Abstract
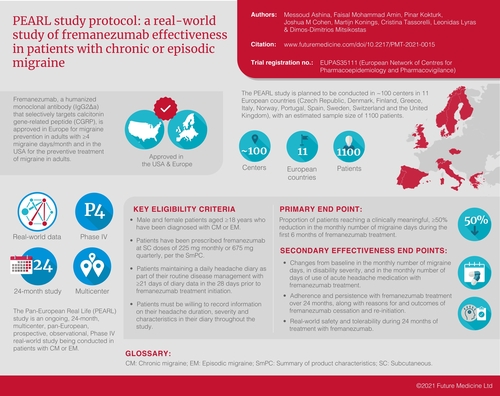
Fremanezumab is a humanized monoclonal antibody (IgG2Δa) that selectively targets calcitonin gene-related peptide and is approved in Europe for migraine prevention in adults with ≥4 migraine days/month. The Pan-European Real Life (PEARL) study is a 24-month, prospective, observational study of fremanezumab in chronic or episodic migraine. End points include proportion of patients with ≥50% reduction in monthly migraine days during 6 months of treatment (primary); changes in monthly migraine days, disability scores and acute headache medication use; adherence and persistence; and effectiveness in patients switching from another calcitonin gene-related peptide pathway-targeting monoclonal antibody. PEARL is being conducted in approximately 100 centers in 11 European countries (estimated n = 1100). PEARL will generate important real-world data on effectiveness of fremanezumab and treatment patterns in patients with chronic migraine or episodic migraine.
Lay abstract
Fremanezumab is an injectable biologic medication that targets calcitonin gene-related peptide, a substance released in the nerves and blood vessels during a migraine attack that plays a role in migraine pain. Fremanezumab is approved in Europe for preventing migraine in adults who experience ≥4 migraine days/month. The Pan-European Real Life (PEARL) study is a 24-month long study that will observe patients with migraine who are starting treatment with fremanezumab in a clinical practice setting under the care of their treating physician. The major goals of the study are to evaluate the effectiveness of fremanezumab for reducing days with migraine attacks in a month, disability associated with migraine and use of acute headache medications to treat migraine, including in patients switching from other biologic migraine therapies in the same drug class. The extent to which patients follow their recommended treatment schedule per their providers’ instructions and whether patients discontinue treatment will also be evaluated. The PEARL study will include >1000 patients in 100 centers across 11 European countries. The study will provide important information on effectiveness for patients with migraine receiving fremanezumab in the normal course of their treatment, as well as on patients’ use of fremanezumab according to their prescribing physicians’ recommendations.
Trial registration number: EUPAS35111 (European Network of Centres for Pharmacoepidemiology and Pharmacovigilance)
Migraine is a prevalent and highly disabling disease.
Migraine preventive treatments are not disease specific and are often unsatisfactory due to intolerability or lack of efficacy; adherence and persistence to these preventive treatments are low.
Fremanezumab is a humanized monoclonal antibody (mAb; IgG2Δa) that selectively targets calcitonin gene-related peptide (CGRP) and is approved in Europe for migraine prevention in adults with ≥4 migraine days/month and in the USA for the preventive treatment of migraine in adults.
The Pan-European Real Life (PEARL) study aims to provide real-world evidence of fremanezumab treatment outcomes in European clinical practice.
PEARL is an ongoing, 24-month, multicenter, pan-European, prospective, observational, Phase IV study being conducted in patients with chronic migraine or episodic migraine.
The primary end point of the PEARL study is the proportion of patients reaching ≥50% reduction in the monthly average number of migraine days during the 6-month period after the first dose of study drug.
Secondary effectiveness end points include changes from baseline in the monthly average number of migraine days, disability scores and the monthly average number of days of acute headache medication use.
Adherence and persistence with fremanezumab treatment over the 24-month follow-up period, as well as reasons for and outcomes of fremanezumab cessation and re-initiation, are also being examined.
Effectiveness will also be evaluated separately for patients switching from another CGRP pathway-targeting mAb.
The tolerability and safety of fremanezumab treatment will be assessed based on adverse event reporting.
The PEARL study is planned to be conducted in approximately 100 centers in 11 European countries, with an estimated sample size of 1100 patients.
