Abstract
Aim: To describe physical, social and emotional aspects of pain self-reported by patients with multiple myeloma (MM), and patient–physician communication of physical pain. Materials & methods: We analyzed self-reported data from 330 adults receiving anti-MM therapy in Germany and Italy on health-related quality of life (European Organisation for Research and Treatment of Cancer Quality of Life Core-30 Questionnaire version 3, -MY20) and bone pain symptoms. Results: Patients experienced clinically important physical (69%), emotional (58%) and social (22%) pain. Less than three-quarters of physicians’ records matched patients’ perception of bone pain (71.5%), with bone pain not recorded in 19.7% of patients experiencing it. Nearly half of physicians underestimated bone pain severity. Conclusion: Patients with MM experience physical, social and emotional pain. Discordance regarding bone pain symptoms and severity was observed, suggesting the need for improved communication.
Pain is commonly experienced by people living with cancer, contributing to poor health-related quality of life (HRQoL) [Citation1]. Despite pain management being an integral part of cancer care, undertreatment of pain in cancer is common [Citation1] with treatment often not proportional to the pain intensity experienced [Citation2]. Some reported reasons for undertreatment of pain include a lack of knowledge and understanding of pain, a patient’s reluctance to admit pain, a healthcare professional’s (HCP) failure to understand the impact of pain on the person with cancer, and ultimately poor communication between patients and HCPs [Citation3,Citation4]. HCPs are more likely to focus on physical pain and not incorporate the more holistic aspects of pain [Citation4,Citation5]. To comprehensively assess the patient experience of pain, it is important to look beyond the physical component of pain and to understand what this pain means to the patient, including how it impacts the person both socially and emotionally.
The different aspects of pain require the HCP and the clinical team to be aware of the physical, social, emotional and spiritual components of pain [Citation3,Citation4]. Patients living with multiple myeloma (MM) typically have a high symptom burden; most will experience chronic pain associated with their disease, with up to 90% experiencing bone pain [Citation6]. Appropriately capturing the patients’ experience of pain can provide insights into the patients’ perceptions of their disease, the impact of treatment, any existing co-morbidities and a basis for appropriate patient management. This may be of benefit to patients and HCPs when choosing treatment options to reduce pain at rest or movement, to prevent skeletal-related events or progression of bone pain using bone-targeting agents (BTA) [Citation7], and to improve function and productivity. There is a growing awareness of the importance of educating patients about pain associated with cancer and the available management options [Citation8] and educating HCPs about the total experience of pain [Citation4]. The patient’s emotional needs and their experiences throughout different stages of their treatment (such as remission or relapse) are often overlooked and poorly addressed by HCPs and are not adequately addressed as part of routine disease management [Citation9]. There is a lack of published studies describing the different aspects of pain as reported by people living with cancer.
In the current study, we sought to describe the physical, social and emotional aspects of pain experienced by patients with symptomatic MM who self-reported the severity of their physical pain (including bone pain) at their routine face-to-face consultation. In addition, we describe patient–physician communication via concordance of the patient and physician perspective, specifically on bone pain symptoms and severity.
Materials & methods
Recruitment, participants & procedures
This study analyzed secondary data collected in the Adelphi Multiple Myeloma Disease Specific Programme™ (DSP™) patient-level database. DSPs are large, independent, multinational point-in-time surveys undertaken in clinical practice. Details of the full DSP methodology have previously been described and validated [Citation10–12]. The current study was based on cross-sectional survey data collected from 20 November 2017 through 1 February 2018 across hospitals in Germany and Italy. Participants and outcomes assessed are summarized in .
Supplementary Table 1 presents a short description of the questionnaires used for collecting outcomes data.
*Reported symptom concordance was based on data for 330 patient–physician pairs. Each physician could be matched with more than one patient.
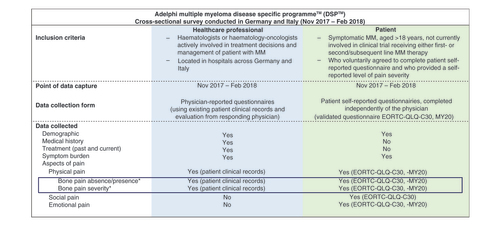
A representative sample of physicians responsible for treatment decisions and management of patients with MM were recruited and gave consent to participate in the DSP. Physicians were instructed to complete a record form for the next eight consecutively consenting patients who visited the physician for routine care, to generate a sample reflective of real-world clinical practice and to mitigate against potential selection bias. Patients were eligible if they were ≥18 years at time of survey, had symptomatic MM, and received either first-line or second/subsequent-line MM therapy at time of data collection. Half of the physician record forms were completed for first-line (1L) patients, with the remaining half of patients receiving second-line or greater (2L+) treatment. Patients and physicians could not be identified; all data were aggregated and de-identified before receipt.
The patient data analyzed within this sample was a subset derived from the Adelphi MM DSP based on outcomes of interest, using patients who agreed to complete the patient-reported questionnaire and who provided a self-reported level of pain severity. No formal sample size was defined in advance as the initial survey was not hypothesis driven.
