Abstract
Aim: It is argued that chronic pain patients who reduce/eliminate their opioids may have compromised pain relief. This study therefore aimed to analyze if reduced opioid consumption associated with 10-kHz spinal cord stimulation adversely affected pain relief. Methods:Post hoc analysis was performed on data from two prospective studies in subjects with upper limbs and neck pain conducted in USA. Results & conclusion: A 10-kHz spinal cord stimulation treatment was associated with reduction in mean visual analog scale scores for upper limbs and neck pain and mean daily opioid consumption. Pain scores decreased in subjects who decreased opioid use and in those who maintained/increased use. Opioid reduction and pain relief was also achieved in subjects taking >90 mg morphine equivalents of opioids at baseline.
Lay abstract
Weaning off opioids or reducing the dose in patients with chronic pain is believed to increase the pain intensity. The guidance issued by Health and Human Services in 2019 highlighted the need to balance risks and benefits during tapering, and the importance of incorporating opioid-sparing treatments for chronic pain. 10-kHz spinal cord stimulation (SCS) has been previously shown to provide durable pain relief and help in reducing dependence on opioids for pain management. Current study further showed that 10-kHz SCS treatment helped patients with chronic upper limb and neck pain in reducing opioid dependence. More importantly, current study demonstrated that there was no rebound increase in 10-kHz SCS treated patients who reduced/eliminated opioid use.
Keywords::
Chronic pain of the upper limbs and neck (ULN) is relatively prevalent in the general population and is associated with physical impairment in patients [Citation1,Citation2]. Opioid analgesics have commonly been prescribed to control chronic pain once other analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) alone are no longer effective [Citation3]. While opioids are effective at treating acute pain, evidence shows only a minority of patients benefit from long-term opioid treatment [Citation4] and the overall reduction in pain may be small [Citation5]. In addition, long-term opioid use is associated with serious adverse events including pain sensitization, overdose and addictive behavior [Citation6,Citation7]. It is critical to develop opioid-sparing treatment options for chronic pain, including ULN pain.
Spinal cord stimulation (SCS) is a nonpharmacologic treatment that has been successfully used for treating chronic, intractable pain and several prospective studies have shown it can be effective for treating ULN pain [Citation8–10]. However, conventional, low-frequency SCS is paresthesia dependent, and this may preclude the use of this therapy in patients who find the sensation uncomfortable. High-frequency SCS, delivered at a frequency of 10-kilohertz (10-kHz SCS), is a paresthesia independent treatment that has been shown to successfully treat other types of chronic, intractable pain, including low back and leg pain [Citation11–13].
In addition to effective pain relief, 10-kHz SCS has been associated with decreased opioid consumption [Citation14]. Data from clinical trials [Citation11,Citation12,Citation15] and retrospective studies [Citation16–18] have shown that 10-kHz SCS is associated with reduced pain and decreased opioid consumption in all subjects, including those taking high-risk doses (>90-mg morphine equivalents [MME] per day). However, previous studies [Citation11,Citation12,Citation15–18] did not analyze pain relief in a subset of subjects who decreased or eliminated their opioid use. In order to address concerns of physicians and patients on the possibility of rebound increase in pain upon reduction or elimination of opioids and encourage weaning opioids in patients treated with 10-kHz SCS, it is important to show that the pain relief is not compromised in patients who decreased or eliminated their opioid use. Further, it is also important to determine whether 10 kHz SCS has similar pain relieving and opioid-sparing effects in patients with ULN pain because many of these patients are on opioids by the time they seek alternative treatments for chronic pain, including some taking high-risk doses. This post hoc sub-analysis hypothesized that 10-kHz SCS-treated patients who reduce or eliminate use of opioids would have similar pain relief and response rate compared with patients who did not change their opioid dose and who increased their opioid dose. The study used data from two prospective studies of 10-kHz SCS in ULN pain and analyzed pain relief and changes in daily opioid dose following 10 kHz SCS treatment. The study further compared reduction in pain scores and response rate in patients who reduced or eliminated opioids use to patients who did not change or increased their daily opioid dose.
