Abstract
Aims: Interventional pain treatments range from injections to established radiofrequency ablation techniques and finally neuromodulation. In addition to safety, efficacy and cost dominance, patient preference for type of treatment is important. Methods: Chronic pain patients (n = 129) completed a preference scale to determine which interventional pain management procedures they would prefer from among radiofrequency ablation, temporary (60-day) peripheral nerve stimulation (PNS), conventional PNS and spinal cord stimulation/dorsal root ganglion stimulation. A second survey (n = 347) specific to assessing the preference for radiofrequency ablation or temporary PNS treatment was completed by patients with low back pain. Results: On the basis of mean rank, temporary PNS percutaneously implanted for up to 60 days was the most preferred treatment compared with the other options presented (p = 0.002). Conclusions: Patient preference should be unbiased and considered as an independent variable for physician discussion in treatment options and future research.
Lay abstract
Patient preference is an important variable for physicians to consider when discussing treatment options for low back pain. A consumer survey study was completed discussing patient preference among various invasive treatments for low back pain. When given scenarios discussing risks and benefits of each procedure (temporary peripheral nerve stimulation for 60 days, heat ablation of small back nerves and permanently implanted back pain devices) temporary peripheral nerve stimulation was considered the preferred option.
That patient preference should play a role in pain treatment-related decision-making is paramount to the concept of patient-centric care in which individual patient preferences, needs and values guide all clinical decisions [Citation1,Citation2]. In fact, the use of quantitative patient preference data is increasingly being considered or proposed by health technology assessment agencies in many European countries [Citation3]. Multiple new interventional pain management therapies have emerged over the past several years including, but not limited to, high frequency and burst spinal cord stimulation [Citation4–7], dorsal root ganglion stimulation [Citation8,Citation9], cooled radiofrequency ablation [Citation10,Citation11], and temporary and permanent percutaneously implanted peripheral nerve stimulation (PNS) [Citation12,Citation13]. While the data in support of these therapies is growing at a rapid pace [Citation14–21], insights into patient-preferences that may impact physician guidance and choice of treatment have not yet been characterized.
To our knowledge, patient preference for choosing various neuroablative and neuromodulation treatments for chronic pain has not been discussed in the literature. To that end, a survey research project was initiated to help characterize patient insights into multiple interventional pain management treatment choices. The purpose of this project was to characterize patient preferences from among several interventional pain management treatment options to include radiofrequency ablation, temporary 60-day PNS treatment, permanently implanted PNS systems and permanently implanted dorsal root ganglion/spinal cord stimulation systems and for practitioners to understand how treatment-related details and nuances can influence these preferences.
Methods
Survey 1
Between September 2019 and October 2019, 129 panelists with chronic pain completed an online survey. A total of 2402 panelists aged 34–75 years were recruited from the American Consumer Opinion panel (a marketing research firm owned by Decision Analyst designed to research and optimize consumer experiences) after confirming the self-reported presence of moderate to severe pain (pain >4 on a 0–10-point scale) of at least 6 months’ duration that prevented participation in activities they enjoyed. On the basis of a set of screener questions, 154 panelists reported pain to be present only in the axial back (it did not radiate into their legs); had sought the care of a medical doctor specializing in pain, orthopedic surgery, spine surgery or neurosurgery; had completed physical therapy; had not completed an interventional back treatment (including radiofrequency ablation [RFA]); and reported health insurance to be in place. Answering all questions in the online survey was mandatory, and responses were deemed theoretical; as such, only the responses of those who provided a complete data set were included in the analysis. Of the eligible panelists, 129 completed the full survey and are included in the present analysis.
This study was determined to be exempt from institutional review board (IRB) approval per 45 CFR 46.101(b) (Categories of Exempt Human Subjects Research) based on its use of anonymized survey procedures [Citation22]. A medical ethicist reviewed and constructed the questions to minimize the internal bias in the questions that were presented to the participants.
Serial ranking of three peripheral-nerve-oriented interventional pain management pain therapies was conducted: RFA, temporary neurostimulation and permanently implanted neurostimulation. The layperson definitions used to describe each therapy within the online survey were as follows:
RFA is a treatment that uses the tip of a needle to burn or ‘ablate’ the nerve in an attempt to stop the pain signal. The procedure is performed via a series of four to six needle sticks and is performed with the patient asleep (under conscious sedation). A small needle is inserted very close to a nerve under image guidance. The target nerve(s) carrying the pain signal are burned (destroyed). Fifty percent of patients who receive RFA obtain relief of 50% of their pain or more for 6–12 months and often longer. In most patients the nerve grows back, and the procedure may need to be repeated.