Patient’s clinical characteristics might underscore important differences in the way pain was perceived and experienced. To assess and account for the impact of these characteristics, we stratified patients into: those diagnosed with MM <1 year or ≥1 year; those with no record of bone pain prior to or at MM diagnosis; those with self-reported bone pain, those with a record BTA use or record of analgesics therapy at time of face-to-face consultation. Subgroups were not mutually exclusive, patients could be included in one or more subgroups mentioned (e.g., have both a record of BTA use and record of analgesics at time of face-to-face consultation).
Outcome measures
Physician-reported outcomes
For each patient recruited, a detailed physician-reported questionnaire was completed by their treating physicians (hematologists or hematology-oncologists) via a standardized electronic form, with information collected on patient demographics, diagnosis, management, clinical status, concomitant conditions, current treatment and treatment history. Completion of the physician-reported questionnaire was undertaken through consultation of existing patient clinical records and the judgement and diagnostic evaluation of the responding physician, which is consistent with decisions made in routine clinical practice.
Physicians were asked to record both historic and current patient symptomatic burden () by responding to the questions “Which of the following symptoms was the patient experiencing at initial diagnosis of multiple myeloma?” and “Which of the following symptoms is the patient currently experiencing?”. If the patient selected “Bone pain” as an experienced symptom at either diagnosis of MM or currently, bone pain severity was quantified by the physician by responding to the question “What was this patient’s level of bone pain at diagnosis of multiple myeloma?” and “What is this patient’s current level of bone pain?” with the physician selecting one response from “mild pain”, “moderate pain” “severe pain” or “not assessed/don’t know”.
Patient-reported outcomes
Patients were invited to complete a standardized patient-reported questionnaire independently of their treating physician immediately after their standard face-to-face consultation (also referred to as time of survey), which were returned in a sealed envelope to ensure confidentiality.
These patient-reported questionnaires collected information on the patient’s generic or disease-specific QoL and symptom burden; patients reported symptoms that they experienced at MM diagnosis and symptoms currently experienced.
Patients self-rated their physical pain severity by responding to the following question: “Please tick the box that best describes the level of pain that you are currently experiencing”. Each patient chose one response, from “no pain”, “mild pain”, “moderate pain” or “severe pain”.
presents the questions used to compare between patient- and physician-recorded pain. Supplementary Table 1 presents a short description of the questionnaires used for collecting outcomes data.
Aspects of pain
Aspects of pain, namely physical, social and emotional, were determined using responses to the validated European Organisation for Research and Treatment of Cancer Quality of Life Core-30 Questionnaire version 3 (EORTC QLQ-C30) [Citation13,Citation14] and the EORTC-QLQ-C30 20-item myeloma-specific questionnaire (EORTC QLQ-MY20) [Citation15]. For each question, patients were asked to respond to each statement “Not at All”, “A Little”, “Quite a Bit” and “Very Much”. Raw scores for each scale were then standardized to a range from 0 to 100, using linear transformation; a higher score represents a higher (“better”) level of functioning or a higher (“worse”) level of symptoms. These aspects of pain (defined as physical [including bone pain], social, emotional pain) were used as proxies, and were defined by responses to the validated EORTC QLQ-C30 and EORTC QLQ-MY20 questionnaires (Supplementary Table 1). The definition of ‘physical pain’ was based on the validated EORTC QLQ-C30 pain scores, with further insights derived from the responses to the EORTC QLQ-MY20 disease symptoms questions. The definition of ‘social pain’ was derived from the validated EORTC QLQ-C30 social functioning scores. The definition of ‘emotional pain’ was based on validated EORTC QLQ-C30 emotional functioning scores, with additional insights on the patients’ future perspective obtained via responses to the EORTC QLQ-MY20 future perspective scores. Base sizes for EORTC QLQ-C30 and MY20 scores varied due to missing responses.
To describe the physical, social and emotional aspects of pain, validated thresholds for clinical importance (TCI) [Citation16] were used to categorize patients into two groups: those with or without a clinically important symptom or functional impairment. TCIs were reported only for EORTC QLQ-C30 scores [Citation16] as such thresholds were not available for EORTC QLQ-MY20. Patients who crossed the following EORTC QLQ-C30 score thresholds were considered to have a clinically important symptom or impairment: physical pain, >25; social pain <58; emotional pain <71 (Supplementary Table 2). Patients were considered to have bone pain in the past 7 days before the survey if they answered “A Little”, “Quite a Bit” or “Very Much” to any combination of EORTC QLQ-MY20 questions 31 to 35 on bone pain/aches (question 31) or site-specific bone pain (questions 32–35).
Ethics
Data collection was undertaken in line with the European Pharmaceutical Market Research Association (EphMRA) Code of Conduct [Citation17]. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act (HIPAA), [Citation18] and Health Information Technology for Economic and Clinical Health (HITECH) Act legislation [Citation19]. Approval for the multinational, cross-sectional studies for the DSP was granted by the Freiburger Ethik Kommission International (FEKI), which is an official independent body that reviewed the DSP methodology and questionnaires offering international coverage.