Through the assessment of a range of effectiveness outcomes and patient-reported measures in clinical practice, PEARL will generate important information about real-world effectiveness, including in patients switching from another CGRP pathway-targeting mAb, as well as treatment adherence and persistence of fremanezumab in patients with chronic migraine or episodic migraine.
The Pan-European Real Life (PEARL) prospective, observational study of fremanezumab effectiveness in patients with chronic migraine (CM) or episodic migraine (EM) is an ongoing, Phase IV, noninterventional study sponsored by Teva Pharmaceuticals Europe BV. It is registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (EUPAS35111). PEARL is being conducted at approximately 100 sites, including a mix of specialized headache centers and general hospitals, across 11 European countries (Czech Republic, Denmark, Finland, Greece, Italy, Norway, Portugal, Spain, Sweden, Switzerland and the UK). The mix of the sites depends on the country due to variations in local reimbursement requirements that determine where calcitonin gene-related peptide (CGRP) pathway-targeted monoclonal antibodies (mAbs) can be prescribed.
Background & rationale
Migraine is a prevalent and highly disabling disease that affects >1 billion people worldwide [Citation1]. In 2019, headache disorders, including migraine, accounted for >46 million years lived with disability [Citation2]. In Europe, migraine is associated with a high disease burden as a result of substantially reduced health-related quality of life, impaired ability to perform daily activities, negative impacts on family life, lost work productivity, reduced educational and career potential and high healthcare resource utilization [Citation3–5]. Furthermore, in the periods between migraine attacks, some patients experience anticipatory anxiety and depression, fearing when their next migraine will occur, and make lifestyle adjustments that adversely affect their social interactions and overall well-being [Citation5,Citation6].
Many traditional treatment options for migraine prevention are not disease specific and are often unsatisfactory due to intolerability and poor treatment adherence and persistence [Citation7–11]. In a retrospective US claims database analysis of >8600 patients who initiated treatment with oral preventive medication for CM, adherence ranged from 26 to 29% at 6 months and from 17 to 20% at 12 months, and treatment persistence was only 25% at 6 months and 14% at 12 months [Citation9,Citation12]. In a separate retrospective, population-based study in Southern Italy that evaluated preventive treatment use in 599 patients, approximately 74% of patients discontinued their initial treatment within 1 year, with 46% of those patients permanently discontinuing treatment over the observation period, 31% restarting treatment with the same preventive medication and 23% switching to another preventive medication [Citation13].
Over the past 3 years, several mAbs targeting the CGRP pathway have been approved for the preventive treatment of migraine in adults based on efficacy and safety results from large-scale, placebo-controlled clinical trials in patients with CM or EM [Citation14,Citation15]. Fremanezumab is a humanized mAb (IgG2Δa) that selectively targets CGRP and is approved in Europe for migraine prevention in adults with ≥4 migraine days/month [Citation16] and in the USA for the preventive treatment of migraine in adults [Citation17]. Fremanezumab can be administered via either monthly or quarterly subcutaneous dosing regimens for the long-acting prevention of migraine [Citation16,Citation18]. In placebo-controlled clinical trials, fremanezumab treatment was associated with reduced headache frequency, severity and duration in patients with CM or EM, including patients with acute medication overuse and patients with difficult-to-treat migraine, such as those with inadequate response to 2–4 classes of migraine preventive medications [Citation19–23]. In a long-term extension study following the pivotal 3-month studies of fremanezumab in CM and EM, efficacy was maintained for a further 12 months of treatment, with no evidence of wearing off at the end of the quarterly or monthly dose intervals [Citation18,Citation24]. Additionally, patients reported high levels of treatment satisfaction, as well as improvements in sleep quality, reduced anxiety and increased quality of time spent with others [Citation25]. However, real-world effectiveness, treatment persistence and adherence data are limited for fremanezumab and other CGRP pathway-targeting mAbs for the prevention of CM or EM.
The PEARL study aims to provide real-world data on fremanezumab treatment outcomes in patients with CM or EM by evaluating effectiveness, adherence and persistence with fremanezumab treatment in European clinical practice, including effectiveness in patients switching from another mAb targeting the CGRP pathway. Here we describe the protocol of the ongoing PEARL study.