Methods
Study design
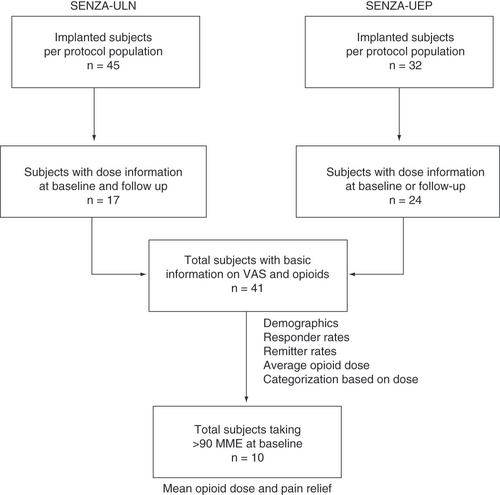
This post hoc analysis was conducted using data from two prospective, multicenter studies of 10-kHz SCS in 1) chronic, refractory pain of the ULN, SENZA-ULN and 2) chronic, refractory upper extremity pain, SENZA-UEP. Both studies collected patient data at baseline and at multiple follow-up visits for 12 months of treatment. For this analysis, patient data from each study was filtered based on the availability of pain scores after 12 months of stimulation as well as data on opioid dose at baseline or 12 months. Patient selection for inclusion in this analysis is shown in . Because the protocols and assessments used in both studies were similar, it was possible to pool data from the studies to increase the number of subjects included in the analysis.
Subjects from the SENZA-ULN and SENZA-UEP studies were selected on the basis of available pain scores and opioid dose information at baseline or after 12 months of stimulation. A total of 41 subjects were included in the current analysis.
MME: Milligram morphine equivalent; VAS: Visual analog scale.
SENZA Upper Limb and Neck study (SENZA-ULN)
This prospective, single-arm multicenter study of 10-kHz SCS in subjects with chronic, intractable pain of the upper limbs or neck, and the protocols are available on ClinicalTrials.gov (NCT02385201) [Citation19]. Study was performed in compliance with US Code of Federal Regulations and recommendations guiding physicians in biomedical research adopted by the 18th World Medical Assembly, Helsinki, Finland. Informed consent forms and study protocols were reviewed and approved by Institutional Review Board prior to the beginning of the study. All patients provided informed consent for data collection including medication for management of pain and for reporting of data. The study included subjects from 6 geographically diverse US centers who had pain intensity scores of ≥5 cm as reported on a 0–10 cm visual analog scale (VAS). In addition, the pain had to be present for at least 3 months and unresponsive to conservative medical treatment. In total, 45 subjects were permanently implanted with a stimulation device (Senza® system, Nevro Corp., CA, USA) and followed up for 12 months.
SENZA Upper Extremity Pain study (SENZA-UEP)
SENZA-UEP was a prospective, post-market, observational study, conducted at five centers in the USA and one in the United Kingdom. The study was registered at ClinicalTrials.gov and the protocols are available there for review (NCT02703818) [Citation20,Citation21]. Study was performed in compliance with US Code of Federal Regulations and recommendations guiding physicians in biomedical research adopted by the 18th World Medical Assembly, Helsinki, Finland. Informed consent forms and study protocols were reviewed and approved by Institutional Review Board (US centers) and Ethics Committee (UK) prior to the beginning of the study. All patients provided informed consent for data collection including medication for management of pain and for reporting of data. All eligible subjects had chronic, intractable pain of the upper extremities and inclusion criteria included VAS scores of ≥5 cm for at least 3 months and pain that did not respond to conventional medical treatment. At the conclusion of the trial, 32 subjects received a permanent implant of the device (Senza® system) and were assessed for 12 months.
Outcomes of interest
Both studies assessed upper limb and neck pain using patient-reported VAS scores ranging from 0 cm (no pain) to 10 cm (worst pain imaginable). Subjects in both source studies were classified as ‘responders’ if they reported pain relief of 50% or more at the follow up assessment, while subjects were considered ‘remitters’ if they had a VAS score of 3.0 cm or less at the follow-up assessment [Citation20]. Finally, opioid use was assessed at baseline and 12 months for all subjects taking opioids daily for management of pain and reported as MME, and changes in opioid use from baseline to follow-up were reported using four categories: eliminated, decreased, no change and increased.
Statistical analysis
Only data from subjects with associated opioid dose information were included in the calculation of mean pain intensity, mean pain relief, responder and remission rates, and mean opioid dose, as well as for the categorization of subjects based on opioid dose. Continuous variables including pain intensity and opioid dose were compared using a two-tailed, paired t-test. Changes in the opioid dose categories from baseline to 12 months were compared using chi-square test with Yates correction. The threshold for statistical significance was defined as p-values of 0.05 or less. Statistical analyses were performed using Excel software (Microsoft, WA, USA).