Temporary neurostimulation is a treatment that uses an implanted leadwire connected to a wearable matchbox-sized stimulator to send mild electrical pulses to a specific nerve to interrupt the pain signal. The leadwire is implanted under local anesthesia through a single needle stick and placed approximately one-half inch away from the nerve. The leadwire is about the size of a human hair and connects to a wearable matchbox-sized stimulator. The site where the leadwire exits the skin is covered by a waterproof bandage. Stimulation intensity can be adjusted using a handheld remote. The leadwire is withdrawn in the doctor’s office after 60 days. Fifty percent of patients who receive temporary neurostimulation obtain relief of 50% or more of their pain for 6–12 months and often longer. If pain returns, the procedure can be repeated or advanced to a permanent implant for patients who obtain pain relief.
Permanently implanted neurostimulation is a treatment that uses a wearable matchbox-sized stimulator and an implanted leadwire to send mild electrical pulses to the nerves to interrupt the pain signal. A leadwire about the diameter of a piece of spaghetti is positioned very closely to a nerve. The leadwire is implanted through two small incisions. A wearable matchbox-sized stimulator delivers energy to the leadwire through the skin. Fifty percent of patients obtain significant pain relief while the device is implanted. The permanently implanted leadwire may be explanted (surgically removed) if not beneficial.
After ranking each of these peripheral-nerve-related therapies, respondents were also asked to consider their interest in a ‘permanently implanted neurostimulation treatment that uses a pacemaker-like device connected to a spaghetti-sized leadwire implanted near your spinal cord to send mild electrical pulses directly to the spinal cord to manage your pain.’ This definition was intended to be used as a proxy description to assess relative interest in spinal cord stimulation (SCS)/dorsal root ganglion stimulation (DRGS)-based therapies.
After each respondent ranked the four treatment options, a follow-on question in regard to potential therapy risks was presented to those with axial low back pain who selected temporary stimulation or RFA as their most-desirable choice. For those who chose temporary stimulation as most desirable, the following additional information was provided:
Temporary neurostimulation follow-on question: ‘In 10% of patients who receive temporary neurostimulation, a piece of the hairlike wire [leadwire] remains in the body when the physician withdraws it. If a small piece does remain in the body, MRI is still possible. There is an extremely low risk that this remnant would cause any complications, but in rare cases an incision may be required to remove the remnant. Note that the remnant is generally much smaller than a commonly used surgical staple.’
Respondents were asked to select one of the following options in response to the additional information:
○ This information does not change my preference for temporary neurostimulation compared with the other options.
○ I would opt to have RFA instead.
○ I would opt to have a device permanently implanted instead.
RFA follow-on question: For those with back pain who ranked RFA higher than temporary neurostimulation, the following additional information was provided: ‘When performed in the lower back, one side effect of RFA is that some core lower back muscles can be temporarily paralyzed until the nerve grows back over the next several months.’
Respondents were asked to select one of the following options in response to the additional information:
| ○ | This information does not change my preference for RFA compared to the other options. | ||||
| ○ | I would opt to have the temporary nerve stimulator for 60 days instead. | ||||
| ○ | I would opt to have a device permanently implanted instead. | ||||
Survey respondents were also asked to characterize the primary benefit they ascribed to temporary PNS via the question ‘What would you consider to be the best outcome with this temporary (60-day) nerve-stimulation device?’ Their response options included the following:
| ○ | The potential for pain relief without the need for a permanently implanted system. | ||||
| ○ | Information regarding whether a permanently implanted system could be helpful. | ||||
For clarity in the remainder of the report on Survey 1, temporary neurostimulation is referred to as ‘temporary PNS,’ permanently implanted neurostimulation is referred to as ‘permanently implanted PNS’ and permanently implanted neurostimulation that uses a pacemaker-like device connected to a spaghetti-sized leadwire implanted near the spinal cord is referred to as ‘permanently implanted SCS/DRGS.’
The mean rank for each treatment option was calculated and compared between treatment options using a nonparametric Mann-Whitney U-test with post hoc Bonferroni correction for multiple comparisons. The distributions of subjects that chose to continue with their first choice, switch to the other therapy or switch to neither option after being provided additional information were compared using a two × three chi-square test. The proportions of low back pain subjects that selected stimulation, ablation and neither as their final choice were compared using a goodness-of-fit test with post hoc Bonferroni correction for multiple pairwise comparisons.