Design
Study design
PEARL is an ongoing, 24-month, multicenter, pan-European, prospective, observational, Phase IV study. The aims of the study are to evaluate the effectiveness of fremanezumab treatment and other measures of clinical treatment, including concomitant preventive and acute migraine medication use and treatment adherence and persistence, in adult patients with CM or EM in European real-world clinical practice.
Eligibility criteria
The study population will be composed of male and female patients aged 18 years or older who have been diagnosed with CM (≥15 headache days/month for >3 months, ≥8 of which meet migraine criteria) or EM (≥4 migraine days/month) and have been prescribed fremanezumab at subcutaneous doses of 225 mg monthly or 675 mg quarterly, according to the summary of product characteristics [Citation16]. Eligible patients are those maintaining a daily headache diary as part of their routine disease management per their treating physician and who have ≥21 days of diary data in the 28 days prior to fremanezumab treatment initiation. Patients must be willing to record information on their headache duration, severity and characteristics in their electronic or paper headache diary throughout the study period. Informed consent will be obtained from all patients for their clinical data to be recorded anonymously and to collect patient questionnaires.
Eligible patients must have been prescribed fremanezumab and must have taken their first dose within 3 months (+7 days) of study enrollment. Before the start of the study, patients may have been prescribed any acute or other preventive migraine treatments by their treating physician. Patients are permitted to continue these treatments, changed or unchanged, throughout the study, and these treatments will be documented. Patients are permitted to continue these treatments, changed or unchanged, throughout the study. Up to 30% of the study population may have previously received preventive migraine treatment with other mAbs targeting the CGRP pathway. When switching from another mAb targeting the CGRP pathway is permitted, it is recommended in the protocol that patients be advised by their physician to wait until their next scheduled dose before starting fremanezumab.
Patients who are participating in an interventional clinical trial in CM or EM or who are unable or unwilling to keep records on headache duration, severity and characteristics in an electronic or paper headache diary for the duration of the study are not eligible to participate in the PEARL study.
Planned sample size
Approximately 1100 patients will be enrolled in this noninterventional study and will be followed for a 24-month observational period. This sample size was not based on statistical considerations but was selected in an effort to include parallel cohorts across all participating countries/regions. It is anticipated that each cohort will have a minimum sample size of approximately 50 patients.
Planned study period
PEARL is an ongoing 24-month study. The first patient was screened and enrolled during the third quarter of 2020. The last patient is expected to complete the study during the first quarter of 2024, and final results should be available in the third quarter of 2024. Interim analyses are planned for when 300, 500 and all enrolled patients have completed 6 months of treatment and when all enrolled patients have completed 12 months of treatment. The first of these interim analyses should be completed in the fourth quarter of 2021.
Study procedures
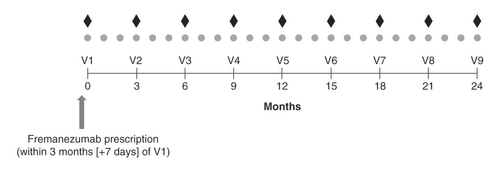
It is suggested that patients schedule clinic visits every 3 months (±15 days) for a total of nine visits () as part of routine disease management and at the discretion of the treating physician. Remote clinic visits are permitted and must be completed by the principal investigator or a subinvestigator and may include trained study staff. Data from remote clinic visits must be captured in an electronic case report form to collect medical history, history of preventive migraine medication classes, comorbidities and current concomitant medications, including acute migraine medications, by appropriately designated and trained personnel for each patient who provided informed consent.
†Diamonds refer to fremanezumab 675 mg quarterly dosing, and circles refer to fremanezumab 225 mg monthly dosing.
†Suggested visit interval is approximately every 3 months (±15 days).
V: Visit.
Baseline is defined as the 28-day period prior to fremanezumab treatment initiation. To provide baseline data, patients must have maintained a headache diary for ≥21 of 28 days during the baseline period. After initiation of treatment with fremanezumab, patients must have maintained a daily headache diary up until the time of study enrollment and must be willing to maintain a daily headache diary during the study as part of their routine disease management and as directed by their treating physician. Ideally, the headache diary is intended to capture information about headache frequency, severity, duration and characteristics, as well as information about concomitant preventive and acute migraine treatments, but individual patient data will be based on which features have been captured in their headache diary prior to study enrollment. Patient-reported outcome measures and validated headache-related disability tools included in the headache diary will provide additional data on the impact of fremanezumab in real-world clinical practice. Safety data will be obtained based on the documentation of adverse events reported in normal clinical practice.
