen pro-peptide) were significantly lower at later K-L stages [Citation74]. Thus, biomarkers have been shown to vary with both pain severity and joint damage, but can they differentiate pain characteristics as well?
Table 1. Biomarkers associated with pain or joint degeneration in osteoarthritis.
Biomarkers & OA pain characteristics
Relationships between biomarkers and specific pain characteristics have indeed been reported: positive relationships with pain on movement were observed for TNF-α, IL-6 and IL-8, as well as a negative relationship for IL-1β; increased levels of TNF-α were also associated with increased pain at rest [Citation56] (). Weight-bearing pain was positively correlated with IL-8 and with a marker for cartilage degradation (CTX-II: carboxy terminal cross-linked telopeptide of type II collagen) [Citation60,Citation67].
Biomarkers have also been associated with processes linked to the shift in pain characteristics over the course of OA. Observed overexpression of inflammatory markers in synovial tissue in early versus late OA may reflect the role of inflammation in OA onset and peripheral sensitization [Citation77]. Levels of a CRP metabolite increase significantly with the degree of central sensitization [Citation75], and serum levels of BDNF may reliably indicate centralization [Citation76]. In both animal and human studies, prostaglandin E2 was found to induce nociceptor sensitization [Citation78,Citation79]. Again, while these markers have been associated with sensitization and centralization in OA, they are not specific to these processes; BDNF, for example, has a range of functions related to neuroplasticity, and has been examined as a biomarker in depression and other neuropsychiatric and neurodegenerative conditions [Citation80,Citation81].
Just a small number of studies linking biomarkers to specific pain characteristics have been published to date; however, the available evidence suggests that different pathophysiologies underlie individual pain characteristics. A consideration of the patient’s pain symptoms in treatment selection may therefore be warranted.
Treatment & pain characteristics
OA treatment guidelines
Pharmacotherapy recommendations from the American College of Rheumatology (ACR) and OARSI are differentiated based on the joint affected and contraindications for medications, but generally do not address differences in patients’ pain characteristics [Citation1,Citation10]. The ACR guidelines strongly recommend topical nonsteroidal anti-inflammatory drugs (NSAIDs), oral NSAIDs and glucocorticoid injections, along with nonpharmacologic approaches including self-management and movement or exercise programs, and weight loss or gait aids where appropriate. The ACR only conditionally recommends duloxetine (due to poor tolerability), and acetaminophen or tramadol (due to weak evidence for efficacy) if other options are contraindicated [Citation1]. OARSI recommendations are similar: topical NSAIDs, oral NSAIDs, COX-2 inhibitors and intra-articular corticosteroids are Level 1 recommendations for patients without comorbidities (depending on the joint involved), in conjunction with core nonpharmacologic therapies such as exercise and weight management [Citation10]. The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) recommends prescription crystalline glucosamine sulfate or chondroitin sulfate or other slow-acting drugs for OA (SYSADOAs) as step 1 pharmacotherapy for treating knee OA pain, together with core nonpharmacologic measures (information/education; weight loss if overweight; exercise program) [Citation9]. ESCEO recommends paracetamol as the first add-on short-term rescue medicine if pain is not controlled by a base of SYSADOAs; a topical NSAID can be added after rescue paracetamol if pain relief is inadequate [Citation9]. The UK National Institute for Health and Care Excellence recommends that clinicians consider offering paracetamol for pain relief in addition to core treatments (information/education, weight loss and exercise); paracetamol and/or topical NSAIDs should be considered ahead of oral NSAIDs, COX-2 inhibitors, or opioids [Citation23]. Due to evidence of cardiovascular risk, oral NSAIDs are contraindicated in patients who are elderly or have a cardiovascular comorbidity and should be used at the lowest effective dose for the shortest possible duration [Citation10,Citation29,Citation82]. The ACR and OARSI recommend against the use of opioids (particularly nontramadol opioids) due to concerns about toxicity and chemical dependency with modest benefit [Citation10,Citation29].
The OARSI treatment algorithm uses joint localization and clinically relevant comorbidities as criteria for selecting patient-centered treatment options; other pharmacotherapies such as intra-articular hyaluronic acid, duloxetine, topical capsaicin, paracetamol and oral opioids, are considered when the patient’s symptoms are unacceptable on re-assessment [Citation10]. The ACR guideline states that choice of interventions ‘may vary over the course of the disease or with patient’ but does not make specific recommendations [Citation1]. However, the new classification of chronic secondary musculoskeletal pain in the 11th edition of the International Classification of Diseases (ICD-11) according to underlying condition, can serve to focus OA treatment on better pain management based on pain pathophysiology in the individual patient [Citation83].