Results
Patient demographics
In the SENZA-ULN study, a total of 45 subjects with chronic, intractable ULN pain were implanted with a 10-kHz SCS device after a successful trial and completed pain assessments after 12 months of stimulation. As shown in , the study records of 17 of these 45 subjects included information on opioid consumption at baseline and after 12 months of stimulation. The SENZA-UEP study, meanwhile, included 32 subjects who were permanently implanted and provided follow-up pain data after 12 months of stimulation, and records for 24 of those subjects had information on opioid consumption at the initiation of stimulation and after 12 months of therapy [Citation20].
The pooled population of 41 subjects included 25 women (61%) and 16 men (39%) with a median age of 51 years, as shown in . Most subjects had chronic, intractable pain in both the neck and one or both upper limbs. The mean daily opioid dose among all subjects at the initiation of 10-kHz SCS was 73.9 MME, including 10 subjects (24%) who were taking high-dose opioids with a daily intake greater than 90 MME.
Table 1. Baseline demographics and clinical characteristics of subjects from SENZA-ULN and SENZA-UEP included in the analysis.
Outcomes in all subjects
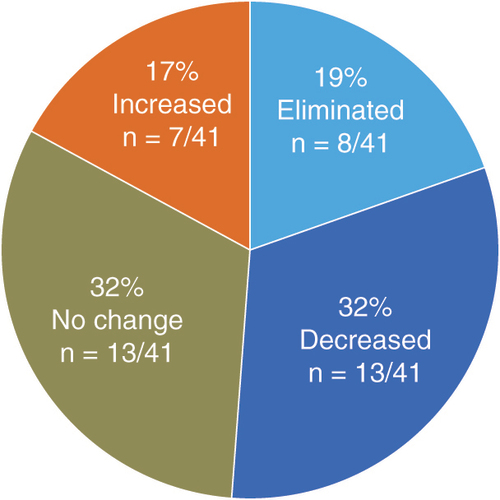
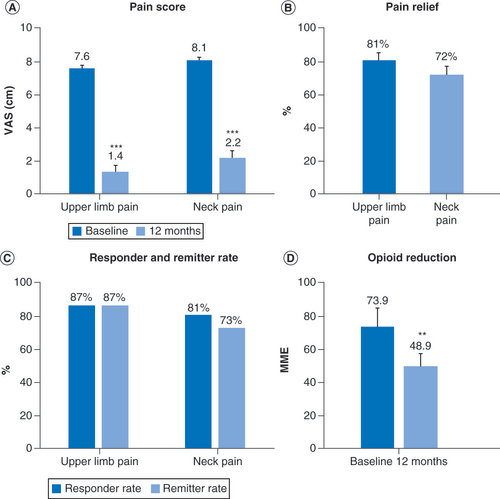
Patient-reported pain scores for upper limb and neck pain decreased significantly (p < 0.001) in the combined overall population following 12 months of treatment with 10-kHz SCS. shows changes in VAS scores, and subjects reported an average 81% reduction in upper limb pain and 72% reduction in neck pain. The proportion of responders, defined as all subjects who reported pain relief of 50% or more following stimulation, were similar for upper limb pain (87%) and neck pain (81%). The remission rate included all subjects who reported VAS scores of 3.0 cm or less at follow up and was 87% for upper limb pain and 71% for neck pain in the overall population. Mean daily opioid dose decreased significantly (p < 0.01) by 34% in the overall population for which medication data was available, from 73.9 MME at baseline to 48.9 MME after 12 months of stimulation (). Individually, 21 subjects (51%) either eliminated or decreased their opioid use over the course of the studies ().
**p < 0.01; ***p < 0.001.
MME: Milligram morphine equivalent; VAS: Visual analog scale.