Survey 2
A subsequent online survey was completed by 347 individuals with moderate to severe low back pain who were identified using the same online recruitment and screening methodology as in Survey 1. The descriptions of stimulation (temporary PNS) and ablation (RFA) were reviewed for balance by all study authors, including experts in pain medicine and medical ethics, before survey administration. Survey 2 was also determined to be exempt from IRB review [Citation22].
Subjects were asked to select their first-choice treatment option, or select neither option, based on the following treatment descriptions:
STIMULATION involves the temporary placement of two hairlike wires (or ‘leads’) using local anesthetics. The leads deliver microdoses of electrical energy to stimulate the nerves that control the back muscles. Stimulation causes the muscles in the low back to comfortably contract while you go about your normal daily routine. It is nondestructive. The leads are covered by a bandage and connected to a small wearable stimulator. Showering is permitted but immersion should be avoided. The leads are withdrawn in your physician’s office after the 60-day treatment period.
ABLATION involves the use of electrical energy to disrupt the nerve. The procedure is typically performed with sedation or local anesthesia. The nerve typically regrows and eventually heals and the procedure may be repeated when necessary.
Follow-on question for those who chose ABLATION as their first choice treatment option:
Please consider the additional details below regarding ablation. With ablation, one of the effects of disrupting the nerve is that impulses which would normally cause the low back muscles to contract and stabilize the spine are temporarily unable to function. Their ability to function again is generally restored after the nerve regrows. Given this additional information, please select your preference for ablation, stimulation or neither.
Follow-on question for those who chose STIMULATION as their first choice treatment option:
Please consider the additional details below regarding stimulation: With stimulation, a small piece of the hairlike lead may remain under the skin in 5–10% of cases when the physician withdraws it. There are no known safety concerns related to the presence of a small remnant. Given this additional information please select your preference for ablation, stimulation or neither.
The proportions of subjects that selected stimulation, ablation and neither were compared using a goodness of fit test with post hoc Bonferroni correction for multiple pairwise comparisons. The distributions of subjects that chose to continue with their first choice, switch to the other therapy or switch to neither option after being provided additional information were compared using a two × three chi-square test. Chi-square tests of independence were used to determine whether first-choice therapy was independent from several baseline variables, including sex, age, duration of low back pain and severity of low back pain.
Results
Survey 1
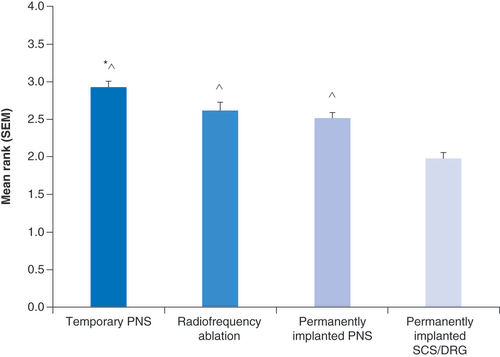
Treatment preferences were collected from 129 axial back pain patients who had moderate to severe pain of at least 6 months duration; 65% of respondents were female, and 45% were younger than 60 years. Treatment options were compared by rank using values from 4 (most desired) to 1 (least desired) (). The mean rank (mean ± SEM) for each group was compared to each other group using a nonparametric Mann-Whitney U-test with post hoc correction for multiple comparisons (). In particular the mean rank of the temporary PNS treatment (2.91 ± 0.09, n = 129) was not significantly different from the mean rank for RFA (2.61 ± 0.11, p = 0.12) but was significantly greater than permanently implanted PNS (2.50 ± 0.08, p = 0.0008), and permanently implanted SCS/DRGS (1.97 ± 0.09, p < 0.0001). The mean ranks for RFA and permanently implanted PNS were also significantly greater (p ≤ 0.0001) compared with permanently implanted SCS/DRGS.
PNS: Peripheral nerve stimulation; SCS/DRG: Spinal cord stimulation/dorsal root ganglion stimulation.