Statistical analysis
Descriptive statistics were presented as frequency, and percentages for categorical variables and mean and standard deviation or median and interquartile range (IQR) for continuous variables. Chi-squared tests for categorical variables and ANOVA tests were used for numeric variables.
A bivariate analysis was performed to determine if self-reported physical pain severity was associated with aspects of pain outcomes. A linear regression was performed that generated a coefficient for each of the pain categories (mild, moderate and severe) which indicated the difference between that severity category and the category without pain.
Concordance analysis was performed to compare the patient and physician perspectives on bone pain symptoms and severity using the symptomatic burden collected via the patient- and physician-reported questionnaire respectively. Results are shown as a calculated κ statistic (or weighted κ statistic) or calculated proportions based on a cross-tabulation of the patient variable with the physician variable. κ statistics were interpreted as follows: <0.0, no agreement; 0.00–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; 0.81–1.00, almost perfect agreement [Citation20].
The main analysis included nonmissing data only. For patients’ demographic or clinical data not reported by their treating physicians, a missing category was added as appropriate. A patient was excluded from analysis in case of form-level missingness (if a whole questionnaire was missing). Missing or incomplete data were not replaced or imputed in case of item-level missingness (where some patients did not answer certain questions). The base sizes therefore varied by analysis. A p-value < 0.05 were considered statistically significant.
Results
Patient demographic & clinical characteristics
In total, data for 330 patients were analyzed. Detailed information on the demographic and clinical characteristics of these 330 patients with MM and all subgroups is reported in . Most patients were female (n = 207; 62.7%) and retired (n = 216; 65.5%). Mean age was 68 years. Cardiovascular disease (n = 169; 51.2%) was the most common comorbidity. Nearly half of patients (48.5%) had lived longer than 1 year with MM and 17.3% had lived with MM for more than 3 years (data not shown). Almost half of patients were at stage III (n = 160; 48.5%) and were receiving second-line treatment or higher (n = 155; 46.9%). The median (IQR) time since MM diagnosis was 0.96 (0.30, 2.07) years. A quarter of patients (n = 82; 24.8%) had moderate-to-severe Eastern Cooperative Oncology Group (ECOG) performance (defined as an ECOG score of 2-4). Combination chemotherapy regimens based on lenalidomide (n = 143; 43.9%) and bortezomib (n = 141; 42.7%) were common (data not shown). Patients took a median (IQR) of 3 (1, 6) months to have a first consultation about their MM concerns with a physician. The most common reason for consultation (60.1%) was due to worry about MM symptoms (data not shown). When patients were asked to define the most important benefit of treatment other than cure, improvements in HRQoL were most important (all patients; 62.1%), followed by improvements in medical condition (24.3%) and improvements in treatment delivery (3.4%), with 20.9% unknown. Differences were seen in the proportion of patients with no pain (29.4%), mild pain (68.8%) and moderate/ severe pain (72.7%) who considered improved HRQoL as the most important treatment benefit (p = 0.0002) (data not shown).
Table 1. Patient demographic and clinical characteristics at face-to-face consultation.
Physical pain
In the past 7 days prior to time of survey, most patients experienced (n = 227; 69%) clinically important physical pain (). Patient self-reported physical pain severity was described as no physical pain, mild, moderate or severe by 22.1% (n = 73), 48.8% (n = 161), 24.6% (n = 81) and 4.6% (n = 15) patients, respectively ().
Table 2. Proportions of patients experiencing clinically important symptom or functional impairmentTable Footnote†.
For physical pain, mean pain scores increased with pain severity (p < 0.0001 all subgroups), and patients classified with mild, moderate or severe pain were over the TCI. Patients diagnosed with MM <1 year with no pain had a mean physical pain score of 9.09, and patients diagnosed with MM ≥1 year had a mean score of 16.67. Patients on analgesic therapy with no pain had a mean physical pain score of 26.67 and those with no pain on BTA had a mean physical pain score of 11.36 (A).
MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. (B) Physical pain (EORTC QLQ-MY20 Disease symptoms) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation. MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. No clinical threshold available for responses to the MY20 questionnaire.
BTA: Bone-targeting agent; MM: Multiple myeloma.
![Figure 2. (A) Physical pain (European Organisation for Research and Treatment of Cancer Quality of Life Core-30 Questionnaire version 3 pain) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation.MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. (B) Physical pain (EORTC QLQ-MY20 Disease symptoms) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation. MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. No clinical threshold available for responses to the MY20 questionnaire.BTA: Bone-targeting agent; MM: Multiple myeloma.](/cms/asset/a177517b-505b-4b52-bec6-9f3a365171ef/ipmt_a_12344458_f0002.jpg)
Mean EORTC disease symptom scores also increased with pain severity (p < 0.0001 all subgroups). Patients with mild pain reported mean disease symptom scores around the TCI, with patients with moderate or severe pain over the clinical threshold. Patients diagnosed with MM <1 year with no pain had a mean disease symptom score of 7.47 and those diagnosed with MM ≥1 year had a mean disease symptom of 12.5. Patients on analgesic therapy with no pain reported had different mean disease symptoms scores than those with no pain on BTA (mean scores: 17.78 vs 10.35, respectively) (B).