Outcomes stratified by change in opioid consumption
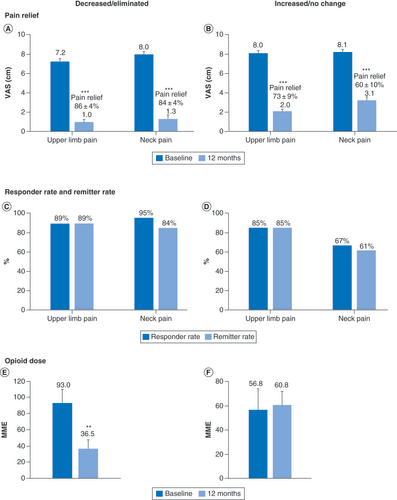
The data were next analyzed after stratifying subjects into two groups: those who decreased or eliminated their opioid use and those who maintained or increased their opioid use. The mean VAS scores for upper limb pain decreased significantly (p < 0.001) by a similar amount in both groups, with a reduction of 6.2 cm in subjects who decreased opioid use and with a reduction of 6.0 cm in those who maintained or increased opioid consumption (). Decreases in VAS scores for neck pain were also similar in the two opioid-use groups, with a reduction of 6.7 cm in subjects who decreased opioid consumption and a reduction of 5.0 cm in those who did not. The proportion of pain relief, responder, and remitter rates were similar in both opioid-use groups for upper limb pain and were slightly lower for neck pain in subjects who maintained or increased their opioid use ().
Pain scores (A & B); Response and remission rates (C & D) and mean daily opioid dose at baseline and 12 months in subjects who decreased or eliminated opioid consumption (E) and in subjects who did not change or increased their opioid consumption (F).
**p < 0.01; ***p < 0.001.
MME: Milligram morphine equivalent; VAS: Visual analog scale.
Outcomes in subjects in high-risk category (>90 MME)
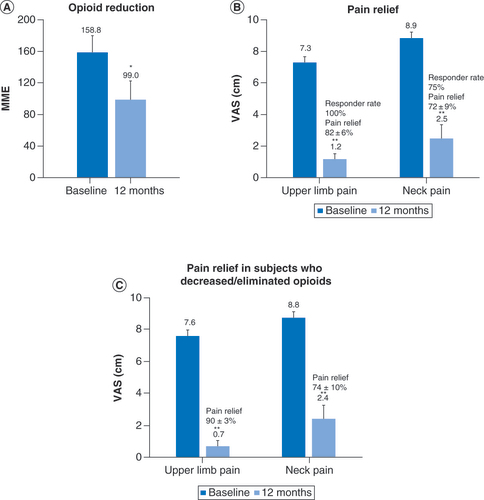
Of the 41 subjects in the pooled population, 10 were taking opioids at high-risk doses of more than 90 MME per day (). At 12 months, seven of the ten subjects in this high-risk group decreased or eliminated their opioid use, and the mean daily dose in this subgroup decreased from 158.8 MME at baseline to 99.0 MME at 12 months, as shown in , a significant decrease in consumption of 37.7% (59.8 MME; p < 0.05). In the entire high-risk subgroup, the mean VAS score significantly (p < 0.01) decreased 6.1 cm for upper limb pain and 6.4 cm for neck pain, while in high-risk subjects who decreased or eliminated opioid use, mean VAS significantly (p < 0.01) decreased 6.9 cm for upper limb pain and 6.4 cm for neck pain. The proportion of pain relief and responder rates in the high-risk group were similar to the overall population.
Subjects with baseline opioid doses of more than 90 mg morphine equivalents per day reduced their mean opioid consumption after 12 months of treatment with 10 kHz spinal cord stimulations (A) and their pain scores were also reduced (B). A similar reduction in pain scores was observed in the subset of subjects taking high-dose opioids who decreased or eliminated opioid (C).
*p < 0.05; **p < 0.01.
MME: Milligram morphine equivalent; VAS: Visual analog scale.
Discussion
The results of this post hoc analysis suggest that the 10-kHz SCS is associated with reduced pain intensity and decreased opioid consumption in subjects with chronic, intractable pain of the ULN. Pain reductions were similar in magnitude to those reported for back and leg pain after 12 months of treatment with 10-kHz SCS in a post hoc analysis of two large studies, SENZA-RCT and SENZA-EU [Citation11,Citation12].
Individually, over 50% of the subjects in the current analysis decreased their use of opioids or eliminated it altogether, while 17% increased their dosage over the 12 months of the study period. These proportions were similar to those reported in the analysis by Al-Kaisy et al., who reported that 53% of subjects decreased or ceased opioid use and 11% increased it [Citation15]. After 12 months of stimulation, our results also show that subjects’ mean opioid consumption decreased by 34% to 49 MME per day, which is below the threshold for increased risk as defined by the US Centers for Disease Control and Prevention (CDC) [Citation22]. The previous analysis in subjects with back and leg pain found higher levels of opioid consumption at baseline, 104 MME, but daily use decreased by a similar proportion of 41% (43 MME) after 12 months of stimulation [Citation15].