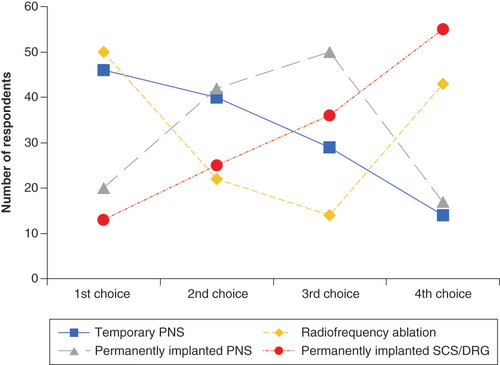
*p = 0.0008 versus permanently implanted PNS. ∧p ≤ 0.0001 versus permanently implanted SCS/DRGS.
PNS: Peripheral nerve stimulation; SCS/DRG: Spinal cord stimulation/dorsal root ganglion stimulation; SEM: Standard error of the mean.
LBP patient follow-on questions
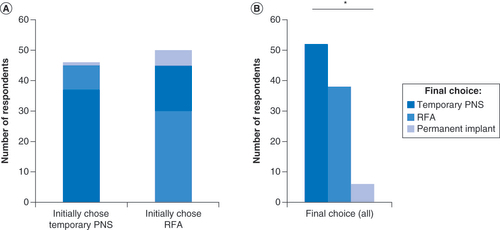
After learning of the proposed 10% chance that a remnant of the hairlike lead may remain following lead removal, 37 of the 46 (80%) respondents with low back pain who ranked temporary PNS as their most desired therapy option retained temporary PNS as their treatment of choice ( & ). Of the remainder who were moved to change, 17% (8 of 46) opted for RFA, and 2% (1 of 46) opted for implantation of a permanent device () (p = 0.068 vs. those who initially selected RFA).
Table 1. Treatment preferences among patients with lower back pain before and after follow-on details about temporary PNS and RFA.
Table 2. Treatment preferences after follow-on details.
Table 3. Demographic information for survey 2 respondents (n = 347).
After learning of the potential for RFA therapy to temporarily denervate the multifidi, of the 50 respondents with low back pain previously ranking RFA as their treatment of choice, 60% (30 of 50) retained RFA as their most desired option, 30% (15 of 50) opted for temporary PNS and 10% (5 of 50) chose implantation of a permanent device ( & ). These changes represented a net increase in the number of respondents choosing temporary PNS as their first choice, and the observed final distribution differed significantly from an expected even distribution (p < 0.0001) ().
(A) Initial and final choices of most desired treatment option among low back pain patients in Survey 1, stratified by their initial choice of Temporary PNS or RFA. Final choices reflect changes after additional information was provided about a potential side effect of each treatment option. p = 0.068. (B) Final choices of all low back pain patients who initially chose temporary PNS or RFA.
*p < 0.0001.
PNS: Peripheral nerve stimulation; RFA: Radiofrequency ablation.
Temporary PNS follow-on questions
Of the subjects asked to characterize the primary benefit they ascribed to temporary PNS, 65% were interested in the ability of the temporary (60-day) PNS system to help them avoid a permanent implant, and 35% were interested in obtaining information regarding the potential for a permanently implanted system to be beneficial.
Survey 2
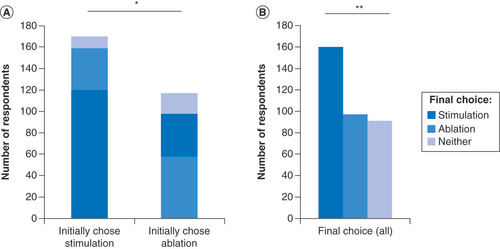
Treatment preferences were collected from 347 respondents with low back pain (). Following the preliminary treatment descriptions, 48.7% of respondents (n = 169) selected stimulation as their first-choice treatment option, 33.7% (n = 117) selected ablation and 17.6% (n = 61) selected neither. These observed proportions differed significantly from an expected equal distribution in a goodness-of-fit test (p = 0.002). Each group was significantly different from each other (p < 0.0001) based on post hoc pairwise tests that were adjusted for multiple comparisons.
Following the provision of additional details about each therapy, 70% (n = 119) of those that selected stimulation and 50% (n = 58) of those that selected ablation continued with their first choice; the remainder switched treatment preferences or switched to desire neither therapy (). The distributions of respondents continuing with their first choice, switching preferences or desiring neither option were significantly different between the stimulation and ablation groups (p = 0.0008), with a greater proportion of respondents choosing to remain with stimulation as their first choice (). In total, after being given the option to revise their treatment preference following the presentation of additional information, 159 respondents (45.8%) preferred stimulation, 97 (28.0%) preferred ablation and 91 (26.2%) preferred neither (p < 0.0001) (). Of those who desired to advance to treatment, 62% preferred stimulation and 38% preferred ablation.