A linear regression revealed that when compared with patients that did not self-report physical pain, patients that self-reported physical pain had a significant increase in mean differences in physical aspects of pain and disease symptom scores (all p < 0.0001) (Supplementary Table 3).
Bone pain
Most patients (n = 289; 87.6%) reported experiencing bone pain during the past 7 days prior to the survey (), with 252 patients (76.4%) reporting bone pain or aches. Vertebral (back) pain was reported by most patients (n = 238; 72.1%). Other sites of bone pain reported were hip pain (n = 185; 56.1%) and chest/rib pain (n = 110; 33.3%). Over half (n = 186; 56.4%) of patients had a record of bone lesions and 38 patients (11.5%) patients had a record of a skeletal-related event at any time.
Increasing physical pain severity was associated with an increased EORTC bone aches or pain score (mean score 1.38–3.0; p < 0.0001). The increase in physical pain severity was more pronounced in patients with vertebral pain (mean score 1.32–2.80; p < 0.0001) than nonvertebral pain (1.25–2.07; p < 0.0001). The mean score for physical pain was consistently higher in patients experiencing vertebral pain than nonvertebral pain.
Patient–physician communication on bone pain symptoms & severity
We assessed concordance between patients and physicians in reporting of bone pain symptoms and bone pain severity (). Not all symptoms reported by patients via the patient-reported questionnaire were recorded by the physician in the physician-reported questionnaire.
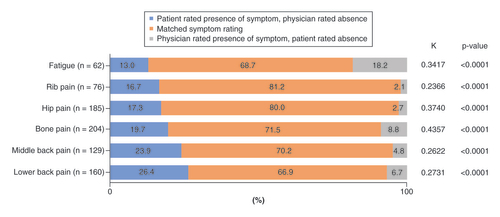
Reported symptom concordance was 66.9% for lower back pain, 70.2% for middle back pain, 71.5% for bone pain, 80.0% for hip pain and 81.2% for rib pain ().
The proportion of discordance varied considerably depending on the symptom, with the lowest level seen for rib pain (16.7%) and the highest for lower back pain (26.4%). In a smaller number of cases, the physician recorded the presence of a symptom, however this was not independently reported by the patient. Patient–physician discordance was higher for nonspecific bone pain (8.8%), and lowest for rib pain (2.1%) and hip pain (2.7%) (). The poor concordance between patients and physicians is reflected in the generally low kappa values (κ <0.40) observed for most symptoms.
Physicians were more likely to record bone pain or aches when patients reported bone pain or aches in general (physicians vs patients; 39.6 vs 37.2%; p < 0.0001) or pain in the back (36.5 vs 35.9%; p < 0.0001) than when patients reported pain in the chest (14.0 vs 19.5%; p = 0.5132), arm/shoulder (19.8 vs 23.7%; p = 0.129) or hip (27.7 vs 28.7%; p = 0.0015). Physicians generally did not specify the site of bone pain in their records. For instance, where 45.4% of patients self-reported hip pain, there was no physician record of hip pain, and where only 11.0% of physicians recorded hip pain, their patients also reported hip pain (Supplementary Table 4).
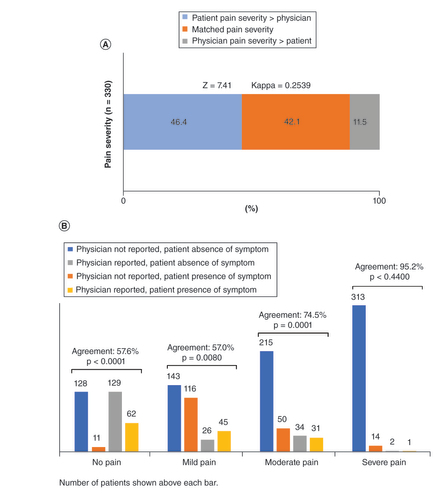
Only 42.1% of physicians reported the same bone pain severity as experienced by their patients. Nearly half of physicians (46.4%) underestimated the severity of bone pain experienced by their patients, and 11.5% overestimated the bone pain severity (A). When stratified by pain severity category, agreement between patients and physicians was highest among severe pain (n = 314, 95.2%) and lowest for no pain (n = 190, 57.6%) (B) (Supplementary Table 5).
Patient–physician communication on other symptoms
Patient–physician reported symptom concordance for communication on other symptoms was also investigated. We observed a low level of agreement (κ = 0.25) between patient and physician responses. Where both patients and physicians recorded symptom presence, the highest proportions were for fatigue (22.7%), followed by weight loss (10.6%) and loss of appetite (10.3%). Where patients recorded symptom presence but physicians did not, the highest proportion of discordance were seen for fatigue (13.0%), tingling of hands and feet (12.1%) and shortness of breath (10.3%). Where physicians recorded symptom presence but patients did not, the highest proportion of discordance were seen for fatigue (18.2%), pallor/pale skin (16.1%) and loss of appetite (11.8%) (Supplementary Table 6).