The guidance issued by Health and Human Services in 2019 highlighted the dangers of rapid opioid tapering, the need to balance risks and benefits during tapering, and the importance of incorporating opioid-sparing treatments for chronic pain, regardless of whether patients reduce opioid use or not [Citation23,Citation24]. When stratified by change in opioid use, our results showed that pain relief, including responder and remitter rates, were similar regardless of whether subjects decreased their opioid use or not. Pain relief using 10-kHz SCS was, therefore, not compromised in those who decreased or eliminated opioid use in contrast to conventional SCS, which has been associated with worse pain outcomes and increased explant rates in patients with chronic pain who take high doses of opioid analgesics [Citation25,Citation26].
Multiple studies have indicated lower baseline opioid is a predictive factor for reduced opioid consumption in response to alternative therapies including SCS and intrathecal drug delivery. A recent retrospective analysis of insurance claim data in patients who were chronic opioid users before receiving conventional SCS showed that daily opioid doses of less than 65 MME at the start of SCS treatment were predictive of reduced opioid use in response to stimulation therapy [Citation27]. A single-center retrospective study of patients who received low-frequency SCS for refractory neuropathic pain similarly reported that baseline opioid use of ≤30 MME daily was predictive of opioid cessation and that most responders (≥50% pain relief) did not reduce opioid use over the course of the study [Citation28]. Likewise, a retrospective study in over 631 patients who received intrathecal drug administration for chronic, noncancer pain also found that baseline systemic opioid dose was strongly correlated with whether patients would discontinue opioid use during the following year, with those taking less than 50 MME being twofold more likely to stop opioid use than those taking 90 MME or more [Citation29].
In contrast, the present data show that 10-kHz SCS was associated with reduced opioid use in subjects with ULN pain taking more than 90 MME at baseline, and the mean daily opioid dose decreased by 38% (60 MME), which was similar to the 34% (25 MME) decrease in the mean dose of all subjects. These results are in agreement with those from SENZA-RCT and SENZA-EU, which showed that 10 kHz SCS was associated with reduced opioid consumption in subjects with low back and leg pain, including those taking high-risk doses [Citation15]. Other retrospective studies have, likewise, shown that 10-kHz SCS is associated with decreased opioid consumption with similar effects regardless of baseline opioids use [Citation16,Citation17]. Finally, a retrospective study of 23 consecutive patients who underwent 10-kHz SCS for ULN pain reported a 56% reduction in opioid dose in a population taking over 1000 MME per day at the start of treatment [Citation30]. The results of our analysis is in agreement with this body of data and suggest that 10-kHz SCS was associated with reduced opioid use in subjects with ULN pain taking both high- and low-dose opioids.
More importantly, pain relief was not compromised in subjects taking high doses of opioids, and these add to the body of evidence that the opioid-sparing effects of 10-kHz SCS are independent of baseline opioid consumption. An important unanswered question for future studies regards to the ability of counseling and behavioral support to increase the opioid-sparing effects of 10-kHz SCS. The current patient population were not actively encouraged or supported in decreasing opioid use, so it is reasonable to hypothesize that making active support for opioid tapering accessible to patients along with 10-kHz SCS might improve the results in terms of reducing opioid consumption.
This study was primarily limited by the design of SENZA-ULN and SENZA-UEP, which were not designed to investigate the safety and efficacy of 10-kHz SCS in reducing opioid use in this population and did not exclusively recruit subjects taking opioids at baseline and include a opioid reduction protocol as part of the study design. Collection of opioid usage information was observational in all subjects and any voluntary changes in opioid dose during the study were recorded. The analysis is post hoc in nature and it lacked a prospectively defined statistical analysis plan for opioid analysis. Finally, the number of subjects in the combined dataset used in this analysis was small, with 41 subjects including 10 in the high-risk subgroup, which reduces the precision of the results obtained. Additional studies with appropriately powered sample size and design could help to validate these findings in this condition.
Conclusion
These results add to a growing body of evidence supporting 10-kHz SCS as a safe and effective nonpharmacologic alternative for treating chronic, refractory pain of several types, including upper limb and neck pain. The results of this study suggest that subjects taking high-dose opioids (>90 MME) benefit from the pain relieving and opioid-sparing effects of 10-kHz SCS as well as subjects taking lower doses. The amount of pain relief from 10-kHz SCS was also not dependent on reducing opioid consumption during the study, indicating that patients unable or unwilling to reduce opioid use still benefited from high-frequency stimulation. 10-kHz SCS is a promising treatment modality for patients with chronic, intractable upper limb and neck pain, and further research in this patient population is warranted.