Table 4. Treatment preference among respondents with low back pain after follow-on details.
(A) Initial and final choices of most desired treatment option among low back pain patients in Survey 2, stratified by their initial choice of stimulation or ablation. Final choices reflect changes after additional information was provided about a potential side effect of each treatment option. (B) Final choices of all low back pain patients who initially chose stimulation or ablation.
*p = 0.0007; **p = 0.0001.
Respondents’ initial and final treatment preferences were independent of age, sex, duration of low back pain and severity of low back pain.
Discussion
This is the first study of its kind assessing patient preference among various interventional pain management options for the treatment of axial back pain without radiculopathy. Outcomes infer that treatment options that avoid permanent implantation are more desirable to pain patients, with 74% of first-choice treatment preferences being for temporary PNS or RFA, the two options that do not involve a permanently implanted system. In addition to preferring to avoid a permanent implant where possible, there was also a preference for stimulation-based versus ablative therapies. RFA became less attractive among the respondents with low back pain in both surveys upon learning of the potential for transient core muscle (multifidus) denervation after treatment ( & ). It is important to note that patients were not necessarily approached in physician clinics, and thus patient preference may not correlate with physician preference in the treatment discussion.
Although no published reports of long-term sequelae following RFA-related denervation have been identified, RFA may be associated with recurrent low back pain [Citation23–29]. In comparing activation of the multifidus in healthy versus RFA or posterior lumbar fusion patients, there was decreased muscle contraction of multifidus in patients following both RFA and posterior lumbar fusion [Citation30]. Multifidus atrophy produced by RFA can be objectively measured through radiographic cross-sectional areas, and statistically significant reductions in size have been demonstrated following RFA [Citation31,Citation32]. This concern may be explored in a discussion with patients along with consideration of conservative therapies and physical therapy before RFA [Citation33].
Additionally, low back pain appears to be associated with differential changes in muscle recruitment and corticomotor excitability, as evidenced by lumbar paraspinal motor evoked potentials in response to transcranial magnetic stimulation [Citation34]. Given the foregoing data, it is reasonable to discuss the potential effects of muscle denervation resulting from RFA of the dorsal ramus or medial branch nerve for the treatment of axial low back pain. Further, recent clinical data indicate that stimulation of the medial branch resulting in activation of the multifidus may play an important role in the management of low back pain [Citation17,Citation35].
Similarly, risks of temporary PNS for the treatment of axial back pain should be discussed when patients are offered the two choices for pain control. Although lead fracture and risk of infection are low, this should be balanced with the risks for RFA and denervation of the axial muscles. The present data suggest that patients may have a preference based on the presentation of both procedures that includes discussion of the risks of the procedures. In this study, temporary PNS was preferred over RFA when both risk and benefits are discussed in an unbiased manner.
Understanding risks and benefits of procedures is a critical component of informed consent, but the presentation of this information without bias is of utmost importance. The current study suggests that patients should be made aware of risks associated with a variety of interventional options including sequelae of procedures, the possibility that permanent implants may not be effective, and the potential for a lead remnant when considering temporary or permanently implanted PNS [Citation36]. For this study, care was taken in constructing the questions with a medical ethicist in an attempt to reduce bias. It may be incumbent on on our speciality to develop guidelines in presenting interventional pain procedures to patients in a consistent and unbiased manner. One risk in this process is the inability of every practitioner to provide each option for axial back pain treatment. As such, various techniques may not be accessible to all patients. In any case, we recommend that practitioners still provide reasonable alternative treatment options, each of which includes full disclosure, allowing patients to make adequately informed decisions.
Respondents indicated that their primary desires in pursuing temporary (60-day) PNS may be varied. For example, most patients (65%) reported being more interested in the potential for long-term pain relief offered by the short-term PNS treatment, and others may be more motivated by the notion that a permanently implanted option is also available if pain returns following the 60-day treatment. In other words, some patients may be attracted to the idea that a permanent implant may be obviated following the 60-day treatment period, whereas others may be more attracted to the idea that a permanent system may be validated. In either case, the patient’s preference should be understood and the potential for adverse events should be discussed with the patient.