Patients who discontinue fremanezumab treatment and subsequently begin newly prescribed preventive treatment for migraine with another preventive migraine medication will be discontinued from this noninterventional study, unless there is an ongoing adverse event that requires follow-up. With the exception of those who start a newly prescribed migraine preventive treatment, patients who discontinue treatment with fremanezumab will remain in the study, will be followed during the observational period according to the clinic visit schedule and will be encouraged to continue completing the daily headache diary as part of routine disease management. Treatment with fremanezumab may be resumed at any time, based on the decision of the treating physician per their clinical judgment and discussions with the patient. All headache diary data will be collected, regardless of missed clinic visits. Reasons for loss of follow-up will be captured.
Outcome measures
The primary end point of this study is the proportion of patients reaching ≥50% reduction in the monthly average number of migraine days during the 6-month period after the first dose of fremanezumab. Secondary clinical effectiveness end points evaluated during the 24-month follow-up period include the mean change from baseline in the monthly average number of migraine days, the proportion of patients reaching ≥50% reduction in mean number of migraine days, the change from baseline in the monthly average number of days of acute headache medication use and the change from baseline in disability scores measured using the 6-item Headache Impact Test and the Migraine Disability Assessment. The 6-item Headache Impact Test is a validated questionnaire that measures headache severity and the adverse impact of headache on social functioning, role functioning, vitality, cognitive functioning and psychological distress [Citation26,Citation27]. The Migraine Disability Assessment is a 5-item instrument that assesses headache-related disability based on lost days of activity in three domains (work, household work and nonwork) over the previous 3 months [Citation28,Citation29]. All secondary clinical effectiveness end points are evaluated at months 3, 6, 9, 12, 15, 18, 21 and 24; reductions in migraine days and days of acute headache medication use are also evaluated at month 1.
For the subgroup of patients with no prior exposure to a mAb targeting the CGRP pathway, the proportion of patients reaching ≥50% reduction in the monthly average number of migraine days during the 6-month period after the first dose of fremanezumab and during the 24-month follow-up period at months 1, 3, 6, 9, 12, 15, 18, 21 and 24 will be assessed as secondary end points.
Secondary end points related to patient adherence and persistence, respectively, with fremanezumab include whether or not the patient takes their prescribed dose of medication within ±5 days of the due date per their monthly or quarterly prescribed dosing regimen and whether or not the patient continues administration of fremanezumab as prescribed, unless told by a healthcare professional to discontinue treatment due to a successful course of treatment or local reimbursement conditions. Treatment adherence and persistence will be assessed at months 3, 6, 9, 12, 15, 18, 21 and 24.
Exploratory end points evaluating the impact of fremanezumab on reducing migraine severity and duration include the change from baseline in mean peak headache severity (assessed using an 11-point numerical rating scale) and duration of the remaining attacks at months 1, 3, 6, 9, 12, 15, 18, 21 and 24 and the proportion of patients with a downward shift from baseline of ≥1 category of the most frequently reported monthly peak headache severity on a mild, moderate or severe pain scale at months 1, 3, 6, 9, 12, 15, 18, 21 and 24. The change from baseline in the number and classes of concomitant preventive and acute medications used at months 1, 3, 6, 9, 12, 15, 18, 21 and 24 are also being assessed as exploratory end points.
Several exploratory end points assess cessation and re-initiation of fremanezumab treatment, including: the proportion of patients relapsing, defined as a ≥50% increase in migraine days/month and ≥4 migraine days/month at 1, 2, 3, 6 and 12 months after treatment cessation as compared with the last month before cessation; the mean change in monthly migraine days from the time of cessation to 1, 2, 3, 6 and 12 months after cessation; the proportion of patients with ≥50% reduction in the number of migraine days from the 28-day period before fremanezumab re-initiation to 1, 2, 3, 6 and 12 months after fremanezumab re-initiation; the mean change in monthly migraine days from the 28-day period before fremanezumab re-initiation to 1, 2, 3, 6 and 12 months after fremanezumab re-initiation; the reasons for fremanezumab cessation or re-initiation; the impact of reasons for fremanezumab cessation or re-initiation on patient outcomes; the number of months of fremanezumab treatment prior to cessation or prior to the end of the observational period; and the number of months off fremanezumab treatment prior to re-initiation or prior to the end of the observational period.