Pain characteristics in OA treatment selection
Recommended OA pain medications are associated with significant improvement in pain scale scores compared with placebo in clinical studies, but generally with small to moderate effect sizes, and with wide variation between studies [Citation84–94]. The modest effect sizes associated with pharmacologic treatments for OA, indicating a small to moderate benefit overall, may reflect the fact that a given treatment may provide meaningful improvement in pain for some patients with OA but little to no effect for others, or that responses may vary over time for a given patient. For example, number needed to treat for benefit calculations from meta-analyses have shown that 11 patients must be treated with celecoxib in order for one to be positively affected [Citation90]; similarly, eight patients must be treated with intra-articular corticosteroid [Citation93] and six or seven must be treated with duloxetine to see a positive effect versus placebo [Citation91]. The number needed to treat for improvement in chronic musculoskeletal or OA pain is 4 to 10 for topical NSAIDs [Citation87,Citation95,Citation96] and 4 to 5 for oral NSAIDs [Citation97]. An approach to treatment selection that takes into account the pain characteristics of the individual patient, the possible underlying mechanisms and the OA treatment guidelines, might increase the likelihood of choosing a treatment that is effective for the individual patient [Citation11–13,Citation52]. This approach is illustrated in Boxes 1 and 2 for 2 hypothetical patients whose differing pain characteristics shift over the course of OA [Citation1,Citation10,Citation12,Citation24–28,Citation30–33].
Biomarker studies suggest that when pain is localized at the joint (Box 1, Figure 2A; Box 2, Figure 3A), peripherally directed therapies that reduce proinflammatory markers can alleviate nociceptive pain and inflammation. Topical NSAIDs, which provide continuous therapeutic concentrations at the synovium [Citation98] while reducing the risks of systemic exposure, are recommended where practical to target the joint [Citation1,Citation10]. Because topical NSAIDs inhibit synthesis of prostaglandins [Citation99], which modulate tissue destruction and nociceptor sensitization [Citation28], it has been suggested that they could potentially slow early tissue destruction and help avoid peripheral or central sensitization, as well as reduce OA pain [Citation100–102]. To date, however, the evidence for such effects is indirect and/or comes from animal studies using experimental models of OA [Citation100–102]. Intra-articular corticosteroids might also be considered for a patient with OA knee pain localized to the joint [Citation10].
Sensitization can be identified by assessing response to a range of noxious or innocuous stimuli (mechanical, thermal and/or electrical modalities) [Citation19], or by measuring pain thresholds, temporal summation and referred pain areas using pressure stimuli at multiple sites relative to the affected joint [Citation47]. For patients with pain characteristics associated with sensitization such as pain outside the joint, referred pain, or pain at rest, an oral NSAID might be most effective, used at the lowest effective dose for the shortest possible duration (Box 1, Figure 2B) [Citation10,Citation29]. Nonselective oral NSAIDs or COX-2 inhibitors effectively treat pain [Citation88–90] and also reduce inflammation [Citation12,Citation103]. Because they have systemic exposure [Citation1], they can modulate processes both locally at the joint and at the level of the spinal cord. Nonselective oral NSAIDs reduce serum or synovial fluid levels of inflammatory markers (TNF-α, IL-8, IL-6, CRP), as well as markers associated with cartilage and bone turnover [Citation28,Citation104–110], and COX-2 inhibitors reduce IL-6 and TNF-α in synovial fluid [Citation28,Citation109] () [Citation28,Citation104–109,Citation111]. An oral NSAID (aceclofenac) and a COX-2 inhibitor (celecoxib) were also shown to reduce levels of prostaglandin E2 [Citation28]. One study further demonstrated that decreasing synovial fluid IL-6, TNF-α and VEGF levels during oral NSAID treatment was correlated with improvement in pain scale scores in patients with knee OA [Citation111]. Blockade of TNF-α has been demonstrated to directly reduce nociceptive response in patients with rheumatoid arthritis [Citation112]; however, the ACR recommends strongly against TNF-α inhibitors (such as adalimumab or etanercept) or interleukin receptor antagonists (such as anakinra) for treating OA pain due to lack of efficacy in clinical trials [Citation1,Citation113].
Table 2. Effects of analgesics on biomarkers with a demonstrated association with osteoarthritis pain severity or degree of joint degradation.