Procedural efficacy is a paramount part of the patient discussion when considering competing treatment options. PNS and RFA have reasonable evidence in the literature, although as of this publication, no head-to-head trial has been performed. The decision as to whether a patient fits into a particular trial’s inclusion and exclusion criteria to determine therapeutic efficacy is physician dependent. A patient may not possess the discernment to conclude efficacy, at which time the individual physician’s experience may be added to the discussion. The goal of the present study was to minimize bias to determine qualities for patient preference among competing therapies for low back pain. Efficacy could also be considered an indeterminate variable which changes with experience. For example, if a physician has not performed PNS for low back pain, the inherent bias will understandably be towards RFA. In the end, therapies were presented as they exist to determine patient preference.
Of note, the mechanisms of action for temporary PNS may be related to the direct activation of sensory afferents and reduction of the spinothalamic tract’s activity (the gate control theory); it is also reasonable to discuss the effect of medial branch stimulation and the resultant tension produced in the multifidis, resulting in the indirect activation of afferent and proprioceptive fibers that may affect CNS changes, resulting in pain relief [Citation37]. Temporary and permanent PNS systems may achieve the direct and indirect activation of sensory afferents with waveform techniques such as choosing higher (40–150 Hz) and lower (2–20 Hz) frequencies, respectively [Citation38]. This concept may be an interesting future study as frequency choice, in addition to anatomic nerve targeting, does seem to have an impact on resultant pain relief, especially considering relief that endures after stimulation has been discontinued.
There are several limitations with this study. First, although attempts were made to characterize each therapy accurately, the highly variable reported rates of therapy-specific outcomes made it difficult to provide specific data to accompany the descriptions. More detailed differentiation outside of device and procedure-related attributes including higher or lower therapy-specific efficacy outcomes may have produced different results. The ethical principle of beneficence dictates that a given therapy or intervention be employed in a fashion where the most good is probable to be carried out. This is done in conjunction with the principle of nonmaleficence, which suggests that regard be taken for potential harm and that this risk be minimized in concert with the choice of the given intervention.
Additionally, although the rate of retreatment is quite well understood in regard to RFA, the rate of retreatment following temporary (60-day) PNS or its conversion to a permanently implanted PNS system has not yet been characterized. Finally, although efforts were made to describe the therapy options factually, the language used in describing the adverse events may have affected patient responses, and different word choices may have resulted in variable patient responses.
Presenting options to the patient
There are multiple interventional pain management options available to treat pain after initial, less invasive attempts have not produced durable relief. Each option includes pros and cons that should be clearly articulated and understood by the patient while indicating that the success of any specific intervention is difficult to predict. Healthcare providers and their patients should jointly consider which treatment option is preferred.
Since deploying the initial survey, new information regarding lead fracture and surgical removal of permanently implanted PNS systems has been presented [Citation39]. Although this particular characteristic was not included or assessed as part of the survey information of this nature should also be considered by clinicians when describing treatment attributes and helping patients discern treatment choices in light of full disclosure of the risks and benefits.
Developing studies to evaluate patient preferences is critical to understanding a physicians influence during patient care. Techniques to validate preference questions such as the treatment acceptability and preferences measure will support study conclusions [Citation40]. Our group had internal validations with an ethicist to provide a decision on internal validity. Although patient preference studies are critical, no consensus exists in how to execute questionnaires. Bell et al. described a randomized controlled trial in which one study arm uses patient preferences to determine the treatment, whereas the other arm is determined by physicians [Citation41]. This study design introduces patient control on treatments as an outcome measure and perhaps this is the future of pain studies in general.
Conclusion
The demographics seemed as an independent variable. Practitioners may use demographic data to choose a specific device versus RFA such as the ability to manage a PNS system. However, the design of the study was meant to be more general and focus on consumer preference independent of physician preference. With each new pain management intervention comes the physician challenge of characterizing each option in a manner that affords the patient the opportunity to optimally participate in their care. This research highlights some of the areas in which the patient-centered physician can help the patient participate in making the most informed decision. Further research assessing the importance of verbal descriptors may be beneficial when seeking to help patients evaluate their interventional pain management options.
Patient preference between the use of temporary peripheral nerve stimulation (PNS) and thermal ablation of the medial branch nerve yields panelists to select temporary PNS over ablation for the the treatment of low back pain.
Presenting risks and benefits of one intervention compared with another for the treatment of low back pain leads to patient decisions that change according to the data delivered.