For the subgroup of patients with prior exposure to a mAb targeting the CGRP pathway, the proportion of patients reaching ≥50% reduction in the monthly average number of migraine days during the 6-month period after the first dose of fremanezumab and during the 24-month follow-up period at months 1, 3, 6, 9, 12, 15, 18, 21 and 24 will be assessed as exploratory end points.
Only adverse events reported in normal clinical practice are being collected; investigators are not soliciting information on adverse events.
Statistics
In this noninterventional, observational study, all variables will be summarized descriptively. For continuous variables, descriptive statistics (n, mean, standard deviation, standard error of the mean, median, minimum and maximum) will be provided for actual values and changes from baseline to each visit. For categorical variables, frequency and percentage will be provided. The 95% CIs will be provided for point estimates, if appropriate. Nominal p-values for comparisons to baseline or for testing some hypotheses may be provided. This approach will apply for all interim analyses and the final analysis. Data analyses will be performed based on the data available from individual patient diaries.
The time of cessation is defined as the day of the first missed scheduled dose, as compared with the patient’s last dose, or 1 month after the patient’s last dose for monthly dosing and 3 months after the patient’s last dose for quarterly dosing. The time of re-initiation is defined as the day the patient resumed dosing after having previously ceased dosing.
Concomitant migraine medications will be summarized by therapeutic class using descriptive statistics.
Descriptive summaries will be provided for reported adverse events, serious adverse events, adverse events of special interest (i.e., ophthalmic adverse events of at least moderate severity, events of anaphylaxis and severe hypersensitivity reactions) and patient withdrawals due to adverse events.
No adjustments will be made for the preplanned multiple comparisons/end points, and no sensitivity analyses are planned.
For monthly and quarterly fremanezumab dosing groups, data will be combined for all analyses. Data will also be evaluated separately for the monthly and quarterly fremanezumab dosing subgroups, the CM and EM subgroups and the subgroup of patients who did not miss any fremanezumab doses.
The enrolled and safety analysis sets include all patients who enrolled in the study. The full analysis set includes all patients in the safety analysis set who have ≥10 days of diary entry data on the primary end point after treatment initiation.
Conclusion
Through the prospective assessment of a range of effectiveness outcomes and patient-reported measures in clinical practice, the PEARL study will generate important information about the real-world effectiveness, including in patients switching from another CGRP pathway-targeting mAb, and treatment adherence and persistence of fremanezumab in patients with CM or EM. There is limited real-world data on the use of CGRP pathway-targeting mAbs in clinical practice, and the majority of effectiveness data are based on retrospective studies or studies with a limited population size [Citation30–33]. Real-world data on adherence and persistence to treatment and on effectiveness after switching from another prior CGRP pathway-targeting mAb are lacking. Furthermore, for fremanezumab, few real-world effectiveness data are available to support the efficacy demonstrated in clinical trials [Citation18–21]. Thus, the results of the PEARL study will fill crucial gaps in the understanding of the real-world effectiveness of fremanezumab and in treatment patterns and effectiveness after switching from another CGRP pathway-targeting treatment. The findings from the PEARL study may also be used to optimize treatment choices in clinical practice and to inform available treatment guidelines [Citation34], as well as to provide real-world evidence for optimizing and standardizing prescription procedures across countries.
Author contributions
All authors provided input into the design of the study, participated in the critical review of this manuscript and approved the final draft for submission.