Widespread, multifocal pain and concomitant nonpain symptoms such as fatigue or depression are suggestive of centralization (Box 2, Figure 3B). A centrally acting drug, such as an SNRI, that addresses underlying neurotransmitter dysfunction might best target centralized pain [Citation11]. Additionally, while first-line OA medications are unlikely to be effective for pain with neuropathic features [Citation114], duloxetine has demonstrated efficacy for treating both pain in OA [Citation91,Citation115,Citation116] and neuropathic pain [Citation117]. The ACR guideline conditionally recommends duloxetine alone or in combination with NSAIDs for some patients with OA but side effects must be considered [Citation1,Citation116]. Other treatments such as psychological counselling, exercise, lifestyle modifications, smoking cessation, healthy diet, corrective measures to restore sleep, relaxation techniques, etc, should also be considered to address the concomitant symptoms. For chronic pain, nonpharmacologic management strategies are particularly important [Citation1], and referral to a multidisciplinary pain management program is appropriate for patients with complex pain and mood symptoms [Citation10].
This discussion is not meant to recommend specific OA medications other than those endorsed by guidelines, but rather to describe an approach to applying the existing guidelines to individual patients when starting OA treatment or when shifts in pain indicate a need for a change in pain therapy (Boxes 1 and 2). An understanding of the mechanisms underlying pain characteristics offers insight for selecting OA treatments for acute localized pain, or for pain with characteristics associated with sensitization or centralization, or with neuropathic features [Citation11]. However, clinical trials using subgroup analyses based on clinical features and/or biomarkers will still be needed to confirm the efficacy of specific drugs in patients with these characteristics [Citation118]. Finally, work examining biomarkers for OA risk factors, such as biomarkers linking obesity and OA [Citation119,Citation120] or markers that might elucidate connections between OA and aging [Citation121] or knee injury [Citation122,Citation123], could support clinical guidance for the treatment of OA in specific patient subgroups.
Conclusion
Pain in OA is complex, and its characteristics vary between patients and over the progression of the disease. Drug classes used for treating OA might address pain symptoms at different sites and at different points in the patient’s course of OA, depending on their mechanisms of action. Patients with pain characteristics indicating underlying mechanisms that differ from those targeted by a drug might not be expected to benefit; therefore, matching OA drugs to subsets of patients most likely to benefit could improve patient outcomes and support targeted drug treatment over the course of the disease.
Future perspective
Numerous studies have demonstrated associations between biomarkers and pain severity or OA stage [Citation56–74], but few have made the more specific connections between markers and type of pain or presence of sensitization or centralization [Citation56,Citation60,Citation67,Citation75,Citation76]. Additional studies differentiating biomarkers associated with the range of pain characteristics outlined in might allow for the use of markers and pain descriptions together in treatment selection for individual patients, and perhaps eventually contribute to the development of more targeted OA pain treatment strategies.
Financial & competing interests disclosure
Martina Hagen and Marina Fayet are employees of GSK Consumer Healthcare S.A., Nyon, Switzerland. GSK manufactures a wide range of OTC analgesics including various topical and oral NSAIDs and paracetamol. The manuscript is funded by GSK Consumer Healthcare S.A., Nyon, Switzerland. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by Kathleen Dorries of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by GSK Consumer Healthcare S.A., Nyon, Switzerland.
Additional information
Funding
References
- Kolasinski SL , NeogiT , HochbergMCet.al 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis. Rheumatol.72(2), 220–233 (2020).
- Smith L , WittenauerR , AdenK , DutheyB. Osteoarthritis. In: Priority Medicines for Europe and the World 2013 Update.KaplanW, WirtzVJ, Mantel-TeeuwisseA, StolkP, DutheyB, LaingR ( Eds). WHO, Geneva, Switzerland, 126–128 (2013).
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet390(10100), 1211–1259 (2017).
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet388(10053), 1545–1602 (2016).
- Callhoff J , AlbrechtK , RedekerIet.al Disease burden of patients with osteoarthritis: results of a cross-sectional survey linked to claims data. Arthritis Care Res. (Hoboken)72(2), 193–200 (2020).
- Sparkes V , WhatlingGM , BiggsPet.al Comparison of gait, functional activities, and patient-reported outcome measures in patients with knee osteoarthritis and healthy adults using 3D motion analysis and activity monitoring: an exploratory case-control analysis. Orthop. Res. Rev.11, 129–140 (2019).