Emotional pain
Of the 327 patients who responded, 189 (58%) experienced clinically important emotional pain (). For emotional pain, mean scores based on responses on the EORTC QLQ-C30 emotional functioning decreased with pain severity (p < 0.0001 all subgroups). Patients classified with mild, moderate or severe pain (with exception of mild pain MM diagnosis <1 year) were below the TCI. Patients diagnosed with MM <1 year with no pain had a mean emotional pain score of 76.26, and those diagnosed with MM ≥1 year had a mean score of 79.58. Patients on analgesic therapy with no pain had a mean emotional pain score of 80.83 and those with no pain on BTA had a mean score of 78.41 (A).
MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; Bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. (B) Emotional pain (EORTC QLQ-MY20 Future perspective) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation. MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for one year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. No clinical threshold available for responses to the MY20 questionnaire.
BTA: Bone-targeting agent; MM: Multiple myeloma.
![Figure 5. (A) Emotional pain (European Organisation for Research and Treatment of Cancer Quality of Life Core-30 Questionnaire version 3 Emotional functioning) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation.MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; Bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. (B) Emotional pain (EORTC QLQ-MY20 Future perspective) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation. MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for one year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. No clinical threshold available for responses to the MY20 questionnaire.BTA: Bone-targeting agent; MM: Multiple myeloma.](/cms/asset/d71659f6-887f-416e-8378-f0b121171586/ipmt_a_12344458_f0005.jpg)
As pain severity increased from no pain to moderate pain, mean EORTC QLQ-MY20 future perspective scores decreased (p < 0.01 all subgroups). There was little difference between those with mild, moderate or severe pain across subgroups analysed. Patients diagnosed with MM <1 year with severe pain had a mean future perspective score of 51.85 and those diagnosed with MM ≥1 year had a score of 44.44 (B).
Social pain
Out of the 329 patients who responded, 74 (22%) experienced clinically important social pain (). For social pain, as pain severity increased mean scores based on responses to the EORTC QLQ-C30 social functioning decreased. All patients with severe pain had a mean score below the TCI (p < 0.0001 all subgroups except for patients with MM diagnosis ≥1 year). Patients with no pain and mild pain diagnosed with MM <1 year had different mean social functioning scores than those diagnosed with MM ≥1 year (no pain: 83.84 vs 78.75, mild pain: 79.82 vs 72.82, respectively). Patients with moderate pain diagnosed with MM <1 year had a mean social functioning score of 62.15 and those diagnosed with MM ≥1 year had a score of 69.70. Patients on analgesic therapy with no pain reported a mean social functioning score of 88.33 and those with no pain on BTA reported a score of 81.06 ().
MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation.
BTA: Bone-targeting agent; MM: Multiple myeloma.
![Figure 6. Social pain (European Organisation for Research and Treatment of Cancer Quality of Life Core-30 Questionnaire version 3 Social functioning) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation.MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation.BTA: Bone-targeting agent; MM: Multiple myeloma.](/cms/asset/a48492f0-9633-4d61-a96d-95e685db3960/ipmt_a_12344458_f0006.jpg)
Discussion
This real-world cross-sectional study of patients with MM and their consulting physicians revealed that patients experience different aspects of pain (defined here as physical, emotional and social aspects of pain). When assessing patient–physician communication via concordance analyses, we observed discordance between patient- and physician-reported bone pain severity and bone pain symptoms. Almost half of physicians underestimated the severity of bone pain experienced by their patients, while not all patients experiencing bone pain had a physician record for the symptom. Since bone pain symptoms are subjective in nature, these findings indicate a need for improved pain communication between patients and physicians.
Patients experienced not only physical pain, but also emotional pain (derived from EORTC QLQ-C30 emotional functioning score and EORTC QLQ-MY20 future perspectives scores) and social pain (derived from EORTC QLQ-C30 social functioning scores); for some patients these were also clinically important. Each of these aspects of pain became progressively worse with increasing disease severity. Hence, simply relying on the physical aspect of pain may not adequately reflect the true severity and impact of pain in clinical practice [Citation5,Citation21] . Our findings suggest that a comprehensive assessment of pain must consider not just physical symptoms but also other dimensions, including social and emotional factors relating to burden of disease and quality of life. Tools such as the Managing Advanced Cancer Pain Together (MACPT) tool allow patients to express how their pain manifests itself from a physical, social and emotional and existential perspective, allowing freedom to express the impact and burden of their symptoms in a real-world setting [Citation3,Citation4,Citation22].