Ethical conduct of research
This study is being conducted in a manner that is consistent with all relevant global, regional, and national guidelines and regulations for conducting studies with humans. This is a noninterventional (observational) study that complies with Article 2(c) of Directive 2001/20/EC, the 2012 Guideline on Good Pharmacovigilance Practice and the 2016 Guideline on Good Pharmacoepidemiology Practice. The study also complies with the nature of noninterventional (observational) studies referred to in the International Conference on Harmonisation Harmonised Tripartite Guideline for Pharmacovigilance Planning (E2E). The study protocol was reviewed and approved by appropriate independent ethics committees and/or institutional review boards in the appropriate geographies, as required by local law. All study participants provided written informed consent for their clinical data to be recorded anonymously and for patient questionnaires (e.g., a headache diary) to be collected. Participants were informed of their right to withdraw their consent at any time during the study.
Infographic
Download PDF (887.9 KB)Financial & competing interests disclosure
This study is sponsored by Teva Pharmaceuticals Europe BV. M Ashina serves as a consultant and/or scientific advisor for Allergan/AbbVie, Amgen, Biohaven, Eli Lilly, Lundbeck, Novartis and Teva Pharmaceuticals; a principal investigator for Alder BioPharmaceuticals, Allergan/AbbVie, Amgen, Eli Lilly, Lundbeck, Novartis and Teva Pharmaceuticals; and an associate editor for Cephalalgia and for the Journal of Headache and Pain. M Ashina has no ownership interest and does not own stock in any pharmaceutical company. M Ashina is president of the International Headache Society. FM Amin has served on an advisory board for or received honoraria from Eli Lilly, Lundbeck, Novartis and Teva Pharmaceuticals. FM Amin has also served as a principal investigator for Phase IV trials for Novartis and Teva Pharmaceuticals. P Kokturk, JM Cohen, M Konings and L Lyras are employees of Teva Pharmaceuticals. C Tassorelli serves as a scientific consultant for Allergan/AbbVie, Eli Lilly, Lundbeck, Novartis and Teva Pharmaceuticals; and as a principal investigator or collaborator in clinical trials sponsored by Alder, Allergan/AbbVie, Amgen, Eli Lilly and Teva Pharmaceuticals. C Tassorelli has received research grants from the European Commission, the Italian Ministry of Health and the Italian Ministry of University. C Tassorelli has no ownership interest and does not own stock in any pharmaceutical company. She is president-elect of the International Headache Society. D-D Mitsikostas has received consulting fees, speaking fees and travel grants from Allergan, Amgen, Bayer, Biogen, Cefaly, electroCore, Eli Lilly, Genesis Pharma, Merck Serono, Merz, Mylan, Novartis, Roche, Sanofi Genzyme, Specifar and Teva Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial assistance was provided by C Koch, of Cello Health Communications/MedErgy (Yardley, PA), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by Teva Pharmaceuticals.
Additional information
Funding
References
- Ashina M . Migraine. N. Engl. J. Med.383(19), 1866–1876 (2020).
- Steiner TJ , StovnerLJ , JensenR , UluduzD , KatsaravaZ. Lifting the burden: the global campaign against headache. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J. Headache Pain21(1), 137 (2020).
- Steiner TJ , StovnerLJ , KatsaravaZet al. The impact of headache in Europe: principal results of the Eurolight project. J. Headache Pain15(1), 31 (2014).
- Vo P , FangJ , BilitouA , LaflammeAK , GuptaS. Patients’ perspective on the burden of migraine in Europe: a cross-sectional analysis of survey data in France, Germany, Italy, Spain and the United Kingdom. J. Headache Pain19(1), 82 (2018).
- Stovner LJ , AndréeC. Eurolight Steering Committee. Impact of headache in Europe: a review for the Eurolight project. J. Headache Pain9(3), 139–146 (2008).
- Lampl C , ThomasH , StovnerLJet al. Interictal burden attributable to episodic headache: findings from the Eurolight project. J. Headache Pain17, 9 (2016).
- Tso AR , GoadsbyPJ. Anti-CGRP monoclonal antibodies: the next era of migraine prevention?Curr. Treat. Options Neurol.19(8), 27 (2017).
- Blumenfeld AM , BloudekLM , BeckerWJet al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second International Burden of Migraine Study (IBMS-II). Headache53(4), 644–655 (2013).
- Hepp Z , DodickDW , VaronSFet al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia37(5), 470–485 (2017).
- Seng EK , RainsJA , NicholsonRA , LiptonRB. Improving medication adherence in migraine treatment. Curr. Pain Headache Rep.19(6), 24 (2015).