- Abbott JH , UsiskinIM , WilsonR , HansenP , LosinaE. The quality-of-life burden of knee osteoarthritis in New Zealand adults: a model-based evaluation. PLoS One12(10), e0185676 (2017).
- Bruyere O , CooperC , PelletierJPet.al A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin. Arthritis Rheum.45(Suppl. 4), S3–11 (2016).
- Bruyère O , HonvoG , VeroneseNet.al An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum.49(3), 337–350 (2019).
- Bannuru RR , OsaniMC , VaysbrotEEet.al OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil.27(11), 1578–1589 (2019).
- Clauw DJ , HassettAL. The role of centralised pain in osteoarthritis. Clin. Exp. Rheumatol.35, (Suppl. 107(5)), 79–84 (2017).
- Dell’Isola A , AllanR , SmithSL , MarreirosSS , SteultjensM. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet. Disord.17(1), 425 (2016).
- Eitner A , HofmannGO , SchaibleHG. Mechanisms of osteoarthritic pain. Studies in humans and experimental models. Front. Mol. Neurosci.10, 349 (2017).
- Thakur M , DickensonAH , BaronR. Osteoarthritis pain: nociceptive or neuropathic?Nat. Rev. Rheumatol.10(6), 374–380 (2014).
- Schaible HG . Nociceptive neurons detect cytokines in arthritis. Arthritis Res. Ther.16(5), 470 (2014).
- Miller RE , TranPB , ObeidatAMet.al The role of peripheral nociceptive neurons in the pathophysiology of osteoarthritis pain. Curr. Osteoporosis Rep.13(5), 318–326 (2015).
- Hunter DJ , Bierma-ZeinstraS. Osteoarthritis. Lancet393(10182), 1745–1759 (2019).
- Griffin TM , ScanzelloCR. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin. Exp. Rheumatol.37(Suppl. 120(5)), 57–63 (2019).
- Fingleton C , SmartK , MoloneyN , FullenBM , DoodyC. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr. Cartil.23(7), 1043–1056 (2015).
- Syx D , TranPB , MillerRE , MalfaitAM. Peripheral mechanisms contributing to osteoarthritis pain. Curr. Rheumatol. Rep.20(2), 9 (2018).
- Arendt-Nielsen L . Pain sensitisation in osteoarthritis. Clin. Exp. Rheumatol.35, (Suppl. 107[5]), 68–74 (2017).
- Lluch E , TorresR , NijsJ , Van OosterwijckJ. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur. J. Pain18(10), 1367–1375 (2014).
- National Institute for Health and Care Excellence . Osteoarthritis overview 2020. https://pathways.nice.org.uk/pathways/osteoarthritis/osteoarthritis-overview.
- Poulsen E , GoncalvesGH , BriccaA , RoosEM , ThorlundJB , JuhlCB. Knee osteoarthritis risk is increased 4–6 fold after knee injury - a systematic review and meta-analysis. Br. J. Sports Med.53(23), 1454–1463 (2019).
- Gooberman-Hill R , WoolheadG , MackichanF , AyisS , WilliamsS , DieppeP. Assessing chronic joint pain: lessons from a focus group study. Arthritis Rheum.57(4), 666–671 (2007).
- Gooberman-Hill R , FrenchM , DieppeP , HawkerG. Expressing pain and fatigue: a new method of analysis to explore differences in osteoarthritis experience. Arthritis Rheum.61(3), 353–360 (2009).
- Hawker GA , StewartL , FrenchMRet.al Understanding the pain experience in hip and knee osteoarthritis – an OARSI/OMERACT initiative. Osteoarthr. Cartil.16(4), 415–422 (2008).
- Alvarez-Soria MA , LargoR , SantillanaJet.al Long term NSAID treatment inhibits COX-2 synthesis in the knee synovial membrane of patients with osteoarthritis: differential proinflammatory cytokine profile between celecoxib and aceclofenac. Ann. Rheum. Dis.65(8), 998–1005 (2006).
- Kolasinski SL , NeogiT , HochbergMCet.al 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. (Hoboken)72(2), 149–162 (2020).
- Gignac MAM , IrvinE , CullenKet.al Men and women’s occupational activities and the risk of developing osteoarthritis of the knee, hip, or hands: a systematic review and recommendations for future research. Arthritis Care Res. (Hoboken)72(3), 378–396 (2020).
- Felson DT . Developments in the clinical understanding of osteoarthritis. Arthritis Res. Ther.11(1), 203 (2009).