After being given unbiased analysis of risks and benefits for interventions for the treatment of low back pain, spinal cord stimulation was the least preferred option.
Temporary PNS may have a role in the treatment of low back pain based on patient discussions.
Author contributions
P Staats and A Gulati had primary responsibility for writing this manuscript. E Ottestad, D Spinner and M Erdek had significant contributions in editing and interpreting the data in the manuscript. T Deer designed, executed and analyzed the data for the study
Institutional review board disclosure
This study was determined to be exempt from institutional review board approval per 45 CFR 46.101(b) (Categories of Exempt Human Subjects Research) based on its use of anonymized survey procedures [Citation22]. A medical ethicist reviewed and constructed the questions to minimize the internal bias in the questions that were presented to the participants.
Financial & competing interests disclosure
This work was conducted at SPR Therapeutics, OH, USA, and this study was supported by a grant from SPR Therapeutics. This study was funded by SPR Therapeutics, Inc. P Staats serves as a consultant to electroCore, Medtronic, Nalu, Saluda and SPR Therapeutics. A Gulati is a consultant for Flowonix, Medtronic, Nalu, SPR Therapeutics and Bausch Health and serves on the advisory board for AIS. E Ottestad serves as a consultant to Bioness, Ipsen, Nine Continents, SPR Therapeutics and Tulavi. T Deer serves as consultant to Abbott, Axonics, Boston Scientific, Cornerloc, Medtronic, Nalu, Nevro, Saluda and SPR Therapeutics. Funded research: Abbott, Avanos, Boston Scientific, Saluda and SPR Therapeutics. D Spinner is a consultant for Bioness, SPR Therapeutics and Nalu Medical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Baker A . Crossing the quality chasm: a new health system for the 21st century [book review]. BMJ323, 1192 (2001).
- Manchikanti L , BoswellMV , GiordanoJ. Evidence-based interventional pain management: principles, problems, potential, and applications. Pain Physician.10(2), 329–356 (2007).
- Marsh K . Patient preferences in health technology assessment in Europe recent advances and future potential [white paper] (2019). www.evidera.com/patient-preferences-in-health-technology-assessment-in-europe-recent-advances-and-future-potential/
- Kapural L , YuC , DoustMWet al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology123(4), 851–860 (2015).
- Kapural L , YuC , DoustMWet al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery79(5), 667–677 (2016).
- De Ridder D , PlazierM , KamerlingNet al. Burst spinal cord stimulation for limb and back pain. World Neurosurg.80(5), 642–649. e641 (2013).
- De Ridder D , VannesteS , PlazierMet al. Burst spinal cord stimulation: toward paresthesia-free pain suppression. Neurosurgery66(5), 986–990 (2010).
- Deer TR , LevyRM , KramerJet al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain158(4), 669–681 (2017).
- Deer TR , GrigsbyE , WeinerRLet al. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation16(1), 67–72 (2013).
- Kapural L , NageebF , KapuralMet al. Cooled radiofrequency system for the treatment of chronic pain from sacroiliitis: the first case-series. Pain Pract.8(5), 348–354 (2008).
- Bellini M , BarbieriM. Cooled radiofrequency system relieves chronic knee osteoarthritis pain: the first case-series. Anaesthesiol. Intensive Ther.47(1), 30–33 (2015).
- Rauck RL , CohenSP , GilmoreCAet al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation17(2), 188–197 (2014).
- Deer T , PopeJ , BenyaminRet al. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation.19(1), 91–100 (2016).
- Deer T , SlavinKV , AmirdelfanKet al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation21(1), 56–66 (2018).
- Oswald J , ShahiV , ChakravarthyKV. Prospective case series on the use of peripheral nerve stimulation for focal mononeuropathy treatment. Pain Manage.9(6), 551–558 (2019).
- Gilmore CA , IlfeldBM , RosenowJMet al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic post-amputation pain: a multi-center randomized placebo-controlled trial. Reg. Anesth. Pain Med.44(6), 637–645 (2019).
- Gilmore CA , KapuralL , McGeeMJ , BoggsJW. Percutaneous peripheral nerve stimulation for chronic low back pain: prospective case series with one year of sustained relief following short-term implant. Pain Pract. (2019).
- Gilmore CA , IlfeldBM , RosenowJMet al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg. Anesth. Pain Med.45(1), 44–51 (2020).