- Goadsby PJ , SprengerT. Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol.9(3), 285–298 (2010).
- Hepp Z , DodickDW , VaronSF , GillardP , HansenRN , DevineEB. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia35(6), 478–488 (2015).
- Orlando V , MucherinoS , MonettiVM , TramaU , MendittoE. Treatment patterns and medication adherence among newly diagnosed patients with migraine: a drug utilisation study. BMJ Open10(11), e038972 (2020).
- Diener HC , FörderreutherS , GaulCet al. Prevention of migraine with monoclonal antibodies against CGRP or the CGRP receptor: addition to the S1 guideline: therapy of migraine attacks and prevention of migraine. Recommendations of the Germany Society of Neurology and the German Migraine and Headache Society. Neurol. Res. Pract.2, 11 (2020).
- Yuan H , SpareNM , SilbersteinSD. Targeting CGRP for the prevention of migraine and cluster headache: a narrative review. Headache59(Suppl. 2), 20–32 (2019).
- AJOVY®, summary of product characteristics. Teva Pharmaceuticals GmbH, Ulm, Germany (2019).
- AJOVY®, prescribing information. Teva Pharmaceuticals USA, Inc., North Wales, PA, USA (2020).
- Goadsby PJ , SilbersteinSD , YeungPPet al. Long-term safety, tolerability and efficacy of fremanezumab in migraine: a randomized study. Neurology95(18), e2487–e2499 (2020).
- Ferrari MD , DienerHC , NingXet al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, Phase IIIb trial. Lancet394(10203), 1030–1040 (2019).
- Dodick DW , SilbersteinSD , BigalMEet al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA319(19), 1999–2008 (2018).
- Silberstein SD , DodickDW , BigalMEet al. Fremanezumab for the preventive treatment of chronic migraine. N. Engl. J. Med.377(22), 2113–2122 (2017).
- Silberstein SD , CohenJM , SeminerioMJ , YangR , AshinaS , KatsaravaZ. The impact of fremanezumab on medication overuse in patients with chronic migraine: subgroup analysis of the HALO CM study. J. Headache Pain21(1), 114 (2020).
- Lipton RB , CohenJM , BibeauKet al. Reversion from chronic migraine to episodic migraine in patients treated with fremanezumab: post hoc analysis from HALO CM study. Headache60(10), 2444–2453 (2020).
- Blumenfeld AM , StevanovicDM , OrtegaMet al. No “wearing-off effect” seen in quarterly or monthly dosing of fremanezumab: subanalysis of a randomized long-term study. Headache60(10), 2431–2443 (2020).
- Buse DC , GandhiSK , CohenJMet al. Improvements across a range of patient-reported domains with fremanezumab treatment: results from a patient survey study. J. Headache Pain21(1), 109 (2020).
- Kosinski M , BaylissMS , BjornerJBet al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual. Life Res.12(8), 963–974 (2003).
- Yang M , Rendas-BaumR , VaronSF , KosinskiM. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia31(3), 357–367 (2011).
- Stewart WF , LiptonRB , WhyteJet al. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology53(5), 988–994 (1999).
- Stewart WF , LiptonRB , KolodnerK , LibermanJ , SawyerJ. Reliability of the Migraine Disability Assessment score in a population-based sample of headache sufferers. Cephalalgia19(2), 107–114 (1999).
- Lambru G , HillB , MurphyM , TylovaI , AndreouAP. A prospective real-world analysis of erenumab in refractory chronic migraine. J. Headache Pain21(1), 61 (2020).
- Robblee J , DevickKL , MendezN , PotterJ , SlonakerJ , StarlingAJ. Real-world patient experience with erenumab for the preventive treatment of migraine. Headache60(9), 2014–2025 (2020).
- Kanaan S , HettieG , LoderE , BurchR. Real-world effectiveness and tolerability of erenumab: a retrospective cohort study. Cephalalgia40(13), 1511–1522 (2020).
- Alex A , VaughnC , RayhillM. Safety and tolerability of 3 CGRP monoclonal antibodies in practice: a retrospective cohort study. Headache60(10), 2454–2462 (2020).
- Sacco S , BendtsenL , AshinaMet al. European Headache Federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J. Headache Pain20(1), 6 (2019).