- Ohtori S , OritaS , YamashitaMet.al Existence of a neuropathic pain component in patients with osteoarthritis of the knee. Yonsei Med. J.53(4), 801–805 (2012).
- Attal N , CruccuG , BaronRet.al EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur. J. Neurol17(9), 1113–1111e1188 (2010).
- Kellgren JH , LawrenceJS. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis16(4), 494–502 (1957).
- Kohn MD , SassoonAA , FernandoND. Classifications in Brief: Kellgren-Lawrence classification of osteoarthritis. Clin. Orthop. Relat. Res.474(8), 1886–1893 (2016).
- Bedson J , CroftPR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet. Disord.9, 116 (2008).
- Melzack R . The McGill Pain Questionnaire: major properties and scoring methods. Pain1(3), 277–299 (1975).
- Melzack R . The short-form McGill Pain Questionnaire. Pain30(2), 191–197 (1987).
- De Oliveira Paes Leme M , YuanSLK , OliveiraMagalhães M , Ferreirade Meneses SR , MarquesAP. Pain and quality of life in knee osteoarthritis, chronic low back pain and fibromyalgia: a comparative cross-sectional study. Reumatismo71(2), 68–74 (2019).
- Cedraschi C , DelézayS , MartyMet.al “Let’s talk about OA pain”: a qualitative analysis of the perceptions of people suffering from OA. Towards the development of a specific pain OA-Related questionnaire, the Osteoarthritis Symptom Inventory Scale (OASIS). PLoS One8(11), e79988 (2013).
- Murphy SL , LydenAK , KratzALet.al Characterizing pain flares from the perspective of individuals with symptomatic knee osteoarthritis. Arthritis Care Res. (Hoboken)67(8), 1103–1111 (2015).
- Marques AP , RhodenL , deOliveira Siqueira J , JoãoSM. Pain evaluation of patients with fibromyalgia, osteoarthritis, and low back pain. Rev. Hosp. Clin. Fac. Med. Sao Paulo56(1), 5–10 (2001).
- Hochman JR , FrenchMR , BerminghamSL , HawkerGA. The nerve of osteoarthritis pain. Arthritis Care Res. (Hoboken)62(7), 1019–1023 (2010).
- de Rooij M , vander Leeden M , HeymansMWet.al Prognosis of pain and physical functioning in patients with knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res. (Hoboken)68(4), 481–492 (2016).
- de Rooij M , vander Leeden M , HeymansMWet.al Course and predictors of pain and physical functioning in patients with hip osteoarthritis: Systematic review and meta-analysis. J. Rehabil. Med.48(3), 245–252 (2016).
- Thudium CS , LofvallH , KarsdalMA , Bay-JensenAC , BihletAR. Protein biomarkers associated with pain mechanisms in osteoarthritis. J. Proteomics190, 55–66 (2019).
- Arendt-Nielsen L , NieH , LaursenMBet.al Sensitization in patients with painful knee osteoarthritis. Pain149(3), 573–581 (2010).
- Treede RD , RiefW , BarkeAet.al A classification of chronic pain for ICD-11. Pain156(6), 1003–1007 (2015).
- Neville SJ , ClauwAD , MoserSEet.al Association between the 2011 fibromyalgia survey criteria and multisite pain sensitivity in knee osteoarthritis. Clin. J. Pain34(10), 909–917 (2018).
- French HP , SmartKM , DoyleF. Prevalence of neuropathic pain in knee or hip osteoarthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum.47(1), 1–8 (2017).
- International Association for the Study of Pain . IASP terminology. International Association for the Study of Pain, 2018. https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698
- Trouvin AP , PerrotS. New concepts of pain. Best Pract. Res. Clin. Rheumatol.33(3), 101415 (2019).
- Miller RE , TranPB , IshiharaSet.al Microarray analyses of the dorsal root ganglia support a role for innate neuro-immune pathways in persistent pain in experimental osteoarthritis. Osteoarthr. Cartil.28(5), 581–592 (2020).
- Richter F , NaturaG , LoserS , SchmidtK , ViisanenH , SchaibleHG. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum.62(12), 3806–3814 (2010).
- Konig C , MorchE , EitnerAet.al Involvement of spinal IL-6 trans-signaling in the induction of hyperexcitability of deep dorsal horn neurons by spinal tumor necrosis factor-alpha. J. Neurosci.36(38), 9782–9791 (2016).