In this study, patients self-rated their physical pain severity by responding to the question: “Please tick the box that best describes the level of pain that you are currently experiencing”. As the level of self-reported pain increased, from “no pain” to “mild pain”, “moderate pain” or “severe pain”, patient outcomes worsened across a range of measures. This single question may enable physicians to establishing pain severity during routine face-to-face consultations [Citation23]. In addition, it provides important information on the control of the disease as bone pain in MM usually reflects progressive disease and or fractures. A positive signal requires careful workup to delineate the underlying cause, which is essential for treatment planning. This may result in change of myeloma medical therapy, and in special situations in implementation of palliative radiotherapy and/or surgical interventions such as kyphoplasty and pathologic fracture stabilisation. Admittedly, in spite of all advances in pain treatment with the introduction of new formulations for drug administration such as patches, various formulations for therapy breakthrough pain, and treatments for opiod-induced constipation, pain control may remain unsatisfactory. Hence, prevention of progression of bone disease becomes important. This may also stimulate discussions focused on bone health, covering lifestyle changes, physical activities as prevention techniques, calcium and vitamin D supplements and medications to support bone health and prevent bone complications [Citation24], as well as enabling physicians to enlist the help of dedicated pain control or palliative care specialists who may be better placed to address these symptomatic burdens [Citation25]. Providing patients with relevant information, either through supplementary questionnaires or smartphone apps, may increase patient’s autonomy, reduce anxiety and improve the quality of patient–physician communication thereby improving compliance (see recommendations in Box 1).
| 1. | Consider using the Managing Advanced Cancer Pain Together (MACPT) conversation tool to facilitate communication on total cancer pain between people with cancer and their healthcare professionals [Citation3,Citation4,Citation22] | ||||
| 2. | When discussing physical pain, consider establishing pain severity by asking patients a single question during the routine face-to-face consultation: “How would you rate the severity of your pain: no pain, mild, moderate or severe?” | ||||
| 3. | At multiple myeloma diagnosis, consider having a conversation focused on bone health, covering lifestyle changes, physical activities as prevention techniques, supplements and medications to support bone health and prevent bone complications [Citation26] | ||||
| 4. | As recommended by clinical guidelines, initiate denosumab, pamidronate or zoledronate at diagnosis of multiple myeloma [Citation27] | ||||
| 5. | Consider addressing information gaps by providing patients with supplementary information (e.g. video, pamphlets, website) | ||||
The importance of effective patient–physician communication in the cancer setting has been highlighted by a recent American Society of Clinical Oncology consensus guideline [Citation28]. Strong patient–physician communication can increase treatment adherence [Citation4] and enable patients to discuss pain effectively [Citation29]. Conversely, poor communication may contribute to under treatment of cancer pain [Citation29,Citation30]. Although patient–physician communication has not been studied extensively in MM, a review of the available literature revealed several barriers to communication in the hematologic malignancy setting [Citation31]. Such barriers from the patient perspective include limitations to physician time and availability, and negative emotional states in the patient [Citation24,Citation32]. From the physician perspective, time, specialized training or tools around quantifying aspects of pain may be suboptimal [Citation9]. Pain control in MM is an important treatment goal and may be challenging in patients with unresponsive progressive disease. In these situations, medical pain therapy becomes a priority mandating the use of strong pain medication including opioids, nonopioid analgesics, adjuvant treatments and nonpharmacological interventions. This is a challenge, in part due to the complexities of the disease and its limited prognosis, and addressing all aspects of pain requires a holistic approach [Citation33]. Addressing these communication barriers may improve patient–physician concordance on pain and consequently allow the physician to better tailor their treatment to the patient’s needs. Further studies using questionnaires validated for measuring mood and social support would be warranted.
Concomitant conditions could be linked to the level of patient reported pain experienced. We did collect concomitant conditions as part of the survey, however we did not find anything statistically significant when performing bivariate analysis between pain level groups apart from with hypertension, although this was not relevant to the study outcomes as hypertension does not typically result in pain.
Various caveats must be considered when interpreting our findings. First, the DSP is based on a representative but not true random sample of physicians. Although measures were taken to minimize selection bias of the patients, the current study is based on voluntary survey participants. Hence, the results of this study are not generalizable to nonsurvey participants and the wider MM population. In addition, certain patient subpopulations were small, such as those with ‘severe pain’, and thus should be interpreted with caution. Data were collected at the time of consultation, reducing the potential for recall bias, but rendering any conclusions about causal relationships is impossible due to the point-in-time design of this study.
Despite these limitations, this study generated valuable insights on various aspects of pain. The definition of pain, from both the patient and physician perspectives, can be subjective and open to interpretation. A more holistic approach to pain identification and management is required, with physicians asking open-ended questions to consider all aspects of pain to reduce symptom burden and improve HRQoL [Citation26,Citation34]. Strategies to reduce the burden of bone pain, including patient education surrounding bone health, lifestyle changes, physical activities as prevention techniques, supplements and medications to support bone health and prevent bone complications, should also be implemented [Citation26], as well as optimizing professional qualification of the treatment physician. As healthcare evolves and patient–physician consultations shift from face-to face clinics to virtual consultations, it is even more important that communication on pain and symptom burdens of disease are effective, and the patient voice is captured accurately (Box 1).