- El Majdoub F , NeudorferC , RichterRet al. 10 kHz cervical SCS for chronic neck and upper limb pain: 12 months’ results. Ann. Clin. Transl. Neurol. (2019).
- Gill JS , AsgerallyA , SimopoulosTT. High-frequency spinal cord stimulation at 10 kHz for the treatment of complex regional pain syndrome: a case series of patients with or without previous spinal cord stimulator implantation. Pain Pract.19(3), 289–294 (2019).
- Hunter CW , SayedD , LubenowTet al. DRG FOCUS: a multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation Technol. Neural Interface22(1), 61–79 (2019).
- Ilfeld BM . Informed consent for medical research: an ethical imperative. Reg. Anesth. Pain Med.31(4), 353 (2006).
- Freeman MD , WoodhamMA , WoodhamAW. The role of the lumbar multifidus in chronic low back pain: a review. PM&R2(2), 142–146 (2010).
- Wu P , DateES , KingeryW. The lumbar multifidus muscle in polysegmentally innervated. Electromyogr. Clin. Neurophysiol.40(8), 483–485 (2000).
- Danneels LA , VanderstraetenG , CambierDCet al. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur. Spine J.9(4), 266–272 (2000).
- Hodges P , HolmAK , HanssonT , HolmS. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine.31(25), 2926–2933 (2006).
- MacDonald D , MoseleyGL , HodgesPW. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. PAIN142(3), 183–188 (2009).
- Wallwork TL , StantonWR , FrekeM , HidesJA. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Manual Ther.14(5), 496–500 (2009).
- Kapural L , MekhailN. Radiofrequency ablation for chronic pain control. Curr. Pain Headache Rep.5(6), 517–525 (2001).
- Sadeghi S , BibleJE , CortesDJJoE , DiagnosticsSiM , Therapy. Quantifying Dysfunction of the Lumbar Multifidus Muscle After Radiofrequency Neurotomy and Fusion Surgery: A Preliminary Study. (2020).
- Smuck M , CrisostomoRA , DemirjianR , FitchDS , KennedyDJ , GeisserME. Morphologic changes in the lumbar spine after lumbar medial branch radiofrequency neurotomy: a quantitative radiological study. Spine J.15(6), 1415–1421 (2015).
- Bonython M , NottageT , XuL , ZottiM , FisherT , SelbyM. Magnetic resonance imaging morphology of lumbar paraspinal muscles following successful bilateral facet joint denervation (2019).
- Cohen SP , BhaskarA , BhatiaAet al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg. Anesth. Pain Med.45(6), 424–467 (2020).
- Tsao H , TuckerKJ , HodgesPWJN. Changes in excitability of corticomotor inputs to the trunk muscles during experimentally-induced acute low back pain. 181, 127–133 (2011).
- Deckers K , DeSmedt K , MitchellBet al. New therapy for refractory chronic mechanical low back pain – restorative neurostimulation to activate the lumbar multifidus: one year results of a prospective multicenter clinical trial. Neuromodulation Technol. Neural Interface.21(1), 48–55 (2018).
- Ilfeld BM , GabrielRA , SaidETet al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the sciatic nerve for postoperative analgesia following ambulatory foot surgery, a proof-of-concept study. Reg. Anesth. Pain Med.43(6), 580–589 (2018).
- Lin T , GargyaA , SinghHet al. Mechanism of peripheral nerve stimulation in chronic pain. Pain Med.21(Supp. 1), S6–S12 (2020).
- Silva JR , SilvaML , PradoWA. Analgesia induced by 2-or 100-Hz electroacupuncture in the rat tail-flick test depends on the activation of different descending pain inhibitory mechanisms. J. Pain.12(1), 51–60 (2011).
- MacKenzie K , OttestadE , DerbyRet al. Lead fracture is a common complication of StimRouter explantation. Presented at: 2020 North American Neuromodulation Society Annual Meeting.NV, USA (2020).
- Sidani S , EpsteinDR , BootzinRRet al. Assessment of preferences for treatment: validation of a measure. Res. Nurs. Health.32(4), 419–31 (2009).
- Bell LV , CornishP , FluskDet al. The INternet ThERapy for deprESsion Trial (INTEREST): protocol for a patient-preference, randomised controlled feasibility trial comparing iACT, iCBT and attention control among individuals with comorbid chronic pain and depression. BMJ Open10(2), e033350 (2020).