- Leung YY , HuebnerJL , HaalandB , WongSBS , KrausVB. Synovial fluid pro-inflammatory profile differs according to the characteristics of knee pain. Osteoarthr. Cartil.25(9), 1420–1427 (2017).
- Garnero P , MazieresB , GueguenAet.al Cross-sectional association of 10 molecular markers of bone, cartilage, and synovium with disease activity and radiological joint damage in patients with hip osteoarthritis: the ECHODIAH cohort. J. Rheumatol.32(4), 697–703 (2005).
- Nees TA , RosshirtN , ZhangJAet.al Synovial cytokines significantly correlate with osteoarthritis-related knee pain and disability: inflammatory mediators of potential clinical relevance. J. Clin. Med.8(9), 1343 (2019).
- Ren G , LutzI , RailtonPet.al Serum and synovial fluid cytokine profiling in hip osteoarthritis: distinct from knee osteoarthritis and correlated with pain. BMC Musculoskelet. Disord.19(1), 39 (2018).
- Ruan G , XuJ , WangKet.al Associations between serum IL-8 and knee symptoms, joint structures, and cartilage or bone biomarkers in patients with knee osteoarthritis. Clin. Rheumatol.38(12), 3609–3617 (2019).
- Li L , LiZ , LiY , HuX , ZhangY , FanP. Profiling of inflammatory mediators in the synovial fluid related to pain in knee osteoarthritis. BMC Musculoskelet. Disord.21(1), 99 (2020).
- Haraden CA , HuebnerJL , HsuehMF , LiYJ , KrausVB. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res. Ther.21(1), 146 (2019).
- Ke X , JinG , YangYet.al Synovial fluid HMGB-1 levels are associated with osteoarthritis severity. Clin. Lab.61(7), 809–818 (2015).
- Wang C , GongZ , HuS , ZhangG. Metallothionein-1 is associated with osteoarthritis disease activity and suppresses proinflammatory cytokines production in synovial cells. Int. Immunopharmacol.75, 105815 (2019).
- Zhang PL , LiuJ , XuL , SunY , SunXC. Synovial fluid macrophage migration inhibitory factor levels correlate with severity of self-reported pain in knee osteoarthritis patients. Med. Sci. Monit.22, 2182–2186 (2016).
- Sowers MF , Karvonen-GutierrezCA , YosefMet.al Longitudinal changes of serum COMP and urinary CTX-II predict X-ray defined knee osteoarthritis severity and stiffness in women. Osteoarthr. Cartil.17(12), 1609–1614 (2009).
- Bihlet AR , ByrjalsenI , Bay-JensenACet.al Associations between biomarkers of bone and cartilage turnover, gender, pain categories and radiographic severity in knee osteoarthritis. Arthritis Res. Ther.21(1), 203 (2019).
- Kapetanakis S , DrygiannakisI , KazakosKet.al Serum TGF-beta2 and TGF-beta3 are increased and positively correlated to pain, functionality, and radiographic staging in osteoarthritis. Orthopedics33(8), (2010).
- Attur M , StatnikovA , SamuelsJet.al Plasma levels of interleukin-1 receptor antagonist (IL1Ra) predict radiographic progression of symptomatic knee osteoarthritis. Osteoarthr. Cartil.23(11), 1915–1924 (2015).
- Sachdeva M , AggarwalA , SharmaRet.al Chronic inflammation during osteoarthritis is associated with an increased expression of CD161 during advanced stage. Scand. J. Immunol.90(1), e12770 (2019).
- Tang Q , HuZC , ShenLY , ShangP , XuHZ , LiuHX. Association of osteoarthritis and circulating adiponectin levels: a systematic review and meta-analysis. Lipids Health Dis.17(1), 189 (2018).
- Daghestani HN , PieperCF , KrausVB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. (Hoboken, N.J.)67(4), 956–965 (2015).
- Valdes AM , MeulenbeltI , ChassaingEet.al Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthr. Cartil.22(5), 683–689 (2014).
- Kraus VB , CollinsJE , HargroveDet.al Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann. Rheum. Dis.76(1), 186–195 (2017).
- Arendt-Nielsen L , EskehaveTN , EgsgaardLLet.al Association between experimental pain biomarkers and serologic markers in patients with different degrees of painful knee osteoarthritis. Arthritis Rheumatol. (Hoboken, N.J.)66(12), 3317–3326 (2014).