Conclusion
Our study revealed the complexity of pain experienced by patients with MM. In addition to the widely recognized physical pain due to bone involvement, patients experienced other aspects of pain, including social and emotional pain. For physical pain in particular, patient–physician discordance regarding bone pain symptoms and severity was observed, suggesting the need for patient–physician communication and physician awareness on all aspects of pain in the clinical setting. Improving patient–physician pain communication may result in better understanding of the individual patient’s needs, improved pain management, and better quality of life of MM patients.
Despite pain management being an integral part of cancer care, under treatment of pain in cancer is common, with treatment often not proportional to the pain intensity experienced.
This study reports on the physical, social and emotional aspects of pain self-reported by patients living with multiple myeloma (MM), and patient–physician communication of physical pain.
Data from 330 adults receiving anti-MM therapy in Germany and Italy who voluntarily completed questionnaires on health-related quality of life (European Organisation for Research and Treatment of Cancer Quality of Life Core-30 Questionnaire version 3, MY20) and who self-reported bone pain during routine consultation.
The clinical importance of physical, social or emotional aspects of pain using validated thresholds, and patient–physician communication via concordance analyses of patient- and physician-reports were assessed.
For each of the considered aspects of pain, the proportion of patients experiencing a clinically important level varied with physical pain being the highest (69%), followed by emotional (58%) and social (22%) pain.
Less than three-quarters of physicians’ records matched the patients’ perception of bone pain (71.5%). Patient-reported bone pain was not recorded in 19.7% of patients experiencing it. Nearly half of physicians (46.4%) underestimated bone pain severity.
The complexity of pain experienced by patients with MM and lack of communication between patient and treating physician highlights the need for physician awareness on all aspects of pain in a clinical setting.
Improved communication would lead to a better understanding of individual patient needs, improved pain management and a better quality of life.
Author contributions
All authors were involved in conception or design, or analysis and interpretation of data; drafting and revising the article; providing intellectual content of critical importance to the work described and final approval of the version to be published, and therefore meet the criteria for authorship in accordance with the International Committee of Medical Journal Editors (ICMJE) guidelines. In addition, all named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Ethical conduct of research
Physicians consented to participate and provide patient medical information during screening. Patients provided informed consent prior completion of questionnaires, with all data aggregated and de-identified before receipt. DSP data were collected according to procedures established at Adelphi Real World and are compliant with the European Pharmaceutical Market Research Association (EphMRA) Code of Conduct, the Health Information Technology for Economic and Clinical Health (HITECH) Act and the Health Insurance Portability and Accountability Act (HIPAA) as appropriate in each specific region or territory. International approval for the survey was also granted by the Freiburger Ethik Kommission International (FEKI (017/1791).
Supplemental Document
Download MS Word (48 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/pmt-2021-0013
Financial & competing interests disclosure
Funding for this research was provided by Amgen Ltd. B Quinn is an employee of Queens University Belfast. H Ludwig is an employee of the Wilhelminen Cancer Research Institute. He has received previous research support from Takeda and Amgen. He has also been a Speaker or had Advisory Board Honoraria from Celgene, Bristol-Meyers, Takeda, Janssen, Amgen and Sanofi. He received no direct benefit from any of these companies in relation to this specific manuscript. A Bailey, K Khela and A Rider are employees of Adelphi Real World. A Seesaghur is an employee of Amgen A Marongiu and KB Carlson were Amgen employees at the time of the study but are no longer Amgen employees. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support under the guidance of the authors was provided by D Ho (ScriboMedica Ltd, Helsinki, Finland), on behalf of Adelphi Real World and Amgen, in accordance with Good Publication Practice (GPP3) guidelines. Editorial support was provided by G Sidgwick, of Adelphi Real World. Statistical support was provided by G Milligan, of Adelphi Real World.
Data sharing statement
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Real World Multiple Myeloma Disease Specific Programme. The analysis described in this study used data obtained from this survey and was funded by Amgen Ltd, who did not influence the original survey through either contribution to the design of questionnaires or data collection. All data that support the findings of this study are the intellectual property of Adelphi Real World and so are not publicly available. Data are however available upon reasonable request and with permission of Adelphi Real World.
Additional information
Funding
References
- Fallon M , GiustiR , AielliFet al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol.29(Suppl. 4), iv166–iv191 (2018).
- Greco MT , RobertoA , CorliOet al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J. Clin. Oncol.32(36), 4149–4154 (2014).
- Managing Advanced Cancer Pain Together . Managing Advanced Cancer Pain Together (MACPT): an expert guidance. http://www.macpt.info/download/J009950_CME1029_Pain_Guidence_Doc.pdf
- Quinn B , LuftnerD , DiPalma M , DarganS , DalLago L , Drudges-CoatesL. Managing pain in advanced cancer settings: an expert guidance and conversation tool. Cancer Nursing Practice16(10), 27–34 (2017).
- Mendoza TR , DueckAC , ShiQet al. The contribution of pain in determining the health status of cancer patients with bone metastases: a secondary analysis of data from three Phase III registration trials. Eur. J. Pain.22(3), 565–571 (2018).