Nonopioid alternatives are recommended for the management of chronic noncancer pain but it is argued that pain relief could be compromised in patients who reduce/eliminate opioids.
A 10-kHz spinal cord stimulation (10-kHz SCS) has been shown to have pain relieving and opioid sparing effects in subjects with chronic pain.
Main objective of the study was to analyze pain relief in patients who reduced or eliminated their opioids after 10-kHz SCS treatment.
Data from two prospective studies in subjects with upper limbs and neck (ULN) pain conducted in the USA were used for the analysis.
Results showed that patients receiving 10-kHz SCS treatment were able to reduce their dependence on opioids for management of upper limb and neck pain.
Pain scores decreased in subjects who decreased opioid use during the study as well as in those who maintained or increased use.
Pain scores were decreased even in subjects taking >90 mg morphine equivalents of opioids and 70% of the subjects either reduced or eliminated opioids.
In conclusion, 10-kHz SCS demonstrated pain relieving and opioid sparing effects in subjects with chronic, intractable ULN pain including in subjects taking high-risk (>90 mg morphine equivalents) doses of opioids.
Author contributions
Authors K Amirdelfan, R Vallejo, R Benyamin, S Rosen, P Kosek and A Burgher were involved in data collection, analysis and writing of the manuscript. D Caraway was involved in design, conceptualization and writing of the manuscript. AR was responsible for analysis, design, conceptualization and working with medical writer in drafting the manuscript. All the authors have reviewed and approved the final version of this manuscript.
Ethical conduct of research
Study was performed in compliance with US Code of Federal Regulations and recommendations guiding physicians in biomedical research adopted by the 18th World Medical Assembly, Helsinki, Finland. Informed consent forms and study protocols were reviewed and approved by Institutional Review Board prior to the beginning of the study. All patients provided informed consent for data collection including medication for management of pain and for reporting of data.
Acknowledgments
Authors thank E MacLaren, Galen Medical Writing LLC, for drafting the manuscript and M Bhandaru, for preparing the illustrations for the manuscript.
Financial & competing interests disclosure
This study was funded by Nevro Corp. K Amirdelfan, R Benyamin and S Rosen are consultants of Nevro Corp. D Caraway and A Rotte are employees of Nevro Corp. R Vallejo is an employee of Stimgenics and a consultant for Medtronic and Avanos. P Kosek received research grants from Boston Scientific and Nevro Corp. A Burgher has no conflicts to disclose. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
E MacLaren, Galen Medical Writing LLC, drafted the manuscript, M Bhandaru, prepared the illustrations and Nevro Corp. funded the production of this manuscript.
Additional information
Funding
References
- Bot SD , VanDer Waal JM , TerweeCBet al. Incidence and prevalence of complaints of the neck and upper extremity in general practice. Ann. Rheum. Dis.64(1), 118–123 (2005).
- Gummesson C , AtroshiI , EkdahlC , JohnssonR , OrnsteinE. Chronic upper extremity pain and co-occurring symptoms in a general population. Arthritis Rheum.49(5), 697–702 (2003).
- Hylands-White N , DuarteRV , RaphaelJH. An overview of treatment approaches for chronic pain management. Rheumatol. Int.37(1), 29–42 (2017).
- Kalso E , EdwardsJE , MooreRA , McquayHJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain112(3), 372–380 (2004).
- Busse JW , WangL , KamaleldinMet al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA320(23), 2448–2460 (2018).
- Edlund MJ , MartinBC , RussoJE , DevriesA , BradenJB , SullivanMD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin. J. Pain30(7), 557–564 (2014).
- Tompkins DA , CampbellCM. Opioid-induced hyperalgesia: clinically relevant or extraneous research phenomenon?Curr. Pain Headache Rep.15(2), 129–136 (2011).
- Deer TR , MekhailN , ProvenzanoDet al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation17(6), 515–550 (2014).
- Haider S , Owusu-SarpongS , PerisCelda Met al. A single center prospective observational study of outcomes with tonic cervical spinal cord stimulation. Neuromodulation20(3), 263–268 (2017).