- Deitos A , Dussan-SarriaJA , SouzaAet.al Clinical value of serum neuroplasticity mediators in identifying the central sensitivity syndrome in patients with chronic pain with and without structural pathology. Clin. J. Pain31(11), 959–967 (2015).
- Benito MJ , VealeDJ , FitzGeraldO , vanden Berg WB , BresnihanB. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis.64(9), 1263–1267 (2005).
- Namer B , SchickM , KleggetveitIPet.al Differential sensitization of silent nociceptors to low pH stimulation by prostaglandin E2 in human volunteers. Eur. J. Pain19(2), 159–166 (2015).
- Khasabova IA , UhelskiM , KhasabovSG , GuptaK , SeyboldVS , SimoneDA. Sensitization of nociceptors by prostaglandin E(2)-glycerol contributes to hyperalgesia in mice with sickle cell disease. Blood133(18), 1989–1998 (2019).
- Bathina S , DasUN. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci.11(6), 1164–1178 (2015).
- Lima Giacobbo B , DoorduinJ , KleinHC , DierckxR , BrombergE , de VriesEFJ. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol. Neurobiol.56(5), 3295–3312 (2019).
- Gourlay DL , HeitHA. Pain and addiction: managing risk through comprehensive care. J. Addict. Dis27(3), 23–30 (2008).
- Perrot S , CohenM , BarkeA , KorwisiB , RiefW , TreedeRD. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain160(1), 77–82 (2019).
- Deng ZH , ZengC , YangYet.al Topical diclofenac therapy for osteoarthritis: a meta-analysis of randomized controlled trials. Clin. Rheumatol.35(5), 1253–1261 (2016).
- Persson MSM , StocksJ , WalshDA , DohertyM , ZhangW. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthr. Cartil.26(12), 1575–1582 (2018).
- Zeng C , WeiJ , PerssonMSMet.al Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br. J. Sports Med.52(10), 642–650 (2018).
- Wiffen PJ , XiaJ. Systematic review of topical diclofenac for the treatment of acute and chronic musculoskeletal pain. Curr. Med. Res. Opin. accepted, 1 (2020).
- van Walsem A , PandhiS , NixonRM , GuyotP , KarabisA , MooreRA. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta-analysis. Arthritis Res. Ther.17, 66 (2015).
- da Costa BR , ReichenbachS , KellerNet.al Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet390(10090), E21–E33 (2017).
- Puljak L , MarinA , VrdoljakD , MarkoticF , UtrobicicA , TugwellP. Celecoxib for osteoarthritis. Cochrane Database Syst. Rev.5, Cd009865 (2017).
- Citrome L , Weiss-CitromeA. A systematic review of duloxetine for osteoarthritic pain: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed?Postgrad. Med.124(1), 83–93 (2012).
- Toupin April K , BisaillonJ , WelchVet.al Tramadol for osteoarthritis. Cochrane Database Syst Rev5, Cd005522 (2019).
- Jüni P , HariR , RutjesAWet.al Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev (10), CD005328 (2015).
- Cohen J . The analysis of variance. In: Statistical Power Analysis for the Behavioral Sciences.CohenJ ( Ed.). Lawrence Erlbaum Associates, NJ, USA, 273–406 (1988).
- Mason L , MooreRA , EdwardsJE , DerryS , McQuayHJ. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC. Musculoskelet. Disord5, 28 (2004).
- Baer PA , ThomasLM , ShainhouseZ. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial [ISRCTN53366886]. BMC Musculoskelet. Disord.6, 44 (2005).
- Osiri M , Suarez-AlmazorME , WellsGA , RobinsonV , TugwellP. Number needed to treat (NNT): implication in rheumatology clinical practice. Ann. Rheum. Dis.62(4), 316–321 (2003).
- Hagen M , BakerM. Skin penetration and tissue permeation after topical administration of diclofenac. Curr. Med. Res. Opin.33(9), 1623–1634 (2017).
- Chlud K , WagenerHH. Percutaneous non-steroidal anti-inflammatory drug (NSAID) therapy with particular reference to pharmacokinetic factors. Eular Bull.2, 40–43 (1987).
- Zweers MC , de BoerTN , van RoonJ , BijlsmaJW , LafeberFP , MastbergenSC. Celecoxib: considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res. Ther.13(5), 239 (2011).
- Nakata K , HanaiT , TakeYet.al Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: a systematic review. Osteoarthr. Cartil.26(10), 1263–1273 (2018).