- Coluzzi F , RolkeR , MercadanteS. Pain management in patients with multiple myeloma: an update. Cancers (Basel)11(12), 2037 (2019).
- Von Moos R , BodyJJ , EgerdieBet al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support. Care Cancer21(12), 3497–3507 (2013).
- What is cancer pain? https://www.esmo.org/for-patients/patient-guides/cancer-pain-management
- Hulin C , HansenT , HeronLet al. Living with the burden of relapse in multiple myeloma from the patient and physician perspective. Leuk. Res.59, 75–84 (2017).
- Anderson P , BenfordM , HarrisN , KaravaliM , PiercyJ. Real-world physician and patient behaviour across countries: Disease-Specific Programmes – a means to understand. Curr. Med. Res. Opin.24(11), 3063–3072 (2008).
- Babineaux SM , CurtisB , HolbrookT , MilliganG , PiercyJ. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open6(8), e010352 (2016).
- Higgins V , PiercyJ , RoughleyAet al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab. Syndr. Obes.9, 371–380 (2016).
- Brooks R . EuroQol: the current state of play. Health Policy37(1), 53–72 (1996).
- Fayers PM , AaronsonNK , BjordalKet al. The European Organisation for Research and Treatment of Cancer QLQ-C30: Aquality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst.85, 365–376 (1993).
- Cocks K , CohenD , WisloffFet al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur. J. Cancer43(11), 1670–1678 (2007).
- Giesinger JM , LothFLC , AaronsonNKet al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J. Clin. Epidemiol. doi:10.1016/j.jclinepi.2019.10.003 (2019) ( Epub ahead of print).
- European Pharmaceutical Market Research Association . Code of conduct. https://www.ephmra.org/standards/code-of-conduct/
- United States Department of Health and Human Services . Summary of the HIPAA privacy rule. http://www.hhs.gov/sites/default/files/privacysummary.pdf
- United States Department of Health and Human Services . Health Information Technology Act. https://www.healthit.gov/sites/default/files/hitech_act_excerpt_from_arra_with_index.pdf
- Landis JR , KochGG. The measurement of observer agreement for categorical data. Biometrics33(1), 159–174 (1977).
- Xiao C , PolomanoR , BrunerDW. Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs.36(6), E1–E16 (2013).
- Managing Advanced Cancer Pain Together . Managing advanced cancer pain together conversation tool. http://www.macpt.info/guidance.php
- Ludwig M , BaileyAL , MarongiuAet al. Patient-reported pain severity and health-related quality of life in patients with multiple myeloma in real world clinical practice. Cancer Reportse1429 (2021).
- Tariman JD , DoorenbosA , ScheppKG , BeckerPS , BerryDL. Patient, physician and contextual factors are influential in the treatment decision making of older adults newly diagnosed with symptomatic myeloma. Cancer Treat Commun.2(2-3), 34–47 (2014).
- Kiely F , CranA , FinnertyD , O’brienT. Self-reported quality of life and symptom burden in ambulatory patients with multiple myeloma on disease-modifying treatment. Am. J. Hosp. Palliat. Care34(7), 671–676 (2017).
- Rome S , NoonanK , BertolottiP , TarimanJD , MiceliT. Bone health, pain, and mobility: evidence-based recommendations for patients with multiple myeloma. Clin. J. Oncol. Nurs.21(Suppl. 5), 47–59 (2017).
- Coleman R , HadjiP , BodyJJet al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol.31(12), 1650–1663 (2020).
- Gilligan T , CoyleN , FrankelRMet al. Patient-Clinician Communication: American Society of Clinical Oncology Consensus Guideline. J. Clin. Oncol.35(31), 3618–3632 (2017).
- Whitten CE , EvansCM , CristobalK. Pain management doesn’t have to be a pain: working and communicating effectively with patients who have chronic pain. Perm. J.9(2), 41–48 (2005).
- Raphael J , HesterJ , AhmedzaiSet al. Cancer pain: part 2: physical, interventional and complimentary therapies; management in the community; acute, treatment-related and complex cancer pain: a perspective from the British Pain Society endorsed by the UK Association of Palliative Medicine and the Royal College of General Practitioners. Pain. Med.11(6), 872–896 (2010).
- Leblanc TW , BaileWF , EgglySet al. Review of the patient-centered communication landscape in multiple myeloma and other hematologic malignancies. Patient Educ. Couns.102(9), 1602–1612 (2019).
- Zaleta AK , MillerMF , JohnsonJet al. Symptom burden, palliative care needs, and patient-provider communication among chronic myeloid leukemia survivors. Blood130, 4704 (2017).
- Baz R , LinHM , HuiAMet al. Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health-related quality of life. Support. Care Cancer23(9), 2789–2797 (2015).
- Carlson KB , FloraDR , LoweKet al. Findings from the Bone Health Education Needs assessment (BEACON) Study: a survey of multiple myeloma and bone metastatic solid tumor patients at risk for skeletal-related events. Presented at: Journal of the Advanced Practitioner in Oncology.Seattle, Washington, USA, 24–27 October 2019.