- Levine AB , ParrentAG , MacdougallKW. Cervical spinal cord and dorsal nerve root stimulation for neuropathic upper limb pain. Can. J. Neurol. Sci.44(1), 83–89 (2017).
- Al-Kaisy A , Van BuytenJP , SmetI , PalmisaniS , PangD , SmithT. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med.15(3), 347–354 (2014).
- Kapural L , YuC , DoustMWet al. Novel 10-kHz high-frequency therapy (HF10 Therapy) Is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology123(4), 851–860 (2015).
- Sayed D , KallewaardJW , RotteA , JamesonJ , CarawayD. Pain relief and improvement in quality of life with 10 kHz SCS therapy: summary of clinical evidence. CNS Neurosci. Ther.26(4), 403–415 (2020).
- Al-Kaisy A , Van BuytenJP , AmirdelfanKet al. Opioid-sparing effects of 10 kHz spinal cord stimulation: a review of clinical evidence. Ann. NY Acad. Sci.1462(1), 53–64 (2020).
- Al-Kaisy A , Van BuytenJP , CarganilloRet al. 10 kHz SCS therapy for chronic pain, effects on opioid usage: post hoc analysis of data from two prospective studies. Sci. Rep.9(1), 11441 (2019).
- Dibenedetto DJ , WawrzyniakKM , SchatmanME , KulichRJ , FinkelmanM. 10 kHz spinal cord stimulation: a retrospective analysis of real-world data from a community-based, interdisciplinary pain facility. J. Pain Res.11, 2929–2941 (2018).
- Salmon J . High-frequency spinal cord stimulation at 10 kHz for widespread pain: a retrospective survey of outcomes from combined cervical and thoracic electrode placements. Postgrad. Med.131(3), 230–238 (2019).
- Sills S . Treatment of painful polyneuropathies of diabetic and other origins with 10 kHz SCS: a case series. Postgrad. Med. doi:10.1080/00325481.2020.1732065 (2020).
- Amirdelfan K , VallejoR , BenyaminRet al. High-frequency spinal cord stimulation at 10 kHz for the treatment of combined neck and arm pain: results from a prospective multicenter study. Neurosurgery87(2), 176–185 (2020).
- Burgher A , KosekP , SurrettSet al. Spinal cord stimulation at 10 kHz for the treatment of chronic pain of the upper extremities: results of a prospective, multicenter, post-market, observational study [abstr]. North American Neuromodulation Society Annual Meeting 2019 (2019).
- Burgher A , KosekP , SurrettSet al. 10kHz SCS for treatment of chronic pain of the upper extremities: a post-market observational study. J. Pain Res.13, 2837–2851 (2020).
- Dowell D , HaegerichTM , ChouR. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA315(15), 1624–1645 (2016).
- Dowell D , ComptonWM , GiroirBP. Patient-Centered reduction or discontinuation of long-term opioid analgesics: the HHS guide for clinicians. JAMA doi:10.1001/jama.2019.164091–3 (2019).
- U.S. Department of Health and Human Services . HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics. (2019).
- Gee L , SmithHC , Ghulam-JelaniZet al. Spinal Cord stimulation for the treatment of chronic pain reduces opioid use and results in superior clinical outcomes when used without opioids. Neurosurgery84(1), 217–226 (2019).
- Sharan AD , RileyJ , FalowskiSet al. Association of Opioid usage with spinal cord stimulation outcomes. Pain Med.19(4), 699–707 (2018).
- Dougherty MC , WoodroffeRW , WilsonS , GillesGT , HowardMA3rd , CarnahanRM. Predictors of reduced opioid use with spinal cord stimulation in patients with chronic opioid use. Neuromodulation doi:10.1111/ner.13054 (2019).
- Simopoulos T , SharmaS , WoottonRJ , OrhurhuV , AnerM , GillJS. Discontinuation of chronic opiate therapy after successful spinal cord stimulation is highly dependent upon the daily opioid dose. Pain Pract.19(8), 794–799 (2019).
- Hatheway JA , BansalM , Nichols-RickerCI. Systemic opioid reduction and discontinuation following implantation of intrathecal drug-delivery systems for chronic pain: a retrospective cohort analysis. Neuromodulation doi:10.1111/ner.13053 (2019).
- El Majdoub F , NeudorferC , RichterR , SchieferdeckerS , MaaroufM. 10 kHz cervical SCS for chronic neck and upper limb pain: 12 months’ results. Ann. Clin. Transl. Neurol.6(11), 2223–2229 (2019).