- Niibori M , KudoY , HayakawaTet.al Mechanism of aspirin-induced inhibition on the secondary hyperalgesia in osteoarthritis model rats. Heliyon6(5), e03963 (2020).
- Park JW , YunYP , ParkKet.al Ibuprofen-loaded porous microspheres suppressed the progression of monosodium iodoacetate-induced osteoarthritis in a rat model. Colloids Surf. B. Biointerfaces147, 265–273 (2016).
- Zhang SL , LiuHQ , XuXZ , ZhiJ , GengJJ , ChenJ. Effects of exercise therapy on knee joint function and synovial fluid cytokine levels in patients with knee osteoarthritis. Mol Med Rep7(1), 183–186 (2013).
- Hussain SA , JassimNA , NumanIT , AlK , AbdullahTAII. Anti-inflammatory activity of silymarin in patients with knee osteoarthritis. A comparative study with piroxicam and meloxicam. Saudi Med. J.30(1), 98–103 (2009).
- Gineyts E , MoJA , KoAet.al Effects of ibuprofen on molecular markers of cartilage and synovium turnover in patients with knee osteoarthritis. Ann. Rheum. Dis.63(7), 857–861 (2004).
- Konstantinidis I , PapageorgiouSN , KyrgidisA , TzellosTG , KouvelasD. Effect of non-steroidal anti-inflammatory drugs on bone turnover: an evidence-based review. Rev. Recent Clin. Trials8(1), 48–60 (2013).
- Manicourt DH , BevilacquaM , RighiniV , FamaeyJP , DevogelaerJP. Comparative effect of nimesulide and ibuprofen on the urinary levels of collagen type II C-telopeptide degradation products and on the serum levels of hyaluronan and matrix metalloproteinases-3 and -13 in patients with flare-up of osteoarthritis. Drugs in R&D6(5), 261–271 (2005).
- Bianchi M , BrogginiM , BalzariniP , FranchiS , SacerdoteP. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int. J. Clin. Pract.61(8), 1270–1277 (2007).
- Pelletier JP , RaynauldJP , CaronJet.al Decrease in serum level of matrix metalloproteinases is predictive of the disease-modifying effect of osteoarthritis drugs assessed by quantitative MRI in patients with knee osteoarthritis. Ann. Rheum. Dis.69(12), 2095–2101 (2010).
- Gallelli L , GalassoO , FalconeDet.al The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthr. Cartil.21(9), 1400–1408 (2013).
- Hess A , AxmannR , RechJet.al Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc. Natl Acad. Sci. USA108(9), 3731–3736 (2011).
- Persson MSM , SarmanovaA , DohertyM , ZhangW. Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology (Oxford)57(10), 1830–1837 (2018).
- Moore RA , ChiCC , WiffenPJ , DerryS , RiceAS. Oral nonsteroidal anti-inflammatory drugs for neuropathic pain. Cochrane Database Syst Rev (10), Cd010902 (2015).
- Wang ZY , ShiSY , LiSJet.al Efficacy and safety of duloxetine on osteoarthritis knee pain: a meta-analysis of randomized controlled trials. Pain Med.16(7), 1373–1385 (2015).
- Osani MC , BannuruRR. Efficacy and safety of duloxetine in osteoarthritis: a systematic review and meta-analysis. Korean J. Intern. Med.34(5), 966–973 (2019).
- Lunn MP , HughesRA , WiffenPJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev (1), Cd007115 (2014).
- Watt FE . Osteoarthritis biomarkers: year in review. Osteoarth. Cartil.26(3), 312–318 (2018).
- Gundogdu G , GundogduK , MilogluFD , TascıSY. A new perspective on the relation between obesity and knee osteoarthritis: omentin. Curr. Rheumatol. Rev. (2019) ( Epub ahead of print) DOI: 10.2174/1573397116666191226122801.
- Poonpet T , HonsawekS. Adipokines: Biomarkers for osteoarthritis?World J. Orthopedics5(3), 319–327 (2014).
- Mosquera A , Rego-PérezI , BlancoFJ , FernándezJL. Leukocyte telomere length in patients with radiographic knee osteoarthritis. Environ. Mol. Mutagen.60(3), 298–301 (2019).
- Griswold AJ , PerezJ , NuytemansKet.al Transcriptomic analysis of synovial extracellular RNA following knee trauma: a pilot study. J. Orthop. Res.36(6), 1659–1665 (2018).
- Catterall JB , StablerTV , FlanneryCR , KrausVB. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res. Ther.12(6), R229 (2010).
